Defining an Optimal Range of Centrifugation Parameters for Canine Semen Processing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection
2.3. Semen Evaluation
2.4. Centrifugation and Evaluation of Post-Centrifugation Sperm Characteristics
2.5. Cooling and Evaluation of Sperm Characteristics at 24 h and 48 h after Cooling
2.6. Statistical Analysis
3. Results
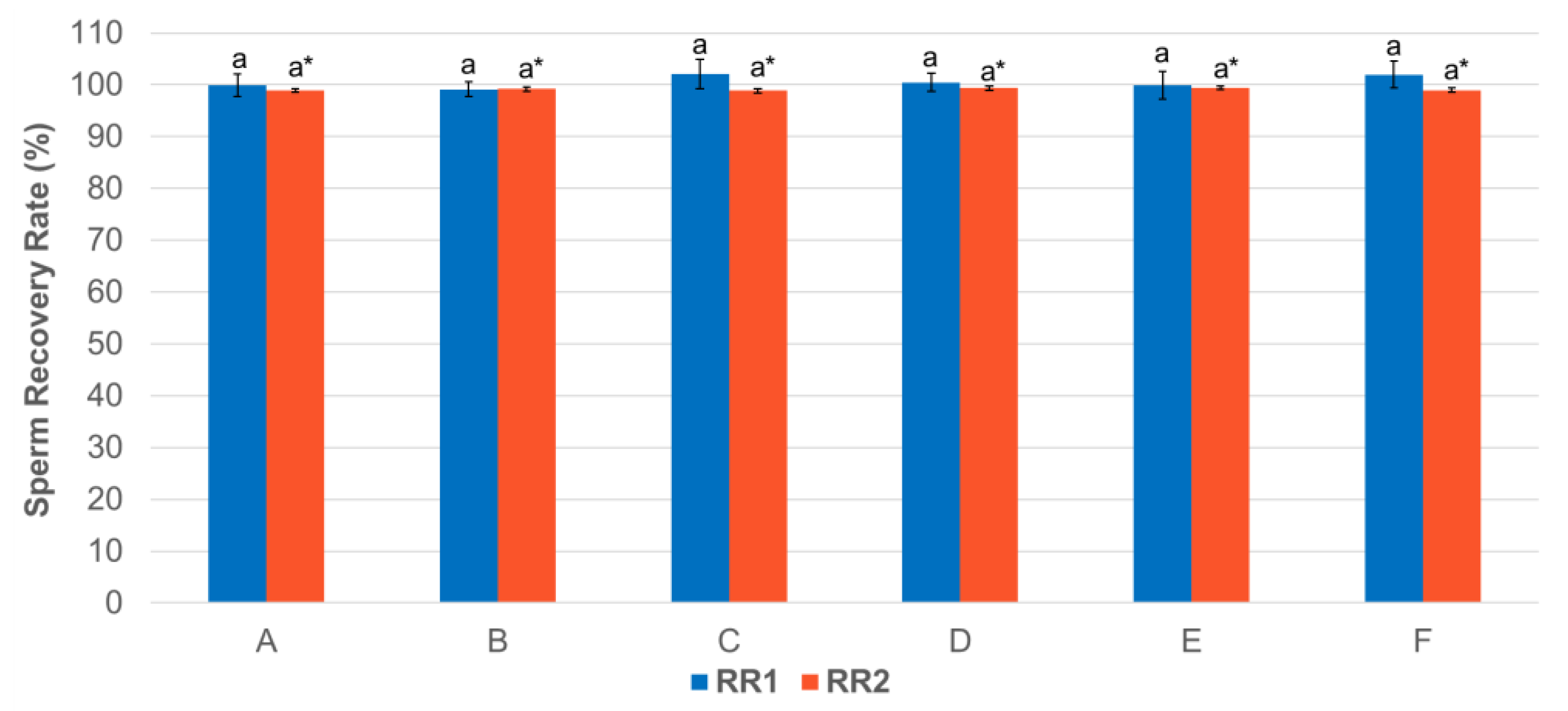
3.1. Effect of Centrifugation Treatment on Spermatozoa Recovery Rate
3.2. Effect of Centrifugation Treatment and Cooling on Sperm Plasma Membrane Integrity
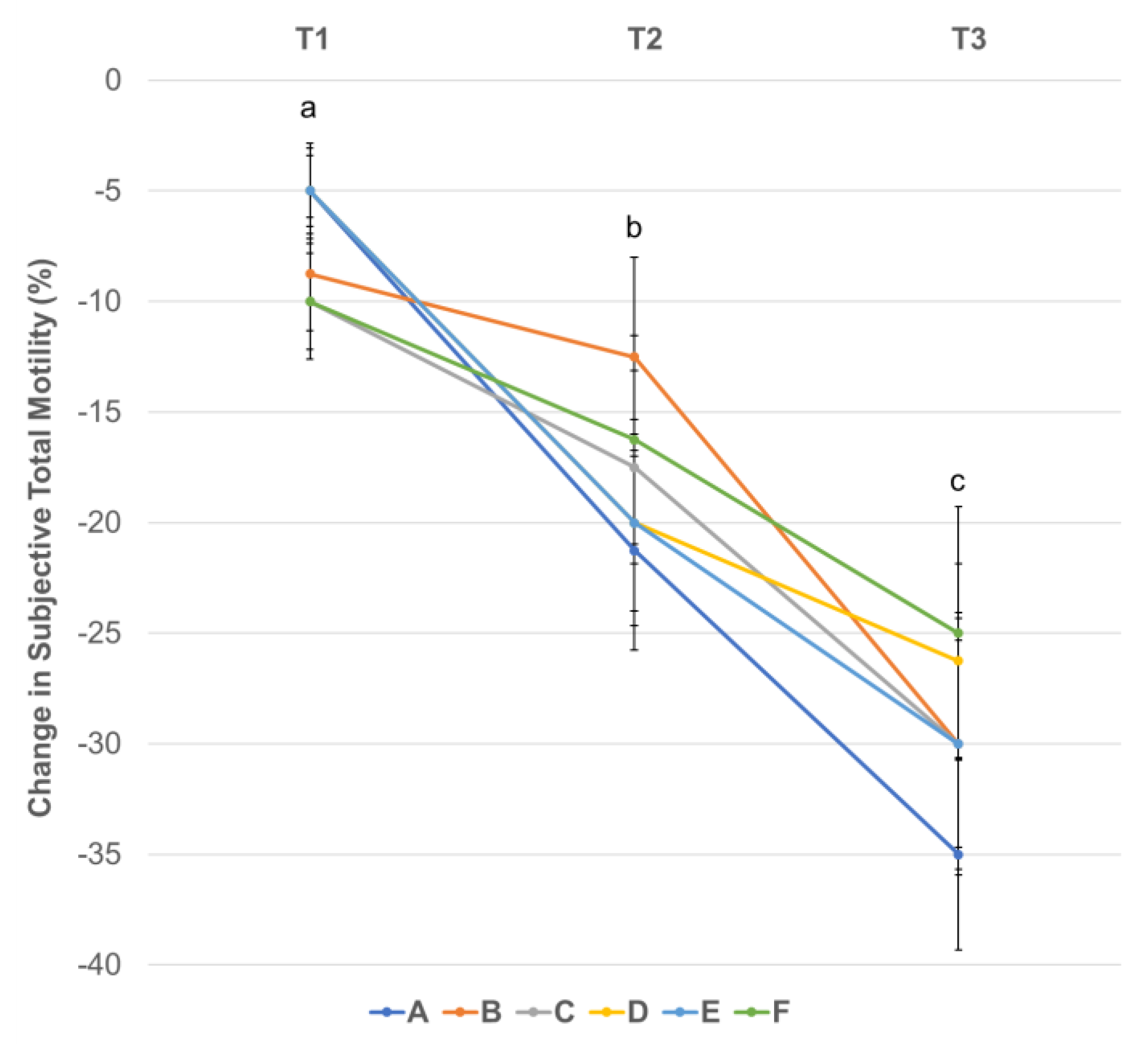
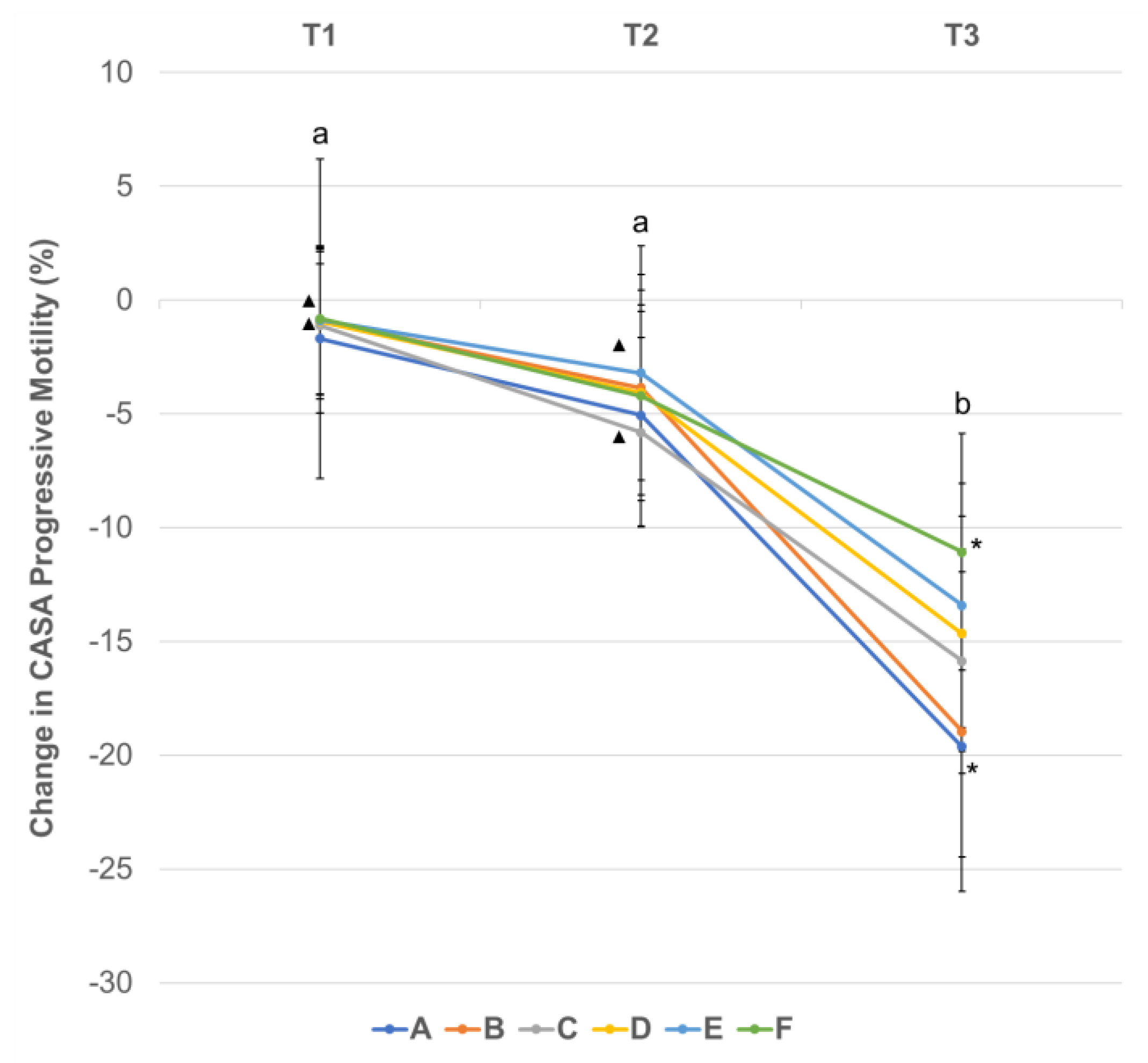
3.3. Effect of Centrifugation Treatment and Cooling on Sperm Motility
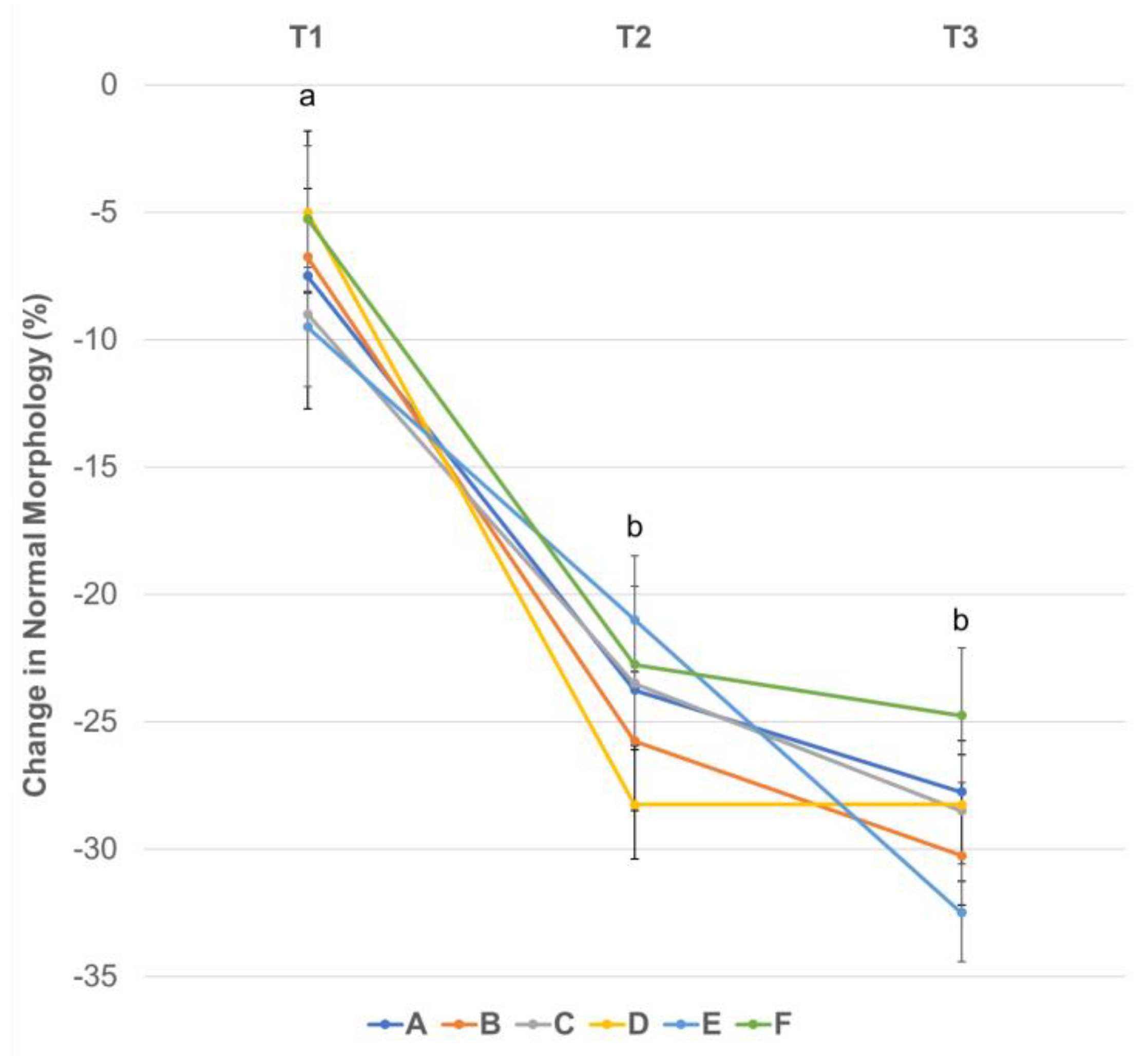
3.4. Effect of Centrifugation Treatment and Cooling on Sperm Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollinshead, F.; Hanlon, D. Factors affecting the reproductive performance of bitches: A prospective cohort study involving 1203 inseminations with fresh and frozen semen. Theriogenology 2017, 101, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Goericke-Pesch, S.; Klaus, D.; Failing, K.; Wehrend, A. Longevity of chilled canine semen comparing different extenders. Anim. Reprod. Sci. 2012, 135, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Strom, B.; Linde-Forsberg, C. Effect of seminal plasma and three extenders on canine semen stored at 4 °C. Theriogenology 1995, 44, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Tesi, M.; Sabatini, C.; Vannozzi, I.; Di Petta, G.; Panzani, D.; Camillo, F.; Rota, A. Variables affecting semen quality and its relation to fertility in the dog: A retrospective study. Theriogenology 2018, 118, 34–39. [Google Scholar] [CrossRef]
- Farstad, W. Cryopreservation of canine semen—New challenges. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 336–341. [Google Scholar] [CrossRef]
