Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Search Methodology
3. Heat Radiation and Its Relationship with Tissular Perfusion
4. Peripheral Vascular Disorders and Thermography
5. Thermal Imaging Applied to Assess Tissular Damage Degree and Microvascular Repair
6. Infrared Thermography Used to Monitor Healing and Surgical Wound Care
7. Technical Factors That Can Influence Thermal Imaging in Animals
8. Perspectives on the Use of IRT in Veterinary Medicine
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early Detection and Prediction of Infection Using Infrared Thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Cook, N.J.N.; Chabot, B.; Lui, T.; Bench, C.C.J.; Schaefer, A.L. AL Infrared Thermography Detects Febrile and Behavioural Responses to Vaccination of Weaned Piglets. Animal 2015, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared Thermal Imaging Associated with Pain in Laboratory Animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary Applications of Infrared Thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Nakagami, G.; Kadono, T.; Ogawa, Y.; Iizaka, S.; Koyanagi, H.; Sasaki, S.; Haga, N.; Sanada, H. Combination of Thermographic and Ultrasonographic Assessments for Early Detection of Deep Tissue Injury. Int. Wound J. 2014, 11, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical Applications of Infrared Thermography: A Review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Tang, Q.; Zeng, G.; Wu, H.; Zhang, N.; Zhong, N. Effectiveness of Digital Infrared Thermal Imaging in Detecting Lower Extremity Deep Venous Thrombosis. Med. Phys. 2015, 42, 2242–2248. [Google Scholar] [CrossRef]
- Lin, P.H.; Echeverria, A.; Poi, M.J. Infrared Thermography in the Diagnosis and Management of Vasculitis. J. Vasc. Surg. Cases Innov. Tech. 2017, 3, 112–114. [Google Scholar] [CrossRef]
- Branco, J.H.L.; Branco, R.L.L.; Siqueira, T.C.; de Souza, L.C.; Dalago, K.M.S.; Andrade, A. Clinical Applicability of Infrared Thermography in Rheumatic Diseases: A Systematic Review. J. Therm. Biol. 2022, 104, 103172. [Google Scholar] [CrossRef]
- Saygin, D.; Highland, K.B.; Tonelli, A.R. Microvascular Involvement in Systemic Sclerosis and Systemic Lupus Erythematosus. Microcirculation 2019, 26, e12440. [Google Scholar] [CrossRef]
- Huang, C.-L.; Wu, Y.-W.; Hwang, C.-L.; Jong, Y.-S.; Chao, C.-L.; Chen, W.-J.; Wu, Y.-T.; Yang, W.-S. The Application of Infrared Thermography in Evaluation of Patients at High Risk for Lower Extremity Peripheral Arterial Disease. J. Vasc. Surg. 2011, 54, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, J.; Militaru, M.; Ciobotaru, E.; Ionascu, I. Thermographic Aspects of Feline Fibrosarcoma Complex. Vet. Dermatol. 2004, 15, 62. [Google Scholar] [CrossRef]
- Nitrini, A.G.C.; Cogliati, B.; Matera, J.M. Thermographic Assessment of Skin and Soft Tissue Tumors in Cats. J. Feline Med. Surg. 2021, 23, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Pouzot-Nevoret, C.; Barthélemy, A.; Goy-Thollot, I.; Boselli, E.; Cambournac, M.; Guillaumin, J.; Bonnet-Garin, J.-M.; Allaouchiche, B. Infrared Thermography: A Rapid and Accurate Technique to Detect Feline Aortic Thromboembolism. J. Feline Med. Surg. 2018, 20, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-GarciaLuna, J.L.; Bartlett, R.; Arriaga-Caballero, J.E.; Fraser, R.D.J.; Saiko, G. Infrared Thermography in Wound Care, Surgery, and Sports Medicine: A Review. Front. Physiol. 2022, 13, 838528. [Google Scholar] [CrossRef]
- Asif, A.; Poyiatzis, C.; Raheman, F.J.; Rojoa, D.M. The Use of Infrared Thermography (IRT) in Burns Depth Assessment: A Diagnostic Accuracy Meta-Analysis. Eur. Burn J. 2022, 3, 432–446. [Google Scholar] [CrossRef]
