Investigations of Histomonosis-Favouring Conditions: A Hypotheses-Generating Case-Series-Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
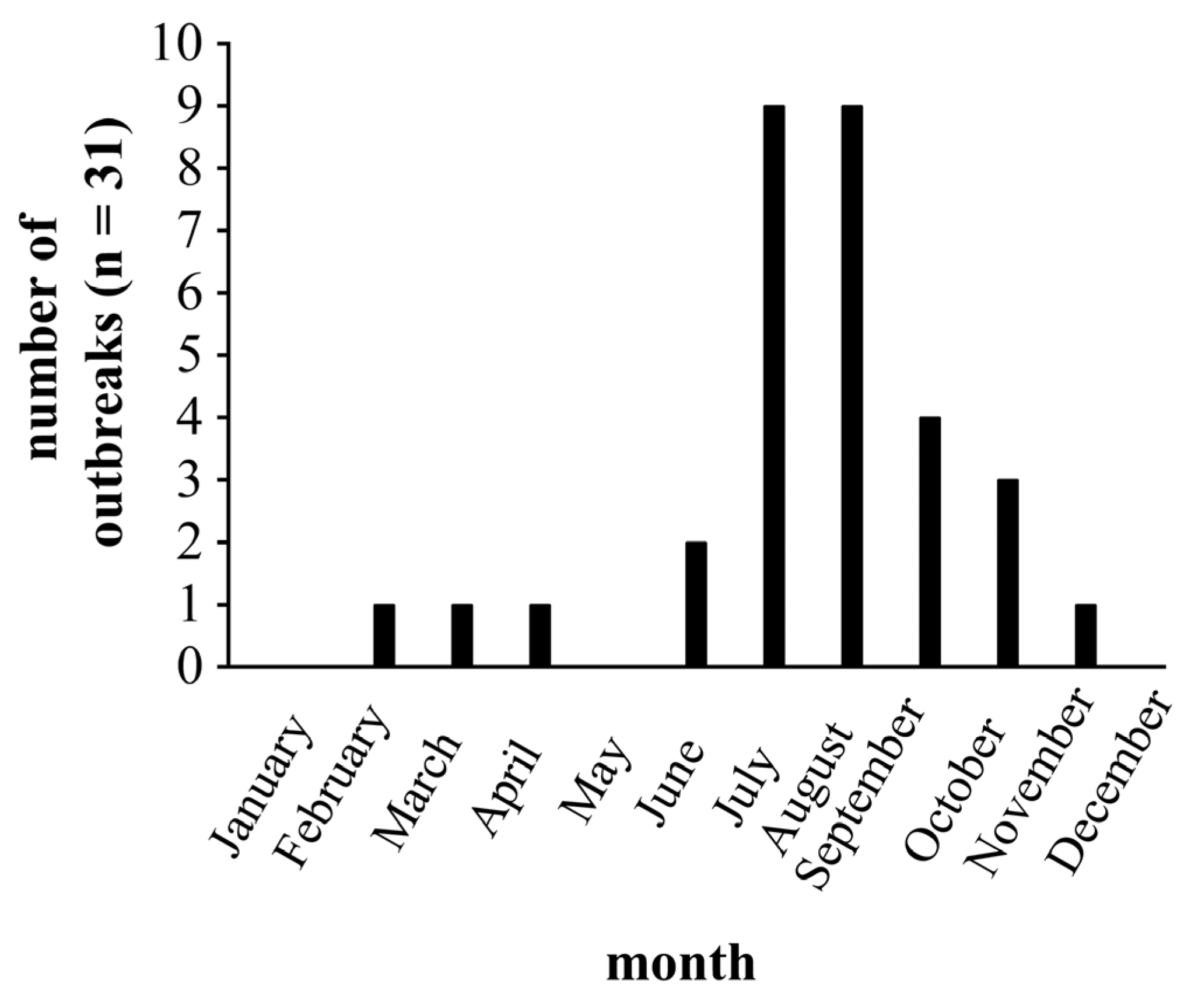
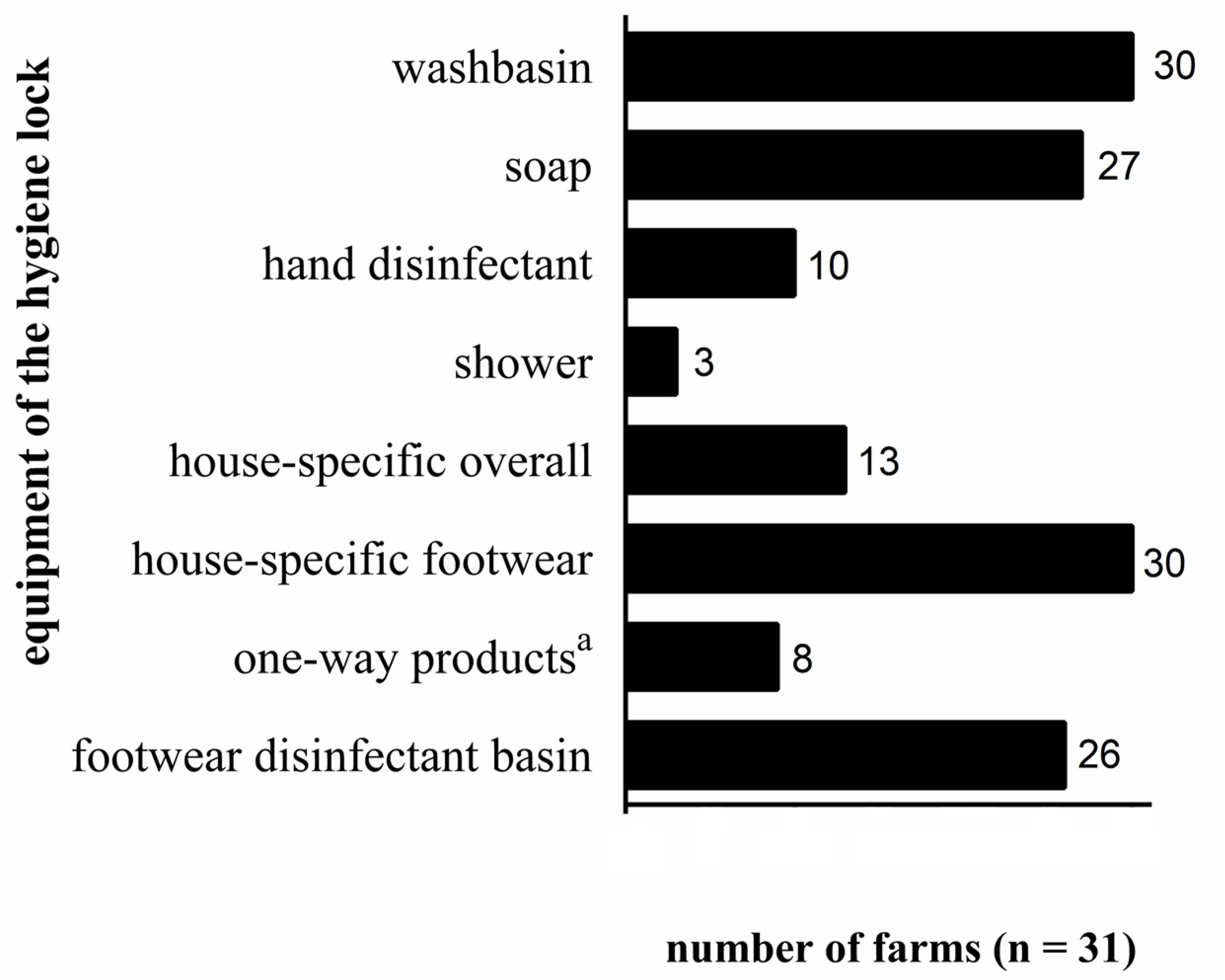
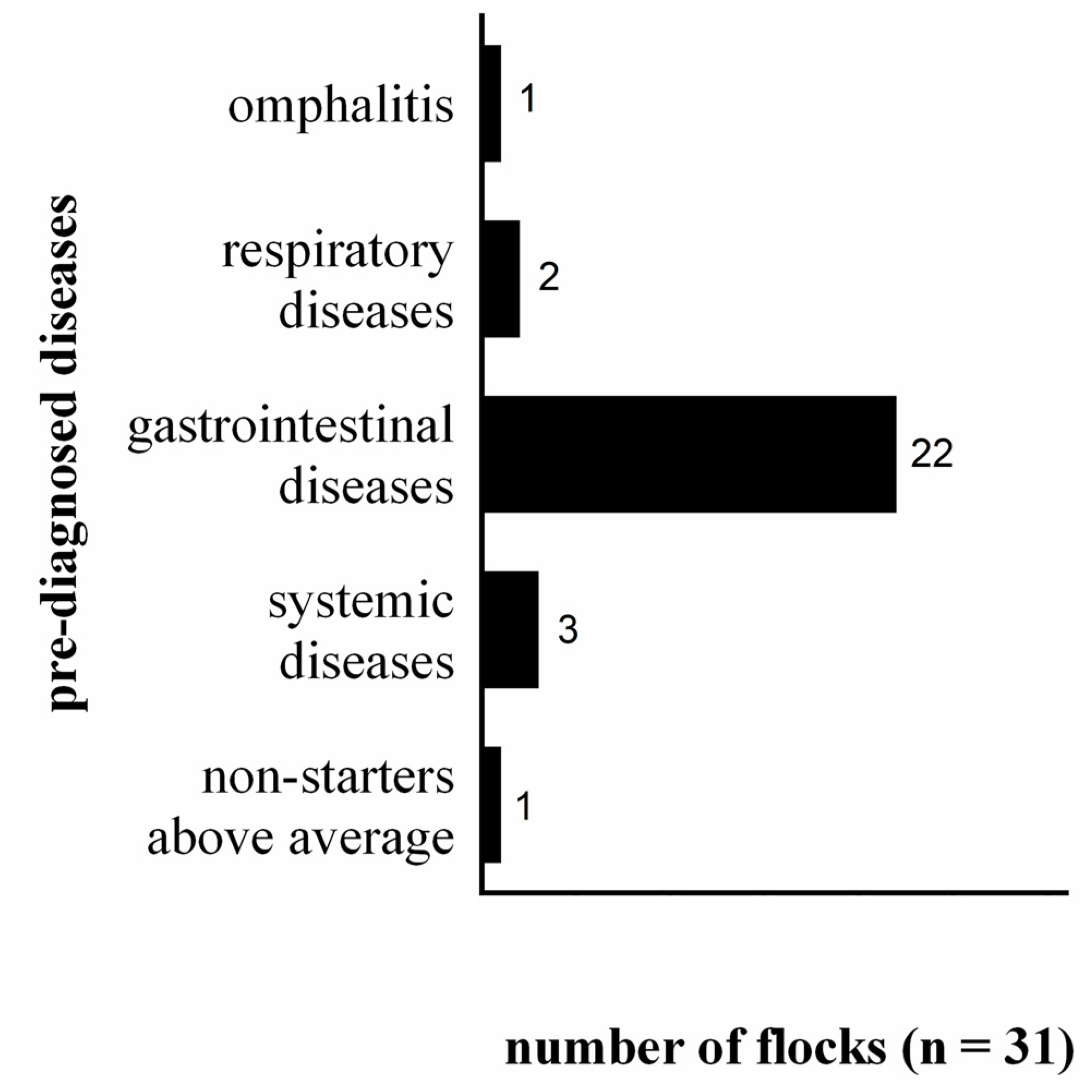
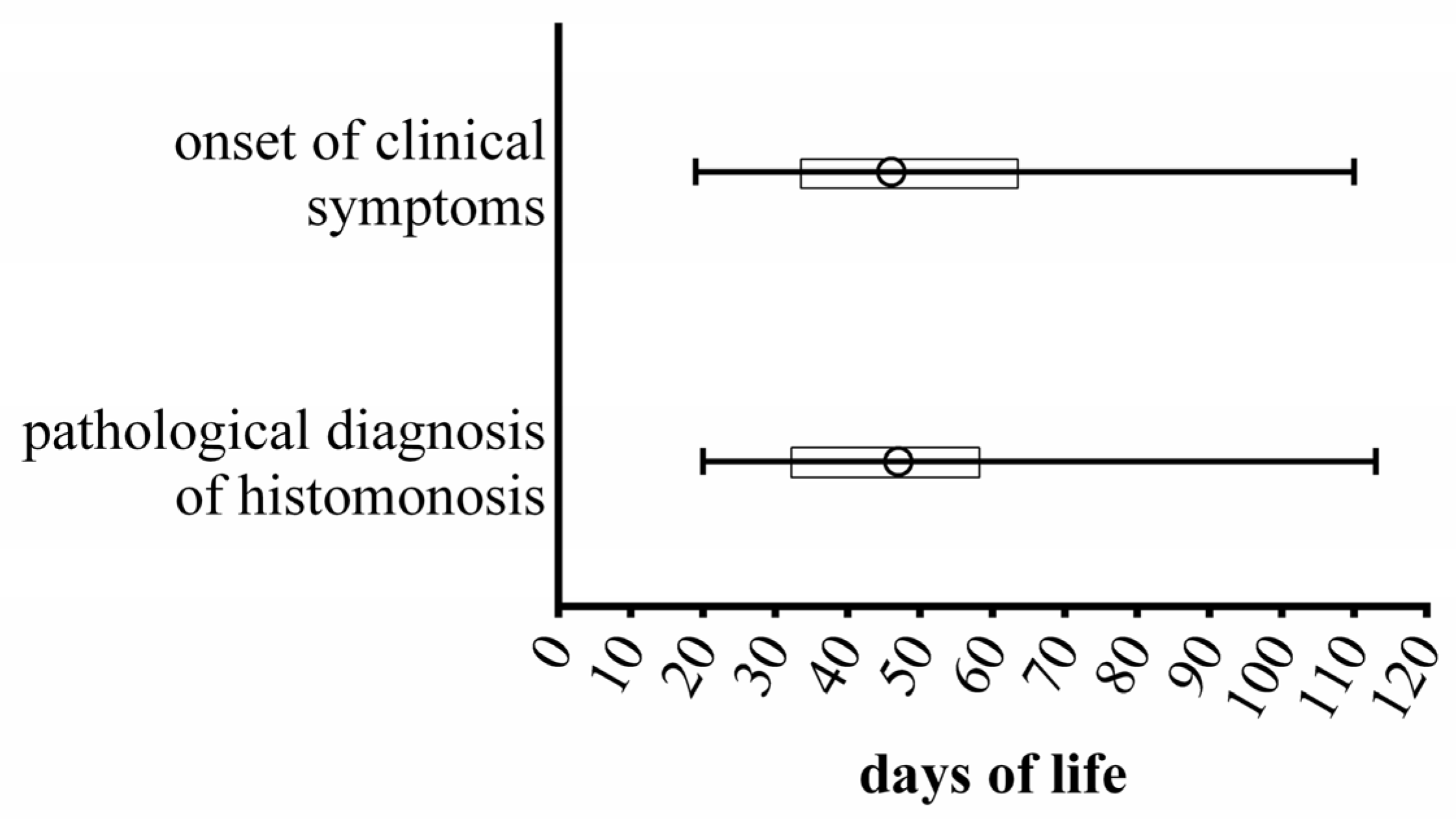
3. Results and Discussion
3.1. Description and Management of the Farm and Flock
3.2. General Biosecurity Measures
3.3. Health Management, Incidence and Therapy of Diseases
3.4. Outbreak Management
3.5. Coincidental Findings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | deoxyribonucleic acid |
| e.g., | exempli gratia (for example) |
| H. meleagridis | Histomonas meleagridis |
| n | number |
| spp. | species |
| etc. | et cetera |
References
- Clark, S.; Kimminau, E. Critical Review: Future Control of Blackhead Disease (Histomoniasis) in Poultry. Avian Dis. 2017, 61, 281–288. [Google Scholar] [CrossRef]
- Liebhart, D.; Ganas, P.; Sulejmanovic, T.; Hess, M. Histomonosis in poultry: Previous and current strategies for prevention and therapy. Avian Pathol. 2017, 46, 1–18. [Google Scholar] [CrossRef]
- Hauck, R.; Hafez, H.M. Experimental infections with the protozoan parasite Histomonas meleagridis: A review. Parasitol. Res. 2013, 112, 19–34. [Google Scholar] [CrossRef]
- Hauck, R.; Balczulat, S.; Hafez, H.M. Detection of DNA of Histomonas meleagridis and Tetratrichomonas gallinarum in German Poultry Flocks Between 2004 and 2008. Avian Dis. 2010, 54, 1021–1025. [Google Scholar] [CrossRef]
- McDougald, L.R. Blackhead Disease (Histomoniasis) in Poultry: A Critical Review. Avian Dis. 2005, 49, 462–476. [Google Scholar] [CrossRef]
- Powell, F.L.; Rothwell, L.; Clarkson, M.J.; Kaiser, P. The turkey, compared to the chicken, fails to mount an effective early immune response to Histomonas meleagridis in the gut. Parasite Immunol. 2009, 31, 312–327. [Google Scholar] [CrossRef]
- Sentíes-Cué, G.; Chin, R.P.; Shivaprasad, H.L. Systemic Histomoniasis Associated with High Mortality and Unusual Lesions in the Bursa of Fabricius, Kidneys, and Lungs in Commercial Turkeys. Avian Dis. 2009, 53, 231–238. [Google Scholar] [CrossRef]