- Santos, I.P.; Silva, C.M.; Carvalho, F.P.; Cunha, I.C.N. Cooling of canine semen contaminated with urine. Braz. J. Vet. Sci. 2017, 24, 48–51. [Google Scholar] [CrossRef]
- Dorado, J.; Galvez, M.J.; Demyda-Peyras, S.; Ortiz, I.; Morrell, J.M.; Crespo, F.; Gosalvez, J.; Hidalgo, M. Differences in preservation of canine chilled semen using simple sperm washing, single-layer centrifugation and modified swim-up preparation techniques. Reprod. Fertil. Dev. 2015, 28, 1545–1552. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function--in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Domain, G.; Ali Hassan, H.; Wydooghe, E.; Bogado Pascottini, O.; Johannisson, A.; Morrell, J.M.; Nizanski, W.; Van Soom, A. Influence of Single Layer Centrifugation with Canicoll on Semen Freezability in Dogs. Animals 2022, 12, 714. [Google Scholar] [CrossRef]
- Rijsselaere, T.; Van Soom, A.; Maes, D.; de Kruif, A. Effect of centrifugation on in vitro survival of fresh diluted canine spermatozoa. Theriogenology 2002, 57, 1669–1681. [Google Scholar] [CrossRef]
- Ponglowhapan, S.; Essen-Gustavsson, B.; Linde Forsberg, C. Influence of glucose and fructose in the extender during long-term storage of chilled canine semen. Theriogenology 2004, 62, 1498–1517. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Simoes, A.; Ferreira, P.; Martins-Bessa, A.; Rocha, A. Differences in preservation of canine chilled semen using different transport containers. Anim. Reprod. Sci. 2009, 112, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hoogewijs, M.; Rijsselaere, T.; De Vliegher, S.; Vanhaesebrouck, E.; De Schauwer, C.; Govaere, J.; Thys, M.; Hoflack, G.; Van Soom, A.; de Kruif, A. Influence of different centrifugation protocols on equine semen preservation. Theriogenology 2010, 74, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Janett, F.; Burger, D.; Hassig, M.; Thun, R. The influence of centrifugation on quality and freezability of stallion semen. Schweiz Arch. Tierheilkd. 