- John, H.E.; Niumsawatt, V.; Rozen, W.M.; Whitaker, I.S. Clinical Applications of Dynamic Infrared Thermography in Plastic Surgery: A Systematic Review. Gland Surg. 2016, 5, 122–132. [Google Scholar] [CrossRef]
- Lawson, R.N.; Wlodek, G.D.; Webster, D.R. Thermographic Assessment of Burns and Frostbite. Can. Med. Assoc. J. 1961, 84, 1129–1131. [Google Scholar]
- Simmons, J.D.; Kahn, S.A.; Vickers, A.L.; Crockett, E.S.; Whitehead, J.D.; Krecker, A.K.; Lee, Y.-L.; Miller, A.N.; Patterson, S.B.; Richards, W.O.; et al. Early Assessment of Burn Depth with Far Infrared Time-Lapse Thermography. J. Am. Coll. Surg. 2018, 226, 687–693. [Google Scholar] [CrossRef]
- Özsoylu, D.; Janus, K.A.; Achtsnicht, S.; Wagner, T.; Keusgen, M.; Schöning, M.J. (Bio-)Sensors for Skin Grafts and Skin Flaps Monitoring. Sens. Actuators Rep. 2023, 6, 100163. [Google Scholar] [CrossRef]
- Klama-Baryła, A.; Kitala, D.; Łabuś, W.; Kraut, M.; Szapski, M.; Smętek, W. Infrared Thermal Imaging as a Method of Improving Skin Graft Qualification Procedure and Skin Graft Survivability. Transplant. Proc. 2020, 52, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, J.; Staffa, E.; Čan, V.; Bernard, V.; Mornstein, V.; Farkašová, M.; Zetelolová, A.; Kala, Z. Intestinal Resection of a Porcine Model under Thermographic Monitoring. Physiol. Meas. 2019, 40, 014003. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, O.; Potter, S.M. Use of Infrared Thermography for the Assessment of Free Flap Perforators in Autologous Breast Reconstruction: A Systematic Review. JPRAS Open 2020, 23, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ilo, A.; Romsi, P.; Mäkelä, J. Infrared Thermography and Vascular Disorders in Diabetic Feet. J. Diabetes Sci. Technol. 2020, 14, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared Thermography: A Non-Invasive Window into Thermal Physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Jorge, J.; Harford, M.; Villarroel, M.; Chaichulee, S.; Davidson, S.; Finnegan, E.; Clark, S.H.; Young, J.D.; Watkinson, P.J.; Tarassenko, L. Non-Contact Assessment of Peripheral Artery Haemodynamics Using Infrared Video Thermography. IEEE Trans. Biomed. Eng. 2021, 68, 276–288. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Mora-Medina, P.; Casas-Alvarado, A.; Rios-Sandoval, J.; de Mira Geraldo, A.; Wang, D. Thermal Imaging to Assess the Health Status in Wildlife Animals under Human Care: Limitations and Perspectives. Animals 2022, 12, 3558. [Google Scholar] [CrossRef]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation Mechanisms and Perspectives for Validating Thermal Windows in Pigs with Hypothermia and Hyperthermia: An Overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef]
- Finstad, J.B.; Cooper, E.; Ten Cate, S.C.; Yaxley, P.; Her, J.; Guillaumin, J. Infrared Thermography Is a Novel Tool to Assess Small Intestinal Surface Temperature in Dogs Undergoing Laparotomy for Foreign Body Obstruction. Am. J. Vet. Res. 2023, 84, ajvr.23.04.0082. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Kashio, M.; Tominaga, M. TRP Channels in Thermosensation. Curr. Opin. Neurobiol. 2022, 75, 102591. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Caterina, M.J. Thermoregulation: Channels That Are Cool to the Core. Curr. Biol. 2007, 17, R885–R887. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Altier, C.; Motta, J.-P.; D’Aldebert, E.; Galeano, S.; Zamponi, G.W.; Vergnolle, N. Potentiation of TRPV4 Signalling by Histamine and Serotonin: An Important Mechanism for Visceral Hypersensitivity. Gut 2010, 59, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin Temperature: Its Role in Thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Sanin, L.Y.; Cabrera, A.M.Z.; Morales, A.M.T. Adaptive Responses to Thermal Stress in Mammals. Rev. Med. Vet. 2016, 31, 121–135. [Google Scholar]