- Windisch, M.; Hess, M. Experimental infection of chickens with Histomonas meleagridis confirms the presence of antibodies in different parts of the intestine. Parasite Immunol. 2010, 32, 29–35. [Google Scholar] [CrossRef]
- Swayne, D.E.; Boulianne, M.; Logue, C.M.; McDougald, L.R.; Nair, V.; Suarez, D.L.; Wit, S.D.; Grimes, T.; Johnson, D.; Kromm, M.; et al. Diseases of Poultry, 14th ed.; Swayne, D.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; Volume 1. [Google Scholar]
- Callait-Cardinal, M.-P.; Leroux, S.; Venereau, E.; Chauve, C.M.; Le Pottier, G.; Zenner, L. Incidence of histomonosis in turkeys in France since the bans of dimetridazole and nifursol. Vet. Rec. 2007, 161, 581–585. [Google Scholar] [CrossRef]
- Aka, J.; Hauck, R.; Blankenstein, P.; Balczulat, S.; Hafez, H.M. [Reoccurrence of histomonosis in turkey breeder farm]. Berl. und Munch. Tierarztl. Wochenschr. 2011, 124, 2–7. [Google Scholar]
- El-Wahab, A.A.; Visscher, C.; Haider, W.; Dimitri, R. A case study of histomoniasis in fattening turkeys identified in histopathological investigations. Ger. J. Vet. Res. 2021, 1, 13–18. [Google Scholar] [CrossRef]
- Lin, G.W. Paromomycin Sulfate Treatment in Histomoniasis Outbreaks in Three Commercial Turkey Flocks in the Fraser Valley of British Columbia, Canada. Avian Dis. 2021, 65, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.; Hauck, R.; Balczulat, S.; Hafez, H.M. Recurring Histomonosis on an Organic Farm. Avian Dis. 2011, 55, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Hauck, R.; Stoute, S.; Chin, R.P.; Sentíes-Cué, C.G.; Shivaprasad, H.L. Retrospective Study of Histomoniasis (Blackhead) in California Turkey Flocks, 2000–2014. Avian Dis. 2018, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, N.; Ramires, M.D.J.; Hess, M.; Bilic, I. Complete genomes of the eukaryotic poultry parasite Histomonas meleagridis: Linking sequence analysis with virulence/attenuation. BMC Genom. 2021, 22, 753. [Google Scholar] [CrossRef]