2004, 146, 285–293. [Google Scholar] [CrossRef]
- Fuchs, B.; Jakop, U.; Goritz, F.; Hermes, R.; Hildebrandt, T.; Schiller, J.; Muller, K. MALDI-TOF “fingerprint” phospholipid mass spectra allow the differentiation between ruminantia and feloideae spermatozoa. Theriogenology 2009, 71, 568–575. [Google Scholar] [CrossRef]
- Neila-Montero, M.; Riesco, M.F.; Montes-Garrido, R.; Palacin-Martinez, C.; Chamorro, C.; de Paz, P.; Alvarez, M.; Anel, L.; Anel-Lopez, L. An optimized centrifugation protocol for ram sperm ensuring high sample yield, quality and fertility. Theriogenology 2022, 191, 179–191. [Google Scholar] [CrossRef]
- Carvajal, G.; Cuello, C.; Ruiz, M.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Effects of centrifugation before freezing on boar sperm cryosurvival. J. Androl. 2004, 25, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lucio, C.F.; Brito, M.M.; Angrimani, D.; Belaz, K.; Morais, D.; Zampieri, D.; Losano, J.; Assumpcao, M.; Nichi, M.; Eberlin, M.N.; et al. Lipid composition of the canine sperm plasma membrane as markers of sperm motility. Reprod. Domest. Anim. 2017, 52 (Suppl. 2), 208–213. [Google Scholar] [CrossRef]
- Zalata, A.; Hafez, T.; Comhaire, F. Evaluation of the role of reactive oxygen species in male infertility. Hum. Reprod. 1995, 10, 1444–1451. [Google Scholar] [CrossRef]
- Ferrer, M.S.; Lyle, S.K.; Eilts, B.E.; Eljarrah, A.H.; Paccamonti, D.L. Factors affecting sperm recovery rates and survival after centrifugation of equine semen. Theriogenology 2012, 78, 1814–1823. [Google Scholar] [CrossRef]