- Sokolova, I. Temperature Regulation. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 633–639. [Google Scholar]
- Pérez de Diego, A.C.; Sánchez-Cordón, P.J.; Pedrera, M.; Martínez-López, B.; Gómez-Villamandos, J.C.; Sánchez-Vizcaíno, J.M. The Use of Infrared Thermography as a Non-Invasive Method for Fever Detection in Sheep Infected with Bluetongue Virus. Vet. J. 2013, 198, 182–186. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Muns, R.; Wang, D.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Mota-Rojas, D. Assessment of Pain and Inflammation in Domestic Animals Using Infrared Thermography: A Narrative Review. Animals 2023, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Mullan, S.; Main, D.C.J. Optimising Lameness Detection in Dairy Cattle by Using Handheld Infrared Thermometers. Vet. Med. Sci. 2018, 4, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in Infrared Thermography: Surgical Aspects, Vascular Changes, and Pain Monitoring in Veterinary Medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef] [PubMed]
- Magnin, M.; Junot, S.; Cardinali, M.; Ayoub, J.Y.; Paquet, C.; Louzier, V.; Garin, J.M.B.; Allaouchiche, B. Use of Infrared Thermography to Detect Early Alterations of Peripheral Perfusion: Evaluation in a Porcine Model. Biomed. Opt. Express 2020, 11, 2431–2446. [Google Scholar] [CrossRef]
- Abbott, C.A.; Carrington, A.L.; Ashe, H.; Bath, S.; Every, L.C.; Griffiths, J.; Hann, A.W.; Hussein, A.; Jackson, N.; Johnson, K.E.; et al. The North-West Diabetes Foot Care Study: Incidence of, and Risk Factors for, New Diabetic Foot Ulceration in a Community-based Patient Cohort. Diabet. Med. 2002, 19, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs-Franssen, M.; Houben, A.; Tooke, J.; Schaper, N. The Effect of Polyneuropathy on Foot Microcirculation in Type II Diabetes. Diabetologia 2002, 45, 1164–1171. [Google Scholar] [CrossRef]
- Abularrage, C.J.; Sidawy, A.N.; Aidinian, G.; Singh, N.; Weiswasser, J.M.; Arora, S. Evaluation of the Microcirculation in Vascular Disease. J. Vasc. Surg. 2005, 42, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Fais, P.; Mazzotti, M.C.; Montisci, M.; Palazzo, C.; Leone, O.; Cecchetto, G.; Viel, G.; Pelotti, S. Post-Mortem Thermal Angiography: A Pilot Study on Swine Coronary Circulation. Int. J. Leg. Med. 2019, 133, 571–581. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Maharjan, S.; Cui, J.; Lu, L.; Gong, X. Evaluating Surgical Delay Using Infrared Thermography in an Island Pedicled Perforator Flap Rat Model. J. Reconstr. Microsurg. 2017, 33, 518–525. [Google Scholar] [CrossRef]
- Łokaj, M.; Czapla, N.; Falkowski, A.; Prowans, P. The Use of Thermography in Early Detection of Tissue Perfusion Disorders in Rats. Videosurgery Other Miniinvasive Tech. 2014, 3, 329–336. [Google Scholar] [CrossRef]
- Caramalac, S.M.; De Bortoli, B.L.; Caramalac, S.M.; Souza, T.J.; Castilho, M.O.; Babo-terra, V.J.; Isa, M.; Palumbo, P. Thromboembolism in a Bitch—Diagnosis by Infrared Thermography. Acta Sci. Vet. 2023, 51, 871. [Google Scholar] [CrossRef]
