Habitat Suitability, Distribution Modelling and GAP Analysis of Przewalski’s Gazelle Conservation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
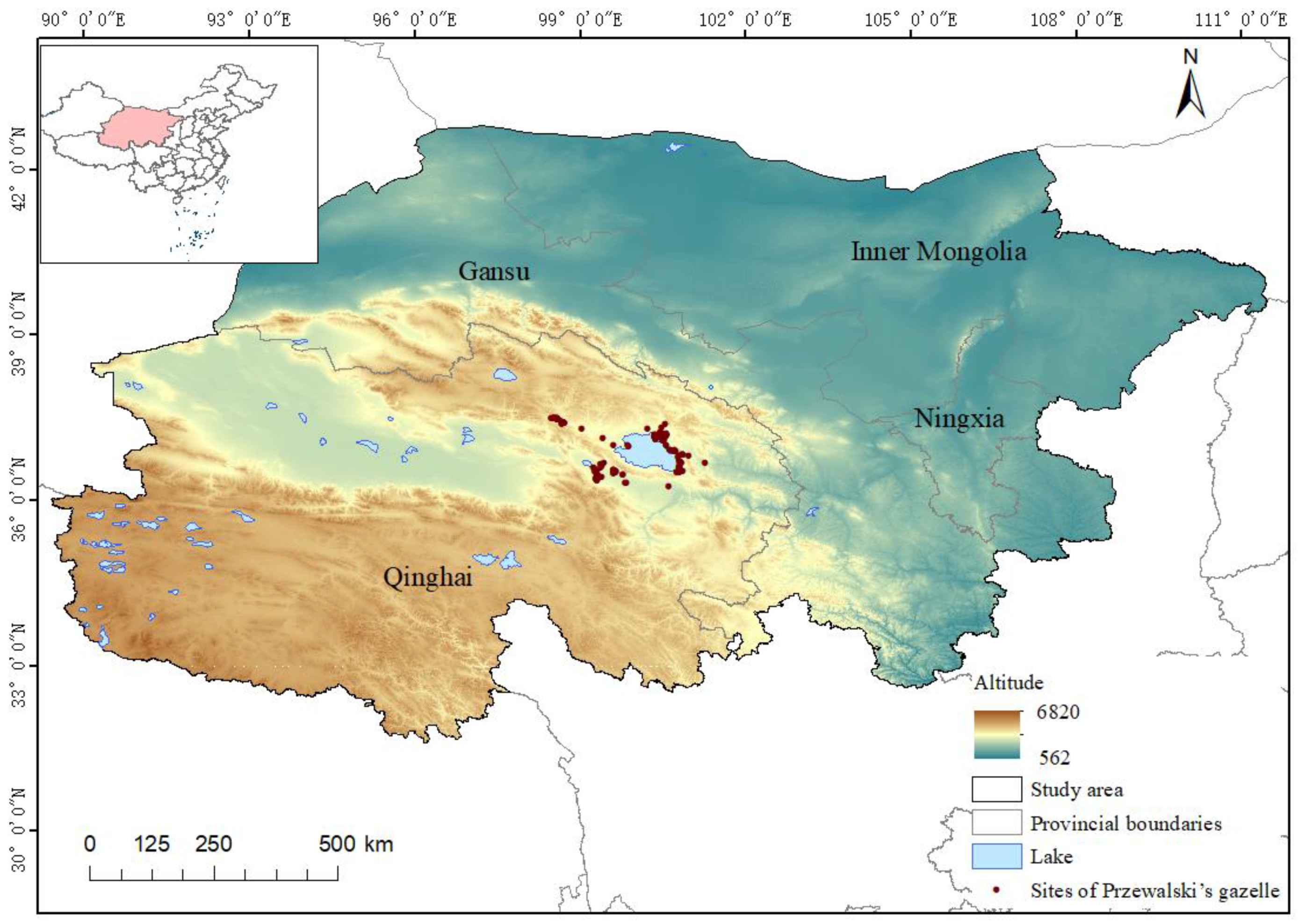
