Spray-Dried Plasma Promotes Broiler Chick Growth by Enhancing Immune Surveillance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Diet, and Bird Management
2.2. Growth Performance
2.3. Blood and Plasma Collection and Preparation
2.4. Differential Leukocyte Count Analysis
2.5. Evaluation of Phaseolus Vulgaris-P-Induced Cutaneous Delayed-Type Hypersensitivity (DTH)
2.6. Assay of Total IgY Concentration
2.7. Pro-Oxidant Capacity
2.8. Microbiological Analysis of Fecal Microbiota
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Differential Leukocyte Counts
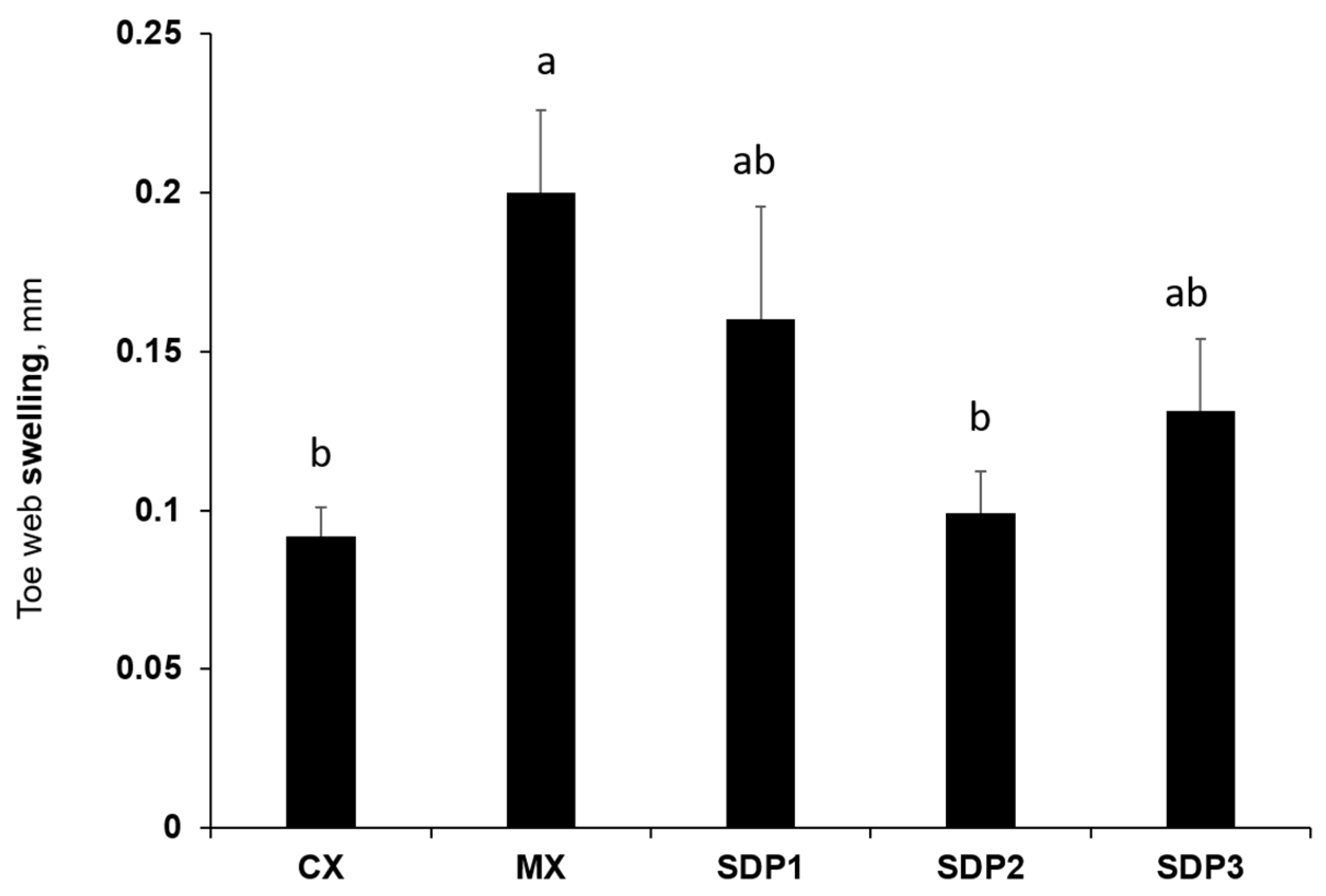
3.3. Delayed-Type Hypersensitivity (DTH) Reaction
3.4. Concentration of Indicator Microorganisms in the Fecal of Broiler Chicks
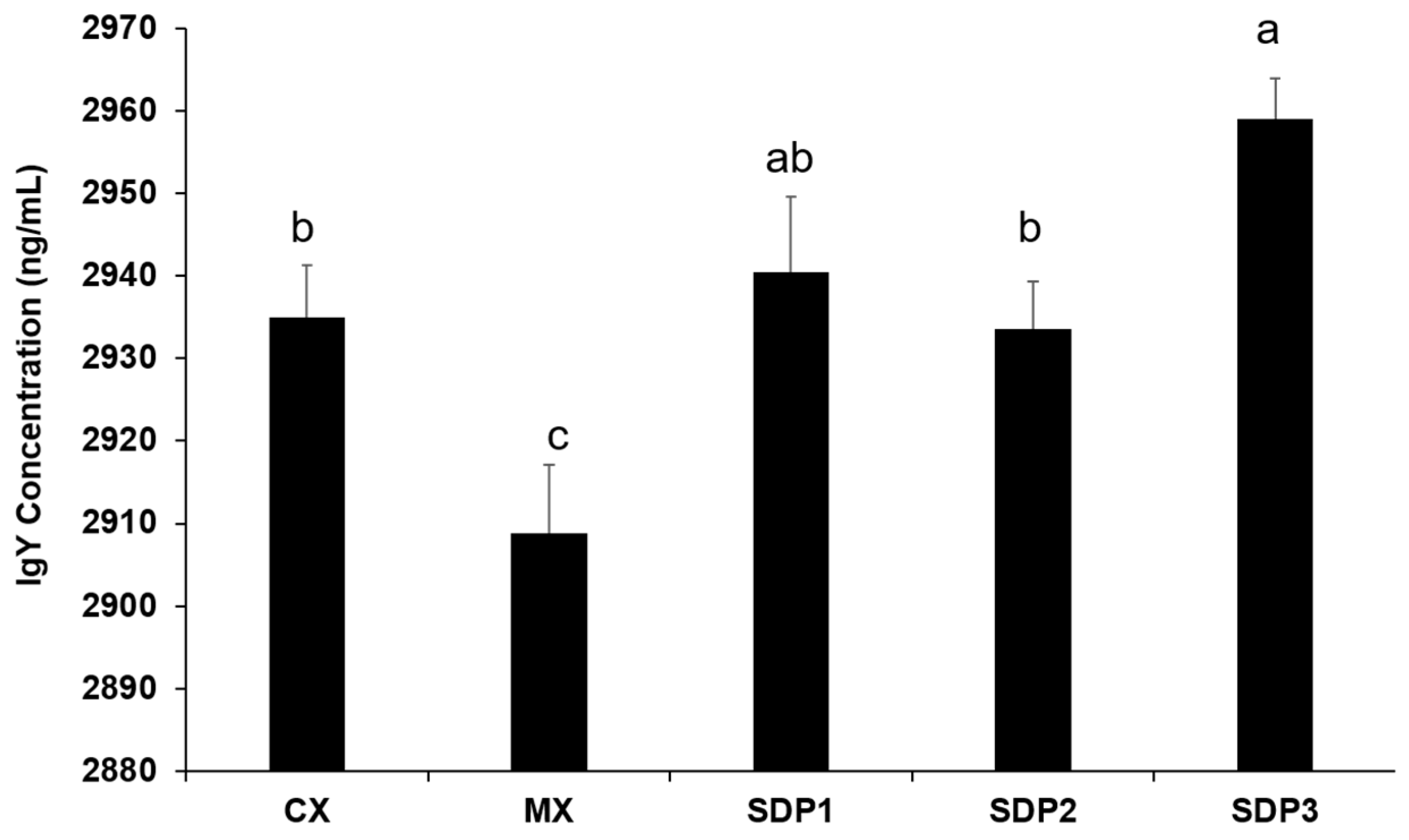
3.5. Plasma Total IgY antibody Concentration
3.6. Pro-Oxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brown, K.; Uwiera, R.R.E.; Kalmokoff, M.L.; Brooks, S.P.J.; Inglis, G.D. Antimicrobial growth promoter use in livestock: A requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob. Agents 2017, 49, 12–24. [Google Scholar] [CrossRef]
- Hasted, T.L.; Sharif, S.; Boerlin, P.; Diarra, M.S. Immunostimulatory Potential of Fruits and Their Extracts in Poultry. Front. Immunol. 2021, 12, 641696. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, P.; Zhang, B.; Kong, L.; Xiao, C.; Song, Z. Progress on Gut Health Maintenance and Antibiotic Alternatives in Broiler Chicken Production. Front. Nutr. 2021, 8, 692839. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Polo, J.; Torrallardona, D. Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porc. Health Manag. 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; González-Esquerra, R.; Polo, J. Impact of Spray-Dried Plasma on Intestinal Health and Broiler Performance. Microorganisms 2019, 7, 219. [Google Scholar] [CrossRef]
- Blázquez, E.; Rodríguez, C.; Ródenas, J.; Segalés, J.; Pujols, J.; Polo, J. Biosafety steps in the manufacturing process of spray-dried plasma: A review with emphasis on the use of ultraviolet irradiation as a redundant biosafety procedure. Porc. Health Manag. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Chuchird, N.; Rairat, T.; Keetanon, A.; Phansawat, P.; Chou, C.C.; Campbell, J. Effects of spray-dried animal plasma on growth performance, survival, feed utilization, immune responses, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0257792. [Google Scholar] [CrossRef] [PubMed]
- Balan, P.; Staincliffe, M.; Moughan, P.J. Effects of spray-dried animal plasma on the growth performance of weaned piglets—A review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Bundy, J.W.; Li, Y.S.; Carney-Hinkle, E.E.; Miller, P.S.; Burkey, T.E. Effects of spray-dried porcine plasma on growth performance, immune response, total antioxidant capacity, and gut morphology of nursery pigs. Anim. Sci. 2014, 92, 4494–4504. [Google Scholar] [CrossRef] [PubMed]