- Prindeze, N.J.; Fathi, P.; Mino, M.J.; Mauskar, N.A.; Travis, T.E.; Paul, D.W.; Moffatt, L.T.; Shupp, J.W. Examination of the Early Diagnostic Applicability of Active Dynamic Thermography for Burn Wound Depth Assessment and Concept Analysis. J. Burn Care Res. 2015, 36, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, M.; Wang, T.; Wang, X.; Ma, X.; He, H.; Ma, G.; Zhao, D.; Yue, Q.; Wang, P.; et al. Smartphone-based Infrared Thermography to Assess Progress in Thoracic Surgical Incision Healing: A Preliminary Study. Int. Wound J. 2023, 20, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tan, C.; Pham, C.; Huang, S.; Yenikomshian, H.; Gillenwater, T.J. Use of Infrared Thermography for Flap Monitoring: A Systematic Review. Plast. Reconstr. Surg.-Glob. Open 2021, 9, 164. [Google Scholar] [CrossRef]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2022, 21, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Prindeze, N.J.; Hoffman, H.A.; Ardanuy, J.G.; Zhang, J.; Carney, B.C.; Moffatt, L.T.; Shupp, J.W. Active Dynamic Thermography Is a Sensitive Method for Distinguishing Burn Wound Conversion. J. Burn Care Res. 2016, 37, e559–e568. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Liu, X.; Chai, J. A Narrative Review of Changes in Microvascular Permeability after Burn. Ann. Transl. Med. 2021, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Miccio, J.; Parikh, S.; Marinaro, X.; Prasad, A.; McClain, S.; Singer, A.J.; Clark, R.A.F. Forward-Looking Infrared Imaging Predicts Ultimate Burn Depth in a Porcine Vertical Injury Progression Model. Burns 2016, 42, 397–404. [Google Scholar] [CrossRef]
- Renkielska, A.; Kaczmarek, M.; Nowakowski, A.; Grudziński, J.; Czapiewski, P.; Krajewski, A.; Grobelny, I. Active Dynamic Infrared Thermal Imaging in Burn Depth Evaluation. J. Burn Care Res. 2014, 35, e294–e303. [Google Scholar] [CrossRef]
- Albernaz, V.G.P.; Castro, J.L.C.; Santalucia, S.; Huppes, R.R.; De Nardi, A.B.; Pazzini, J.M. Reconstructive Surgical Repair of a Forth Degree Iatrogenic Burn in a Dog. Acta Sci. Vet. 2016, 44, 5. [Google Scholar] [CrossRef]
- de Lima, J.G.; Bahr, A.M.V. Medical and Surgical Management of a Large Thermal Burn in a Dog. Acta Sci. Vet. 2015, 43, 103. [Google Scholar]
- Wearn, C.; Lee, K.C.; Hardwicke, J.; Allouni, A.; Bamford, A.; Nightingale, P.; Moiemen, N. Prospective Comparative Evaluation Study of Laser Doppler Imaging and Thermal Imaging in the Assessment of Burn Depth. Burns 2018, 44, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; De, S. Thermal Injury of Skin and Subcutaneous Tissues: A Review of Experimental Approaches and Numerical Models. Burns 2017, 43, 909–932. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Cerna, C.; Becerra, S.C.; Sloan, M.; Wilmink, G.; Christy, R.J. Noninvasive Techniques for the Determination of Burn Severity in Real Time. J. Burn Care Res. 2017, 38, e180–e191. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.; Thomson, R.; Bamford, A.; Moiemen, N. A Pilot Evaluation Study of High Resolution Digital Thermal Imaging in the Assessment of Burn Depth. Burns 2013, 39, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R. Grafts and Flaps. Surgery 2006, 24, 27–32. [Google Scholar] [CrossRef]
- Aisa, J.; Vernon, J. The Use of Skin Grafts in Small Animal Reconstructive Surgery. Companion Anim. 2016, 21, 642–648. [Google Scholar] [CrossRef]
- Martínez-Jiménez, M.A.; Ramirez-GarciaLuna, J.L.; Kolosovas-Machuca, E.S.; Drager, J.; González, F.J. Development and Validation of an Algorithm to Predict the Treatment Modality of Burn Wounds Using Thermographic Scans: Prospective Cohort Study. PLoS ONE 2018, 13, e0206477. [Google Scholar] [CrossRef]
- Kumar, P.; Gaurav, A.; Rajnish, R.K.; Sharma, S.; Kumar, V.; Aggarwal, S.; Patel, S. Applications of Thermal Imaging with Infrared Thermography in Orthopaedics. J. Clin. Orthop. Trauma 2022, 24, 101722. [Google Scholar] [CrossRef]