- Lagler, J.; Schmidt, S.; Mitra, T.; Stadler, M.; Wernsdorf, P.; Grafl, B.; Hatfaludi, T.; Hess, M.; Gerner, W.; Liebhart, D. Comparative investigation of IFN-γ-producing T cells in chickens and turkeys following vaccination and infection with the extracellular parasite Histomonas meleagridis. Dev. Comp. Immunol. 2020, 116, 103949. [Google Scholar] [CrossRef]
- Callait-Cardinal, M.P.; Gilot-Fromont, E.; Chossat, L.; Gonthier, A.; Chauve, C.; Zenner, L. Flock management and histomoniasis in free-range turkeys in France: Description and search for potential risk factors. Epidemiol. Infect. 2010, 138, 353–363. [Google Scholar] [CrossRef]
- Lüning, J.; Wunderl, D.; Rautenschlein, S.; Campe, A. Histomonosis in German turkey flocks: Possible ways of pathogen introduction. Avian Pathol. 2023. [Google Scholar] [CrossRef]
- Krumpal, I. Determinants of social desirability bias in sensitive surveys: A literature review. Qual. Quant. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- Houe, H.; Kjaer Ersboll, A.; Toft, N. Introduction to Veterinary Epidemiology; Biofolia: Frederiksberg, Denmark, 2004; Volume 1. [Google Scholar]
- Zaragatzki, E.; Mehlhorn, H.; Abdel-Ghaffar, F.; Rasheid, K.A.S.; Grabensteiner, E.; Hess, M. Experiments to produce cysts in cultures of Histomonas meleagridis—The agent of histomonosis in poultry. Parasitol. Res. 2010, 106, 1005–1007. [Google Scholar] [CrossRef]
- Zaragatzki, E.; Hess, M.; Grabensteiner, E.; Abdel-Ghaffar, F.; Al-Rasheid, K.A.S.; Mehlhorn, H. Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitol. Res. 2010, 106, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Munsch, M.; Lotfi, A.; Hafez, H.M.; Al-Quraishy, S.; Mehlhorn, H. Light and transmission electron microscopic studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitol. Res. 2009, 104, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kemp, R.L.; Franson, J.C. Transmission of Histomonas meleagridis to Domestic Fowl by Means of Earthworms Recovered from Pheasant Yard Soil. Avian Dis. 1975, 19, 741. [Google Scholar] [CrossRef] [PubMed]