- Maijó, M.; Miró, L.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, J.; Weaver, E.; Moretó, M.; Pérez-Bosque, A. Dietary plasma proteins modulate the adaptive immune response in mice with acute lung inflammation. J. Nutr. 2012, 142, 264–270. [Google Scholar] [CrossRef]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010, 28, 623–667. [Google Scholar] [CrossRef] [PubMed]
- Fasina, Y.O.; Obanla, T.O.; Ferket, P.R.; Shah, D.H. Comparative efficacy of spray-dried plasma and bacitracin methylene disalicylate in reducing cecal colonization by Salmonella Enteritidis in broiler chickens. Poult. Sci. 2021, 100, 101134. [Google Scholar] [CrossRef] [PubMed]
- Aviagen, W. Ross 708: Broiler Nutrition Specifications. 2022. Available online: https://en.aviagen.com/assets/ (accessed on 20 February 2023).
- Thiam, M.; Wang, Q.; Barreto, S.A.L.; Zhang, J.; Ding, J.; Wang, H.; Zhang, Q.; Zhang, N.; Wang, J.; Li, Q.; et al. Heterophil/Lymphocyte Ratio Level Modulates Salmonella Resistance, Cecal Microbiota Composition and Functional Capacity in Infected Chicken. Front. Immunol. 2022, 13, 816689. [Google Scholar] [CrossRef] [PubMed]
- Morucci, G.; Ryskalin, L.; Pratesi, S.; Branca, J.J.V.; Modesti, A.; Modesti, P.A.; Gulisano, M.; Gesi, M. Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects. Medicina 2022, 58, 1341. [Google Scholar] [CrossRef]
- Abbas, M.; Moussa, M.; Akel, H. Type I Hypersensitivity Reaction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560561/ (accessed on 22 February 2023).