- da Silveira, Y.G.; Kajiura, C.; Bernardes, F.J.L.; Maria, S.P.; Fernandes, M.P.; Frasson, M.T.; Cassino, P.; Moreira, S.H.; Gómez, J.L.Á.; Pazzini, J.M.; et al. Use of Thermography in Skin Grafts after the Application of Therapeutic Ultrasound in Wistar Rats. Acta Cirúrgica Bras. 2020, 35, e202000703. [Google Scholar] [CrossRef]
- Lekas, R.; Jakuška, P.; Kriščiukaitis, A.; Veikutis, V.; Dzemyda, G.; Mickevičius, T.; Morkūnaitė, K.; Vilkė, A.; Treigys, P.; Civinskienė, G.; et al. Monitoring changes in heart tissue temperature and evaluation of graft function after coronary artery bypass grafting surgery. Medicina 2009, 45, 221. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.W. Grafting Techniques and Failures in Small Animal Surgery. Probl. Vet. Med. 1990, 2, 413–423. [Google Scholar] [PubMed]
- Hallock, G.G. The Use of Smartphone Thermography to More Safely Unmask and Preserve Circulation to Keystone Advancement Flaps in the Lower Extremity. Injury 2020, 51, S121–S125. [Google Scholar] [CrossRef] [PubMed]
- Sjøberg, T.; Mercer, J.B.; Weum, S.; de Weerd, L. The Value of Dynamic Infrared Thermography in Pedicled Thoracodorsal Artery Perforator Flap Surgery. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2799. [Google Scholar] [CrossRef] [PubMed]
- Miland, Å.O.; de Weerd, L.; Weum, S.; Mercer, J.B. Visualising Skin Perfusion in Isolated Human Abdominal Skin Flaps Using Dynamic Infrared Thermography and Indocyanine Green Fluorescence Video Angiography. Eur. J. Plast. Surg. 2008, 31, 235–242. [Google Scholar] [CrossRef]
- Rabbani, M.J.; Bhatti, A.Z.; Shahzad, A. Flap Monitoring Using Thermal Imaging Camera: A Contactless Method. J. Coll. Physicians Surg. Pak. 2021, 31, 703–706. [Google Scholar] [CrossRef]
- Miland, Å.O.; de Weerd, L.; Mercer, J.B. Intraoperative Use of Dynamic Infrared Thermography and Indocyanine Green Fluorescence Video Angiography to Predict Partial Skin Flap Loss. Eur. J. Plast. Surg. 2008, 30, 269–276. [Google Scholar] [CrossRef]
- Tenorio, X.; Mahajan, A.L.; Wettstein, R.; Harder, Y.; Pawlovski, M.; Pittet, B. Early Detection of Flap Failure Using a New Thermographic Device. J. Surg. Res. 2009, 151, 15–21. [Google Scholar] [CrossRef]
- Czapla, N.; Łokaj, M.; Falkowski, A.; Prowans, P. The Use of Thermography to Design Tissue Flaps–Experimental Studies on Animals. Videosurgery Other Miniinvasive Tech. 2014, 3, 319–328. [Google Scholar] [CrossRef]
- Drvis, P.; Shejbal, D.; Svaic, S.; Boras, I.; Sundov, I.; Susa, M.; Bedekovic, V.; Kalogjera, L.; Petrovic, I.; Sikiric, P. Evaluation of Cutaneous Flap Survival by IR Thermography. In Proceedings of the 2004 International Conference on Quantitative InfraRed Thermography, Zagreb, Croatia, 5–8 January 2004; pp. 1–10. [Google Scholar]
- Shejbal, D.; Drvis, P.; Bedekovic, V. Thermography-measured Effect of Capsaicin, Methylprednisolone and Mitomycin on the Survival of Random Skin Flaps in Rats. Ski. Res. Technol. 2012, 18, 157–161. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Fenner, J.; Sasson, A.; McClain, S.A.; Singer, A.J.; Tonnesen, M.G. Blood Vessel Occlusion with Erythrocyte Aggregates Causes Burn Injury Progression—Microvasculature Dilation as a Possible Therapy. Exp. Dermatol. 2018, 27, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Hurley-Sanders, J.L.; Sladky, K.K.; Nolan, E.C.; Loomis, M.R. Use of Cortical Bone Fenestration, Autogenous Free Skin Graft, and Thermography for Wound Treatment and Monitoring in a Red Wolf (Canis Rufus Gregoryi). J. Zoo Wildl. Med. 2015, 46, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chen, Y.-C.; Cheng, K.-S.; Yu, P.-J.; Wang, J.-L.; Ko, N.