- Sulejmanovic, T.; Liebhart, D.; Magdefrau-Pollan, B.; Sanglhuber, E.M.; Wiesinger, E.; Bilic, I.; Hess, M. Emergence of fatal histomonosis in meat turkey flocks in Austria from 2014 to 2016. Wien. Tierarztl. Monatsschr. 2017, 104, 277–287. [Google Scholar]
- Huneau-Salaün, A.; Scoizec, A.; Thomas, R.; Martenot, C.; Schmitz, A.; Pierre, I.; Allée, C.; Busson, R.; Massin, P.; Briand, F.-X.; et al. Avian influenza outbreaks: Evaluating the efficacy of cleaning and disinfection of vehicles and transport crates. Poult. Sci. 2022, 101, 101569. [Google Scholar] [CrossRef]
- Wein, Y.; Bar Shira, E.; Friedman, A. Avoiding handling-induced stress in poultry: Use of uniform parameters to accurately determine physiological stress. Poult. Sci. 2017, 96, 65–73. [Google Scholar] [CrossRef]
- Shini, S.; Huff, G.R.; Shini, A.; Kaiser, P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010, 89, 841–851. [Google Scholar] [CrossRef]
- Hughes, C.; Gaskell, R.; Jones, R.; Bradbury, J.; Jordan, F. Effects of certain stress factors on the re-excretion of infectious laryngotracheitis virus from latently infected carrier birds. Res. Vet. Sci. 1989, 46, 274–276. [Google Scholar] [CrossRef]
- Hu, J.; McDougald, L.R. Direct Lateral Transmission of Histomonas meleagridis in Turkeys. Avian Dis. 2003, 47, 489–492. [Google Scholar] [CrossRef]
- Hess, M.; Liebhart, D.; Bilic, I.; Ganas, P. Histomonas meleagridis—New insights into an old pathogen. Vet. Parasitol. 2015, 208, 67–76. [Google Scholar] [CrossRef]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Wu, N.; Zhu, G.; Yang, C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 2019, 103, 461–472. [Google Scholar] [CrossRef]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Potts, G.R. Long-term changes in the prevalences of caecal nematodes and histomonosis in gamebirds in the UK and the interaction with poultry. Vet. Rec. 2009, 164, 715–718. [Google Scholar] [CrossRef]
- Adrizal, A.; Patterson, P.H.; Hulet, R.M.; Bates, R.M.; Myers, C.A.; Martin, G.P.; Shockey, R.L.; Van Der Grinten, M.; Anderson, D.A.; Thompson, J.R. Vegetative buffers for fan emissions from poultry farms: 2. ammonia, dust and foliar nitrogen. J. Environ. Sci. Health Part B 2008, 43, 96–103. [Google Scholar] [CrossRef]