- Linde-Forsberg, C. Achieving Canine Pregnancy by Using Frozen or Chilled Extended Semen. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Containers for Transport of Equine Semen. In Equine Reproduction, 2nd ed.; Angus, O., McKinnon, E.L.S., Vaala, W.E., Varner, D.I.D., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 1, pp. 1330–1335. [Google Scholar]
- Koderle, M.; Aurich, C.; Schafer-Somi, S. The influence of cryopreservation and seminal plasma on the chromatin structure of dog spermatozoa. Theriogenology 2009, 72, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Lasso, J.L.; Blasco, L.; Nunez, R.C.; Heyner, S.; Caballero, P.P.; Storey, B.T. Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim-up procedure without centrifugation extends their motile lifetime. Hum. Reprod. 1993, 8, 1087–1092. [Google Scholar] [CrossRef]
- Coetzee, K.; Franken, D.R.; Kruger, T.F.; Lombard, C.J. Effect of multiple centrifugations on the evaluation of the acrosome reaction in human spermatozoa. Andrologia 1992, 24, 331–334. [Google Scholar] [CrossRef]
- Pickett, B.W.; Sullivan, J.J.; Byers, W.W.; Pace, M.M.; Remmenga, E.E. Effect of centrifugation and seminal plasma on motility and fertility of stallion and bull spermatozoa. Fertil. Steril. 1975, 26, 167–174. [Google Scholar] [CrossRef]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K. Influence of semen processing technique on human sperm DNA integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef]
- Katkov, I.I.; Katkova, N.; Critser, J.K.; Mazur, P. Mouse spermatozoa in high concentrations of glycerol: Chemical toxicity vs osmotic shock at normal and reduced oxygen concentrations. Cryobiology 1998, 37, 325–338. [Google Scholar] [CrossRef]
- Knop, K.; Hoffmann, N.; Rath, D.; Sieme, H. Evaluation of slow cooling after centrifugation and glycerol addition at 22 °C versus direct freezing of semen in stallions with good and poor sperm longevity. Anim. Reprod. Sci. 2005, 89, 299–302. [Google Scholar]