-Y. Higher Periwound Temperature Associated with Wound Healing of Pressure Ulcers Detected by Infrared Thermography. J. Clin. Med. 2021, 10, 2883. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Fielding, A.; Mullhi, R.; Chipp, E.; Torlinski, T. Temperature Management of Adult Burn Patients in Intensive Care: Findings from a Retrospective Cohort Study in a Tertiary Centre in the United Kingdom. Anaesthesiol. Intensive Ther. 2022, 54, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Saidu, A.M.; Olorunfemi, J.O.; Laku, D. Infrared Thermography Following Castration, Otectomy and Gastrotomy in Nigerian Indigenous Dogs. Sahel J. Vet. Sci. 2023, 20, 50–56. [Google Scholar] [CrossRef]
- Viscardi, A.V.; Cull, C.A.; Kleinhenz, M.D.; Montgomery, S.; Curtis, A.; Lechtenberg, K.; Coetzee, J.F. Evaluating the Utility of a CO2 Surgical Laser for Piglet Castration to Reduce Pain and Improve Wound Healing: A Pilot Study. J. Anim. Sci. 2020, 98, skaa320. [Google Scholar] [CrossRef]
- Marti, S.; Schwartzkopf-Genswein, K.S.; Janzen, E.D.; Meléndez, D.M.; Gellatly, D.; Pajor, E.A. Use of Topical Healing Agents on Scrotal Wounds after Surgical Castration in Weaned Beef Calves. Can. Vet. J. La Rev. Vet. Can. 2017, 58, 1081–1085. [Google Scholar]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Rosas, M.E.; Miranda-Cortés, A.; Domínguez-Oliva, A.; Mora-Medina, P. Assessment of Thermal Response, Cardiorespiratory Parameters and Post-Operative Analgesia in Dogs Undergoing Ovariohysterectomy with Different Combinations of Epidural Analgesia and Isoflurane. J. Anim. Behav. Biometeorol. 2023, 11, e2023009. [Google Scholar] [CrossRef]
- Nogues, E.; von Keyserlingk, M.A.G.; Weary, D.M. Pain in the Weeks Following Surgical and Rubber Ring Castration in Dairy Calves. J. Dairy Sci. 2021, 104, 12881–12886. [Google Scholar] [CrossRef]
- Loughin, C.A.; Marino, D.J. Evaluation of Thermographic Imaging of the Limbs of Healthy Dogs. Am. J. Vet. Res. 2007, 68, 1064–1069. [Google Scholar] [CrossRef]
- Malafaia, O.; Brioschi, M.L.; Aoki, S.M.S.; Dias, F.G.; Gugelmin, B.S.; Aoki, M.S.; Aoki, Y.S. Infrared Imaging Contribution for Intestinal Ischemia Detection in Wound Healing. Acta Cir. Bras. 2008, 23, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Ringer, S.K.; Lischer, C.J.; Ueltschi, G. Assessment of Scintigraphic and Thermographic Changes after Focused Extracorporeal Shock Wave Therapy on the Origin of the Suspensory Ligament and the Fourth Metatarsal Bone in Horses without Lameness. Am. J. Vet. Res. 2005, 66, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Thielecke, M.; Raharimanga, V.; Ramarokoto, C.E.; Rogier, C.; Krantz, I.; Feldmeier, H. High-Resolution Infrared Thermography: A New Tool to Assess Tungiasis-Associated Inflammation of the Skin. Trop. Med. Health 2017, 45, 23. [Google Scholar] [CrossRef] [PubMed]
- Galvin, E.M.; Niehof, S.; Medina, H.J.; Zijlstra, F.J.; van Bommel, J.; Klein, J.; Verbrugge, S.J.C. Thermographic Temperature Measurement Compared with Pinprick and Cold Sensation in Predicting the Effectiveness of Regional Blocks. Anesth. Analg. 2006, 102, 598–604. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Clausen, S.; Mercer, J.B.; Pedersen, L.J. Determining the Emissivity of Pig Skin for Accurate Infrared Thermography. Comput. Electron. Agric. 2014, 109, 52–58. [Google Scholar] [CrossRef]
- Autio, E.; Neste, R.; Airaksinen, S.; Heiskanen, M.-L. Measuring the Heat Loss in Horses in Different Seasons by Infrared Thermography. J. Appl. Anim. Welf. Sci. 2006, 9, 211–221. [Google Scholar] [CrossRef]