- Walters, H.G.; Jasek, A.; Campbell, J.M.; Coufal, C.; Lee, J.T. Evaluation of spray-dried plasma in broiler diets with or without bacitracin methylene disalicylate. J. Appl. Poult. Res. 2019, 28, 364–373. [Google Scholar] [CrossRef]
- Daneshmand, A.; Sharma, N.K.; Dao, T.H.; Barekatain, R.; Swick, R.A.; Wu, S.B. Spray-dried porcine plasma enhances feed efficiency, intestinal integrity, and immune response of broilers challenged with necrotic enteritis. Poult. Sci. 2022, 102, 102431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, D.W.; Yu, B.; He, J.; Yu, J.; Mao, X.B.; Wang, J.X.; Luo, J.Q.; Huang, Z.Q.; Cheng, G.X.; et al. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J. Anim. Sci. 2015, 93, 2967–2976. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 73, 502–511. [Google Scholar] [CrossRef]
- Adetunji, A.; Casey, T.; Franco, J.; Shah, D.; Fasina, Y. Proteomic Analysis of the Effect of Salmonella Challenge on Broiler Chicken. Molecules 2022, 27, 7277. [Google Scholar] [CrossRef]
- Ma, H.; Tao, W.; Zhu, S. T lymphocytes in the intestinal mucosa: Defense and tolerance. Cell. Mol. Immunol. 2019, 16, 216–224. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Wang, Q.; Zhang, Q.; Thiam, M.; Zhu, B.; Ying, F.; Elsharkawy, M.S.; Zheng, M.; Wen, J.; et al. A heterophil/lymphocyte-selected population reveals the phosphatase PTPRJ is associated with immune defense in chickens. Commun. Biol. 2023, 6, 196. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Zulfiqar, H.; Ramphul, K. Delayed Hypersensitivity Reactions. [Updated 2022 Sep 5]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519023/ (accessed on 27 February 2023).
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B.Y.; Zhang, L.; Dannelly, H.K. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth. Syst. Biotechnol. 2018, 3, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dock, D.B.; Aguilar-Nascimento, J.E.; Latorraca, M.Q. Probiotics enhance the recovery of gut atrophy in experimental malnutrition. Biocell 2004, 28, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Corthesy, B. Novel functions of the polymeric Ig receptor: Well beyond transport of immunoglobulins. Trends Immunol. 2003, 24, 55–58. [Google Scholar] [CrossRef]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef]
- Nakajima, A.; Vogelzang, A.; Maruya, M.; Miyajima, M.; Murata, M.; Son, A.; Kuwahara, T.; Tsuruyama, T.; Yamada, S.; Matsuura, M.; et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 2018, 215, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef]

| Ingredient | Control Diet | BMD Diet 1 | SDP1 Diet 1 | SDP2 Diet 1 | SDP3 Diet 1 |
|---|---|---|---|---|---|
| Corn | 50.59 | 50.59 | 52.07 | 53.54 | 55.02 |
| Soybean meal | 40.67 | 40.67 | 38.72 | 36.78 | 34.83 |
| Spray-dried plasma (SDP, AP920) | 0.00 | 0.00 | 1.00 | 2.00 | 3.00 |
| Poultry fat | 4.53 | 4.53 | 4.10 | 3.68 | 3.26 |
| Limestone | 1.37 | 1.37 | 1.39 | 1.42 | 1.45 |
| Mono-Dicalcium phosphate | 1.51 | 1.51 | 1.47 | 1.43 | 1.39 |
| Salt NaCl | 0.24 | 0.24 | 0.21 | 0.19 | 0.16 |
| Soda bicarbonate | 0.16 | 0.16 | 0.12 | 0.08 | 0.04 |
| L-Lysine HCl 98% | 0.17 | 0.17 | 0.16 | 0.14 | 0.13 |
| DL-Methionine 99.0% | 0.34 | 0.34 | 0.33 | 0.33 | 0.32 |
| L-Threonine 98.5% | 0.10 | 0.10 | 0.09 | 0.08 | 0.07 |