- Dinev, I. The darkling beetle (Alphitobius diaperinus)—A health hazard for broiler chicken production. Trakia J. Sci. 2013, 1, 1–4. [Google Scholar]
- Spindler, L.A. Experimental transmission of Histomonas meleagridis and Heterakis gallinarum by the sow-bug, Porcellio scaber, and its implications for further research. Proceeding Helminthol. Soc. Wash. 1967, 34, 26–29. [Google Scholar]
- Beckmann, J.F.; Dormitorio, T.; Oladipupo, S.O.; Terra, M.T.B.; Lawrence, K.; Macklin, K.S.; Hauck, R. Heterakis gallinarum and Histomonas meleagridis DNA persists in chicken houses years after depopulation. Vet. Parasitol. 2021, 298, 109536. [Google Scholar] [CrossRef]
- Delpont, M.; Guinat, C.; Guérin, J.-L.; Le Leu, E.; Vaillancourt, J.-P.; Paul, M.C. Biosecurity measures in French poultry farms are associated with farm type and location. Prev. Veter-Med. 2021, 195, 105466. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, S.; Yuan, Z.; Yi, J.; Tian, Y.; Wu, J.; Wen, L. Effects of induced stress from the live LaSota Newcastle disease vaccination on the growth performance and immune function in broiler chickens. Poult. Sci. 2020, 99, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- McDougald, L.R.; Hu, J. Blackhead Disease (Histomonas meleagridis) Aggravated in Broiler Chickens by Concurrent Infection with Cecal Coccidiosis (Eimeria tenella). Avian Dis. 2001, 45, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ganas, P.; Liebhart, D.; Glösmann, M.; Hess, C.; Hess, M. Escherichia coli strongly supports the growth of Histomonas meleagridis, in a monoxenic culture, without influence on its pathogenicity. Int. J. Parasitol. 2012, 42, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.K.; Quijada, N.M.; Dzieciol, M.; Hatfaludi, T.; Bilic, I.; Selberherr, E.; Liebhart, D.; Hess, C.; Hess, M.; Paudel, S. Co-infection of Chicken Layers with Histomonas meleagridis and Avian Pathogenic Escherichia coli Is Associated With Dysbiosis, Cecal Colonization and Translocation of the Bacteria From the Gut Lumen. Front. Microbiol. 2020, 11, 586437. [Google Scholar] [CrossRef]