- Ludwig, N.; Gargano, M.; Luzi, F.; Carenzi, C.; Verga, M. Technical Note: Applicability of Infrared Thermography as a Non Invasive Measurements of Stress in Rabbit. World Rabbit Sci. 2010, 15, 588. [Google Scholar] [CrossRef]
- Jørgensen, G.H.; Mejdell, C.M.; Bøe, K.E. Effects of Hair Coat Characteristics on Radiant Surface Temperature in Horses. J. Therm. Biol. 2020, 87, 102474. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Kwon, C.J.; Brundage, C.M. Quantifying Body Surface Temperature Differences in Canine Coat Types Using Infrared Thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef]
- Maśko, M.; Witkowska-Piłaszewicz, O.; Jasiński, T.; Domino, M. Thermal Features, Ambient Temperature and Hair Coat Lengths: Limitations of Infrared Imaging in Pregnant Primitive Breed Mares within a Year. Reprod. Domest. Anim. 2021, 56, 1315–1328. [Google Scholar] [CrossRef] [PubMed]
- Favilla, A.B.; Costa, D.P. Thermoregulatory Strategies of Diving Air-Breathing Marine Vertebrates: A Review. Front. Ecol. Evol. 2020, 8, 555509. [Google Scholar] [CrossRef]
- Gauchet, L.; Jaeger, A.; Grémillet, D. Using Facial Infrared Thermography to Infer Avian Body Temperatures in the Wild. Mar. Biol. 2022, 169, 57. [Google Scholar] [CrossRef]
- McQueen, A.; Barnaby, R.; Symonds, M.R.E.; Tattersall, G.J. Birds Are Better at Regulating Heat Loss through Their Legs than Their Bills: Implications for Body Shape Evolution in Response to Climate. Biol. Lett. 2023, 19, 20230373. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.; McCane, B.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Automated Collection and Analysis of Infrared Thermograms for Measuring Eye and Cheek Temperatures in Calves. Animals 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Olmos-Hernández, A.; Villegas-Juache, J.; Verduzco-Mendoza, A.; Mota-Rojas, D. Thermal Response of Laboratory Rats (Rattus norvegicus) during the Application of Six Methods of Euthanasia Assessed by Infrared Thermography. Animals 2023, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of Environmental Factors on Infrared Eye Temperature Measurements in Cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- de Lima, V.; Piles, M.; Rafel, O.; López-Béjar, M.; Ramón, J.; Velarde, A.; Dalmau, A. Use of Infrared Thermography to Assess the Influence of High Environmental Temperature on Rabbits. Res. Vet. Sci. 2013, 95, 802–810. [Google Scholar] [CrossRef]
- Diakides, N.A.; Bronzino, J.D. Medical Infrared Imaging; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Chung, Y.; Lee, S.; Kim, W. Latest Advances in Common Signal Processing of Pulsed Thermography for Enhanced Detectability: A Review. Appl. Sci. 2021, 11, 12168. [Google Scholar] [CrossRef]
- Zhou, T.; Gan, Z.; Zhang, H.; Liu, Z.; Pu, Y.; Rong, M. A Novel Technique to Harvest Bone Autografts with Mild Local Hyperthermia and Enhanced Osteogenic Bone Quality: A Preclinical Study in Dogs. BMC Oral Health 2023, 23, 838. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.; Salimov, F.; Temmerman, A.; Turer, O.U.; Alkaya, B.; Haytac, M.C. The Evaluation of Different Osteotomy Drilling Speed Protocols on Cortical Bone Temperature, Implant Stability and Bone Healing: An Experimental Study in an Animal Model. J. Oral Implantol. 2022, 48, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Krasnikov, A.V.; Krasnikova, E.S. Use of Infrared Thermography to Control Osteoreparative and Integrative Processes during Implantation in Animals. J. Phys. Conf. Ser. 2020, 1515, 052011. [Google Scholar] [CrossRef]
- Ilo, A.; Romsi, P.; Mäkelä, J. Infrared Thermography as a Diagnostic Tool for Peripheral Artery Disease. Adv. Ski. Wound Care 2020, 33, 482–488. [Google Scholar] [CrossRef]