- Len, J.A.; Jenkins, J.A.; Eilts, B.E.; Paccamonti, D.L.; Lyle, S.K.; Hosgood, G. Centrifugation has minimal effects on motility, viability, and acrosome integrity of equine sperm. Theriogenology 2008, 70, 582–583. [Google Scholar] [CrossRef]
- Bradecamp, E.A. Chapter 149 Centrifugation of Semen: Standard Technique. In Equine Reproductice Procedures, 2nd ed.; Dascanio, J., McCue, P., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 551–555. [Google Scholar]
- Johnston, S.D.; Root Kustritz, M.V.; Olson, P.S. Chapter 16 Semen Collection, Evaluation, and Preservation. In Canine and Feline Theriogenology; Saunders: Philadelphia, PA, USA, 2001; pp. 287–306. [Google Scholar]
- Hermansson, U.; Linde Forsberg, C. Freezing of stored, chilled dog spermatozoa. Theriogenology 2006, 65, 584–593. [Google Scholar] [CrossRef]
- Arlt, S.P.; Reichler, I.M.; Herbel, J.; Schafer-Somi, S.; Riege, L.; Leber, J.; Frehner, B. Diagnostic tests in canine andrology—What do they really tell us about fertility? Theriogenology 2023, 196, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Root Kustritz, M.V. The value of canine semen evaluation for practitioners. Theriogenology 2007, 68, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Koziol, J.H.; Armstrong, C.L. Chapter 1: Factors Evaluated by Observation of Breeding Behavior. In Society for Theriogenology Manual for Breeding Soundness Examination of Bulls, 2nd ed.; Society For Theriogenology: Montgomery, AL, USA, 2018. [Google Scholar]
- Rodenas, C.; Parrilla, I.; Roca, J.; Martinez, E.A.; Lucas, X. Quality of chilled and cold-stored (5 °C) canine spermatozoa submitted to different rapid cooling rates. Theriogenology 2014, 82, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Yoshikuni, R.; Kobayashi, M.; Kawakami, E. Effects of storage temperature and semen extender on stored canine semen. J. Vet.-Med. Sci. 2014, 76, 259–263. [Google Scholar] [CrossRef]