- Abdelhamid, M.K.; Rychlik, I.; Hess, C.; Hatfaludi, T.; Crhanova, M.; Karasova, D.; Lagler, J.; Liebhart, D.; Hess, M.; Paudel, S. Typhlitis induced by Histomonas meleagridis affects relative but not the absolute Escherichia coli counts and invasion in the gut in turkeys. Vet. Res. 2021, 52, 1–12. [Google Scholar] [CrossRef]
- Bilic, I.; Hess, M. Interplay between Histomonas meleagridis and Bacteria: Mutualistic or Predator–Prey? Trends Parasitol. 2020, 36, 232–235. [Google Scholar] [CrossRef]
- Danzeisen, J.L.; Clayton, J.B.; Huang, H.; Knights, D.; McComb, B.; Hayer, S.S.; Johnson, T.J. Temporal Relationships Exist Between Cecum, Ileum, and Litter Bacterial Microbiomes in a Commercial Turkey Flock, and Subtherapeutic Penicillin Treatment Impacts Ileum Bacterial Community Establishment. Front. Vet. Sci. 2015, 2, 56. [Google Scholar] [CrossRef]
- Cortes, P.L.; Chin, R.P.; Bland, M.C.; Crespo, R.; Shivaprasad, H.L. Histomoniasis in the Bursa of Fabricius of Chickens. Avian Dis. 2004, 48, 711–715. [Google Scholar] [CrossRef]
- Hauck, R.; Hafez, H.M. Effect of Coated Plant Extracts on Histomonas Meleagridis and Growth of Bacteria in Vitro. Avian Dis. 2007, 51, 880–883. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How Safe is Chicken Litter for Land Application as an Organic Fertilizer?: A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef] [PubMed]
- Thiel, N.; Münch, S.; Behrens, W.; Junker, V.; Faust, M.; Biniasch, O.; Kabelitz, T.; Siller, P.; Boedeker, C.; Schumann, P.; et al. Airborne bacterial emission fluxes from manure-fertilized agricultural soil. Microb. Biotechnol. 2020, 13, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Frentrup, M.; Thiel, N.; Junker, V.; Behrens, W.; Münch, S.; Siller, P.; Kabelitz, T.; Faust, M.; Indra, A.; Baumgartner, S.; et al. Agricultural fertilization with poultry manure results in persistent environmental contamination with the pathogen Clostridioides Difficile. Environ. Microbiol. 2021, 23, 7591–7602. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.E.; Wehr, E.E.; Ellis, D.J. Earthworm Transmission of Heterakis and Histomonas to Turkeys and Chickens. J. Parasitol. 1966, 52, 899. [Google Scholar] [CrossRef]