- Grossbard, B.P.; Loughin, C.A.; Marino, D.J.; Marino, L.J.; Sackman, J.; Umbaugh, S.E.; Solt, P.S.; Afruz, J.; Leando, P.; Lesser, M.L.; et al. Medical Infrared Imaging (Thermography) of Type I Thoracolumbar Disk Disease in Chondrodystrophic Dogs. Vet. Surg. 2014, 43, 869–876. [Google Scholar] [CrossRef]
- Han, T.; Khavanin, N.; Wu, J.; Zang, M.; Zhu, S.; Chen, B.; Li, S.; Liu, Y.; Sacks, J.M. Indocyanine Green Angiography Predicts Tissue Necrosis More Accurately Than Thermal Imaging and Near-Infrared Spectroscopy in a Rat Perforator Flap Model. Plast. Reconstr. Surg. 2020, 146, 1044–1054. [Google Scholar] [CrossRef]
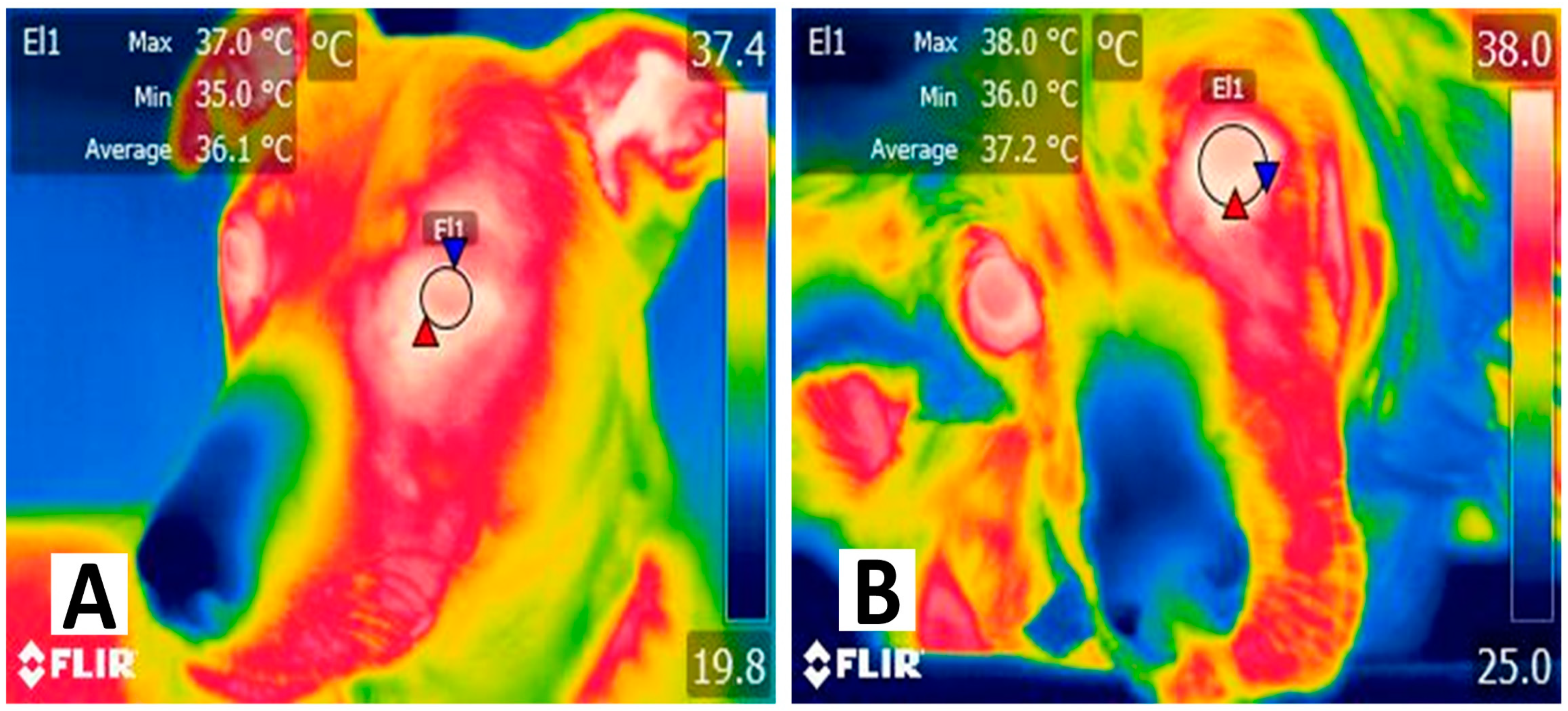


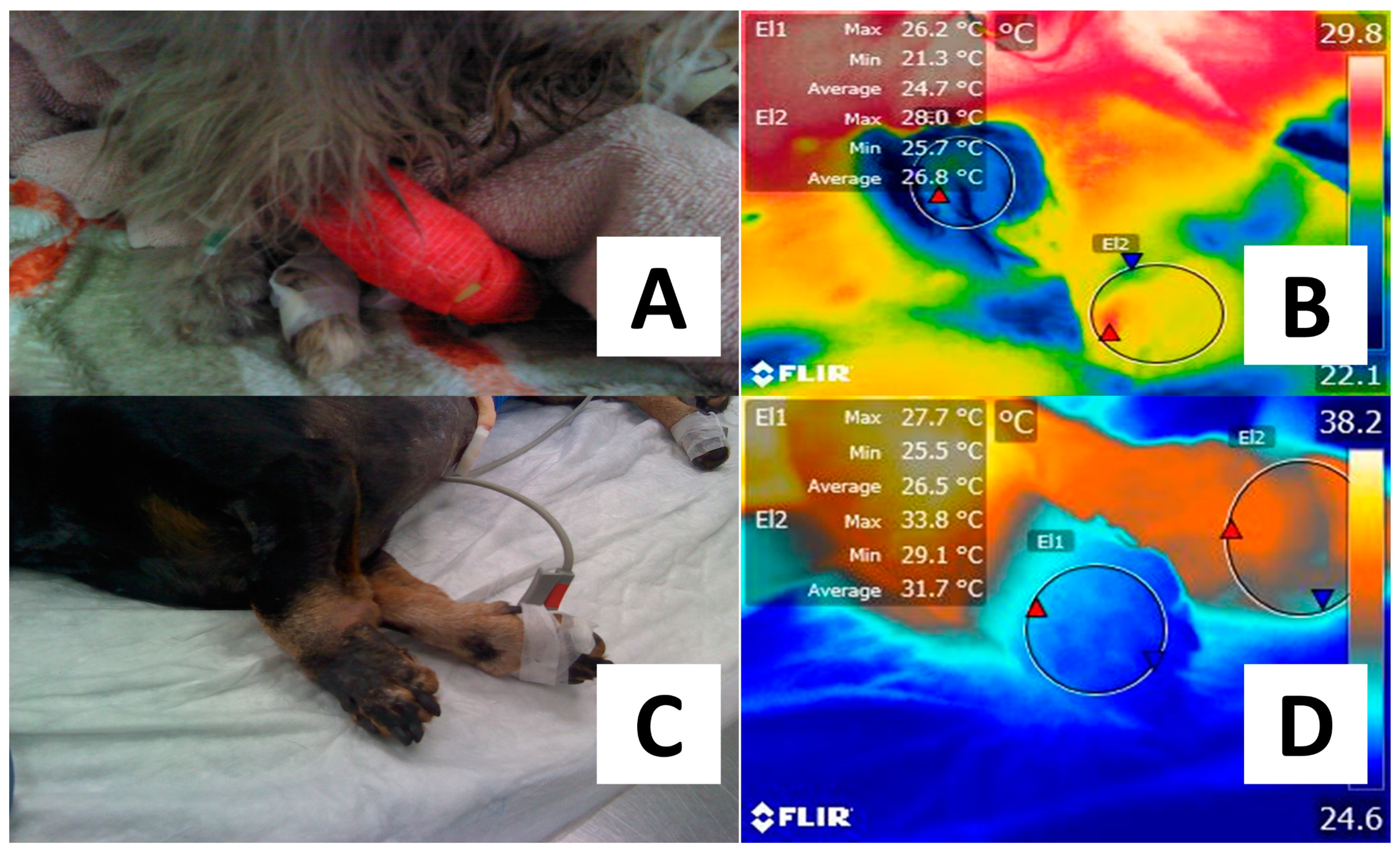
| Species | Camera | Distance (cm) | Emissivity | Ambient Temperature (°C) | Aim | Outcome |
|---|---|---|---|---|---|---|
| Cats [14] | Flir C2 | 75 | 0.95 | 20.0 | Identify aortic thrombosis. | 2.4 °C to differentiate sick animals. |
| Dogs [52] | FlirOne Pro | 30 | NR | 25.0 | Diagnose thromboembolism. | Affected limb had lower temperature (31.3 °C vs. 35.0 °C). |
| Pigs [49] | FlirOne | NR | NR | NR | Establish coronary circulation. | Visible occlusion of coronaries appearing in bright yellow. |
| Pigs [45] | Flir-E6 | 50 | 0.98 | NR | Assess changes in skin temperature in response to blood pressure. | Negative correlation between blood pressure and temperature gradient. |
| Rats [50] | Flir T650SC | 45 | NR | NR | Monitor pedicled island perforator flaps. | 1.4% increase in red zones or hot spots. |
| Rats [51] | Flir 335 | 20 | NR | NR | Detect tissue perfusion disorders in femoral vessels. | Return of warmth in the limb was slower. |
| Species | Camera | Distance (cm) | Emissivity | Ambient Temperature (°C) | Aim | Outcome |
|---|---|---|---|---|---|---|
| Pigs [59] | Flir T300 | 30 | NR | NR | Assess burn depth. | Surface hypothermia (27.31 ± 0.37 °C) predicts larger scar depth. |
| Pigs [60] | ADT | NR | NR | NR | Assess burn depth. | Shorter thermal time (49.1 ± 23 s) related to fast healing. |
| Pigs [65] | Flir A655sc | 40 | NR | NR | Assess burn severity. | Severe burns have lower temperatures (32.4–33 °C). |
| Rats [76] | DIRT Flir ThermaCAM S65 | NR | NR | NR | Predict flap survival. | 74% flap survival. |
| Rats [79] | BioScanIR | NR | NR | NR | Detect flap failure. | Hot spot disappeared, followed by macroscopic congestion. |
| Red wolf [84] | eVs DTIS 500 | NR | NR | NR | Monitor free skin graft. | Neovascularization in the graft becomes thermally equivalent to healthy tissue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Ogi, A.; Villanueva-García, D.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Lendez, P.; Ghezzi, M. Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications. Animals 2024, 14, 142. https://doi.org/10.3390/ani14010142
Mota-Rojas D, Ogi A, Villanueva-García D, Hernández-Ávalos I, Casas-Alvarado A, Domínguez-Oliva A, Lendez P, Ghezzi M. Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications. Animals. 2024; 14(1):142. https://doi.org/10.3390/ani14010142
Chicago/Turabian StyleMota-Rojas, Daniel, Asahi Ogi, Dina Villanueva-García, Ismael Hernández-Ávalos, Alejandro Casas-Alvarado, Adriana Domínguez-Oliva, Pamela Lendez, and Marcelo Ghezzi. 2024. "Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications" Animals 14, no. 1: 142. https://doi.org/10.3390/ani14010142
APA StyleMota-Rojas, D., Ogi, A., Villanueva-García, D., Hernández-Ávalos, I., Casas-Alvarado, A., Domínguez-Oliva, A., Lendez, P., & Ghezzi, M. (2024). Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications. Animals, 14(1), 142. https://doi.org/10.3390/ani14010142