- Len, J.A.; Jenkins, J.A.; Eilts, B.E.; Paccamonti, D.L.; Lyle, S.K.; Hosgood, G. Immediate and delayed (after cooling) effects of centrifugation on equine sperm. Theriogenology 2010, 73, 225–231. [Google Scholar] [CrossRef]
- Pan, C.; Wu, Y.; Yang, Q.; Ye, J. Effects of seminal plasma concentration on sperm motility and plasma and acrosome membrane integrity in chilled canine spermatozoa. Pol. J. Vet.-Sci. 2018, 21, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Belala, R.; Delay, J.; Amirat, L.; Ropers, M.H.; Guillou, J.L.; Anton, M.; Schmitt, E.; Thorin, C.; Michaud, S.; Kaidi, R.; et al. The benefits of liposomes for chilling canine sperm for 4 days at 4 °C. Anim. Reprod. Sci. 2016, 168, 100–109. [Google Scholar] [CrossRef]
- Flechon, J.E. The acrosome of eutherian mammals. Cell Tissue Res. 2016, 363, 147–157. [Google Scholar] [CrossRef]
- Treulen, F.; Sanchez, R.; Risopatron, J. Effects of seminal fluid fractions on plasma and acrosome membrane integrity and mitochondrial membrane potential determined by flow cytometry in chilled canine spermatozoa. Reprod. Domest. Anim. 2012, 47, 1043–1048. [Google Scholar] [CrossRef]
- Kasimanickam, V.R.; Kasimanickam, R.K.; Memon, M.A.; Rogers, H.A. Effect of extenders on sperm mitochondrial membrane, plasma membrane and sperm kinetics during liquid storage of canine semen at 5 °C. Anim. Reprod. Sci. 2012, 136, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.J.; Corselli, J.U.; Jacobson, J.D.; Patton, W.C.; King, A. Spermac stain analysis of human sperm acrosomes. Fertil. Steril. 1999, 72, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Cheuqueman, C.; Bravo, P.; Treulen, F.; Giojalas, L.; Villegas, J.; Sanchez, R.; Risopatron, J. Sperm membrane functionality in the dog assessed by flow cytometry. Reprod. Domest. Anim. 2012, 47, 39–43. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Setting |