- Perreault, J.M.; Whalen, J.K. Earthworm burrowing in laboratory microcosms as influenced by soil temperature and moisture. Pedobiologia 2006, 50, 397–403. [Google Scholar] [CrossRef]
- Noor, R.; Javid, A.; Hussain, A.; Bukhari, S.M.; Suleman, S.; Malik, S.; Amin, F.; Azam, S.M.; Ali, K.; Mustafa, G.; et al. Prevalence of parasites in selected captive bird species. Braz. J. Biol. 2021, 84, e254251. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Meshgi, B.; Rahbari, S.; Ghaemi, P.; Aghaebrahimi-Samani, R. Biodiversity and Prevalence of Parasites of Rook (Corvus frugilegus) in Iran. Iran. J. Parasitol. 2007, 2, 42–43. [Google Scholar]
- Bertran, K.; Balzli, C.; Kwon, Y.-K.; Tumpey, T.M.; Clark, A.; Swayne, D.E. Airborne Transmission of Highly Pathogenic Influenza Virus during Processing of Infected Poultry. Emerg. Infect. Dis. 2017, 23, 1806–1814. [Google Scholar] [CrossRef]
- Kumar, G.D.; Williams, R.C.; Al Qublan, H.M.; Sriranganathan, N.; Boyer, R.R.; Eifert, J.D. Airborne soil particulates as vehicles for Salmonella contamination of tomatoes. Int. J. Food Microbiol. 2017, 243, 90–95. [Google Scholar] [CrossRef]
- Zhao, Y.; Richardson, B.; Takle, E.; Chai, L.; Schmitt, D.; Xin, H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019, 9, 11755. [Google Scholar] [CrossRef]
- Nguyen, X.D.; Zhao, Y.; Evans, J.D.; Lin, J.; Purswell, J.L. Survival of Escherichia coli in Airborne and Settled Poultry Litter Particles. Animals 2022, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Piearce, T.G.; Phillips, M.J. Fate of ciliates in the earthworm gut: An in vitro study. Microb. Ecol. 1980, 5, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Fries, R.; Akcan, M.; Bandick, N.; Kobe, A. Microflora of two different types of poultry litter. Br. Poult. Sci. 2005, 46, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Ostović, M.; Ravić, I.; Kovačić, M.; Kabalin, A.E.; Matković, K.; Sabolek, I.; Pavičić, Ž.; Menčik, S.; Tomić, D.H. Differences in fungal contamination of broiler litter between summer and winter fattening periods. Arh. Hig. Rada Toksikol. 