| NCSU Poultry Vitamin Premix 2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| NCSU Poultry Mineral Premix 3 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Bacitracin (Antibiotic, g/kg) | ---- | 0.055 | ---- | ---- | |
| Choline chloride 60% | 0.07 | 0.07 | 0.10 | 0.10 | 0.10 |
| Analyzed nutrient composition 4 | |||||
| Metabolizable energy (Kcal/kg) | 3146 | 3181 | 3150 | 3111 | 3150 |
| Crude protein, % | 23.71 | 24.86 | 23.79 | 23.96 | 23.86 |
| Crude fat, % | 6.17 | 6.31 | 5.84 | 5.42 | 5.32 |
| Crude fiber, % | 2.3 | 2.3 | 2.3 | 2.2 | 2.2 |
| Ash, % | 5.76 | 5.85 | 5.76 | 5.61 | 5.49 |
| Calculated nutrient composition | |||||
| Total sulfur amino acids, % | 1.04 | 1.04 | 1.06 | 1.06 | 1.06 |
| Lysine, % | 1.42 | 1.42 | 1.43 | 1.43 | 1.43 |
| Calcium, % | 0.96 | 0.96 | 0.95 | 0.95 | 0.95 |
| Available phosphorus, % | 0.48 | 0.48 | 0.48 | 0.48 | 0.48 |
| Treatment | Average Body Weight (kg/bird) 1 | Average Weight Gain (kg/bird) 1 | Average Feed Intake (kg/bird) 1 | FCR (kg:kg) 2 |
|---|---|---|---|---|
| CX | 1.58 | 1.49 | 1.96 | 1.318 a |
| MX | 1.47 | 1.36 | 1.76 | 1.294 ab |
| SDP1 | 1.47 | 1.40 | 1.81 | 1.292 ab |
| SDP2 | 1.44 | 1.42 | 1.86 | 1.311 a |
| SDP3 | 1.57 | 1.52 | 1.91 | 1.258 b |
| SEM | 0.051 | 0.052 | 0.082 | 0.015 |
| p-value | 0.328 | 0.191 | 0.160 | 0.022 |
| Treatment | Heterophils (%) | Lymphocytes (%) | H:L Ratio 1 |
|---|---|---|---|
| CX | 12.25 a | 65.50 | 0.19 |
| MX | 4.92 c | 63.60 | 0.09 |
| SDP1 | 7.90 b | 74.35 | 0.11 |
| SDP2 | 6.40 bc | 73.82 | 0.10 |
| SDP3 | 7.62 b | 72.50 | 0.11 |
| SEM | 1.849 | 4.724 | 0.032 |
| p-value | 0.049 | 0.499 | 0.144 |
| Treatment | Total Bacteria Count | E. coli | Lactobacillus spp. | Bifidobacterium spp. |
|---|---|---|---|---|
| CX | 8.11 ± 0.07 b | 7.49 ± 0.05 c | 9.00 ± 0.18 | 7.22 ± 0.01 b |
| MX | 8.72 ± 0.16 a | 8.86 ± 0.16 a | 8.91 ± 0.05 | 7.98 ± 0.09 a |
| SDP1 | 8.40 ± 0.20 ab | 7.25 ± 0.07 c | 9.07 ± 0.05 | 7.23 ± 0.05 b |
| SDP2 | 6.88 ± 0.05 c | 6.49 ± 0.06 d | 8.99 ± 0.11 | 7.70 ± 0.11 a |
| SDP3 | 8.79 ± 0.17 a | 8.01 ± 0.08 b | 8.96 ± 0.13 | 7.81 ± 0.13 a |
| p-value | 0.0001 | 0.0001 | 0.906 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blue, C.E.C.; Jababu, Y.; Ibrahim, S.A.; Minor, R.C.; Williams, L.L.; Adetunji, A.O.; Ali, R.; Young, L.S.; Fasina, Y.O. Spray-Dried Plasma Promotes Broiler Chick Growth by Enhancing Immune Surveillance. Animals 2023, 13, 1436. https://doi.org/10.3390/ani13091436
Blue CEC, Jababu Y, Ibrahim SA, Minor RC, Williams LL, Adetunji AO, Ali R, Young LS, Fasina YO. Spray-Dried Plasma Promotes Broiler Chick Growth by Enhancing Immune Surveillance. Animals. 2023; 13(9):1436. https://doi.org/10.3390/ani13091436
Chicago/Turabian StyleBlue, Candice E. C., Yasin Jababu, Salam A. Ibrahim, Radiah C. Minor, Leonard L. Williams, Adedeji O. Adetunji, Rizwana Ali, Lea S. Young, and Yewande O. Fasina. 2023. "Spray-Dried Plasma Promotes Broiler Chick Growth by Enhancing Immune Surveillance" Animals 13, no. 9: 1436. https://doi.org/10.3390/ani13091436
APA StyleBlue, C. E. C., Jababu, Y., Ibrahim, S. A., Minor, R. C., Williams, L. L., Adetunji, A. O., Ali, R., Young, L. S., & Fasina, Y. O. (2023). Spray-Dried Plasma Promotes Broiler Chick Growth by Enhancing Immune Surveillance. Animals, 13(9), 1436. https://doi.org/10.3390/ani13091436