|---|---|
| Field-of-view depth (sample chamber depth) | 20 µm |
| Light adjustment | 80–110 |
| Total number of fields | 7 fields |
| Sperm recognition area | 20–60 µm2 |
| Frame rate | 60 frames/s |
| Points assessed for sperm motility | 11 |
| Total motility | Progressive motility + local motility |
| Immotile sperm | AOC* < 9.5 |
| Progressive motility | Every cell that is not “immotile” or “local motile” |
| Parameter | Mean ± S.D. |
|---|---|
| Volume (mL) | 4.25 ± 1.83 |
| Concentration (×106/mL) | 175.33 ± 63.08 |
| Viability (%) | 87.1 ± 6.57 |
| Subjective Total Motility (%) | 83.75 ± 6.85 |
| CASA Total Motility (%) | 86.91 ± 5.05 |
| CASA Progressive Motility (%) | 81.77 ± 11.30 |
| Morphologically Normal Spermatozoa (%) | 51.32 ± 9.52 |
| Acrosome defects (%) | 3.11 ± 2.25 |
| Head defects (%) | 23.86 ± 7.95 |
| Midpiece defects (%) | 16.71 ± 5.17 |
| Tail defects (%) | 9.25 ± 5.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugai, N.; Werre, S.; Cecere, J.; Balogh, O. Defining an Optimal Range of Centrifugation Parameters for Canine Semen Processing. Animals 2023, 13, 1421. https://doi.org/10.3390/ani13081421
Sugai N, Werre S, Cecere J, Balogh O. Defining an Optimal Range of Centrifugation Parameters for Canine Semen Processing. Animals. 2023; 13(8):1421. https://doi.org/10.3390/ani13081421
Chicago/Turabian StyleSugai, Nicole, Stephen Werre, Julie Cecere, and Orsolya Balogh. 2023. "Defining an Optimal Range of Centrifugation Parameters for Canine Semen Processing" Animals 13, no. 8: 1421. https://doi.org/10.3390/ani13081421