2021, 72, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Li, W.L.; Feng, Y.; Yao, J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011, 90, 2740–2746. [Google Scholar] [CrossRef]
| Observation of Insects | ||||
|---|---|---|---|---|
| Yes | No | Σ | ||
| Control of insects | Yes | 4 | 3 | 7 |
| No | 10 | 14 | 24 | |
| Σ | 14 | 17 | 31 | |
| Enrichment Material | ||||
|---|---|---|---|---|
| Easy to Clean a | Difficult to Clean b | Σ | ||
| Re-use of enrichment material | Yes | 3 | 9 | 12 |
| No | 4 | 14 | 18 | |
| Σ | 7 | 23 | 30 | |
| Treatment | ||
|---|---|---|
| Yes | No | |
| Escherichia coli (n = 19) | 18 a | 1 |
| Eimeria spp. (n = 14) | 14 b | 0 |
| Clostridium spp. (n = 8) | 8 a | 0 |
| Flock Mortality | 0–10% | 11–50% | 51–100% |
|---|---|---|---|
| Number of flocks | 8/31 | 14/31 | 9/31 |
| Pre-existing gastrointestinal diseases | 6/8 | 9/14 | 7/9 |
| Treatment against H. meleagridis a | 8/8 | 14/14 | 8/9 |
| Treatment success by rapid clinical improvement | 4/8 | 1/14 | 0/8 |
| Emergency culling | 0/8 | 5/14 | 5/9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüning, J.; Campe, A.; Rautenschlein, S. Investigations of Histomonosis-Favouring Conditions: A Hypotheses-Generating Case-Series-Study. Animals 2023, 13, 1472. https://doi.org/10.3390/ani13091472
Lüning J, Campe A, Rautenschlein S. Investigations of Histomonosis-Favouring Conditions: A Hypotheses-Generating Case-Series-Study. Animals. 2023; 13(9):1472. https://doi.org/10.3390/ani13091472
Chicago/Turabian StyleLüning, Julia, Amely Campe, and Silke Rautenschlein. 2023. "Investigations of Histomonosis-Favouring Conditions: A Hypotheses-Generating Case-Series-Study" Animals 13, no. 9: 1472. https://doi.org/10.3390/ani13091472
APA StyleLüning, J., Campe, A., & Rautenschlein, S. (2023). Investigations of Histomonosis-Favouring Conditions: A Hypotheses-Generating Case-Series-Study. Animals, 13(9), 1472. https://doi.org/10.3390/ani13091472