2.1. Study Area
2.2. Data Collection
2.3. Environment Variable Filtering
2.4. Model Parameter Optimization
2.5. Model Operation
2.6. Result Threshold Division
2.7. Model Accuracy Evaluation
2.8. GAP Analysis
3. Results
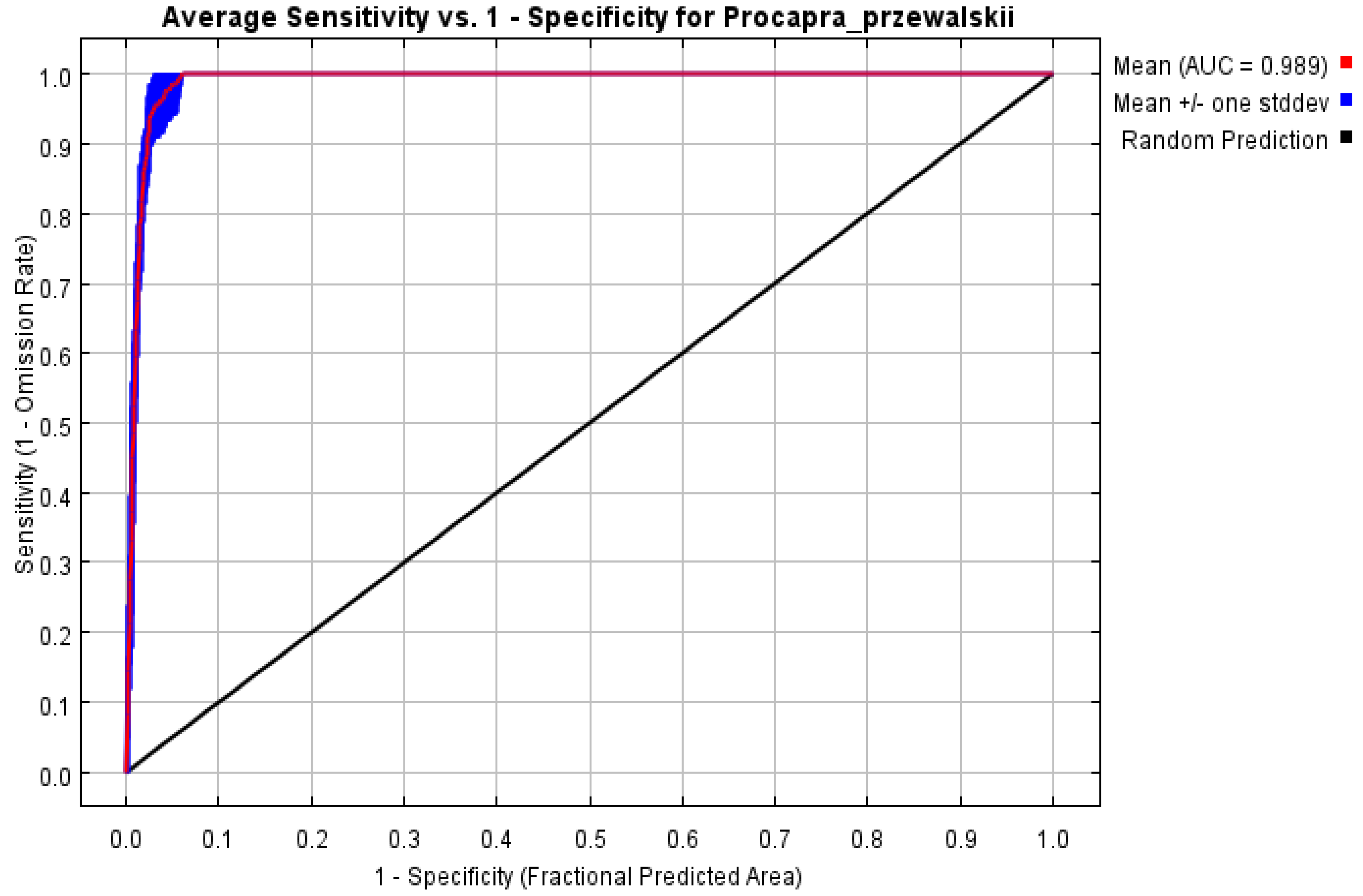
3.1. MaxEnt Result Accuracy Analysis
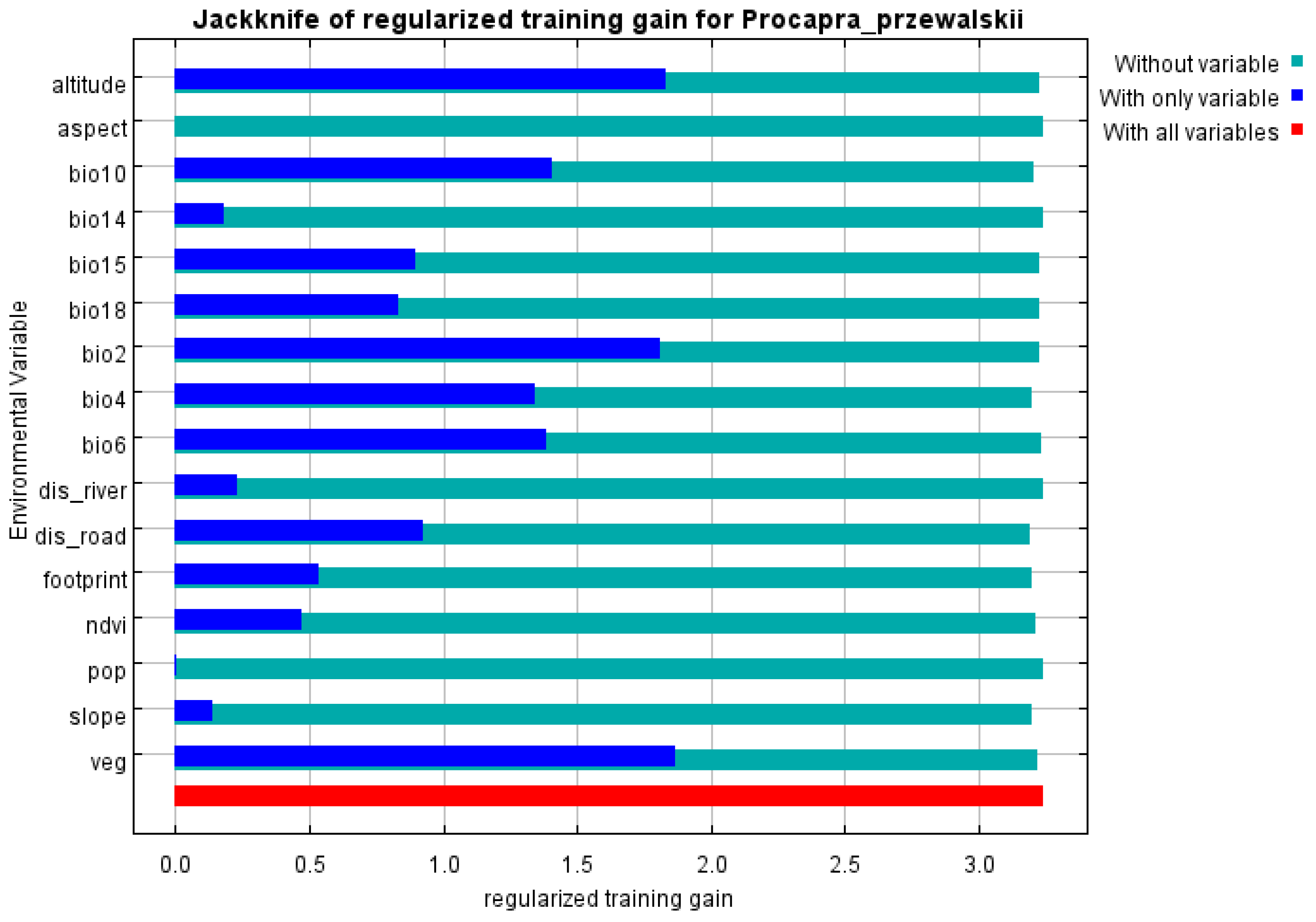
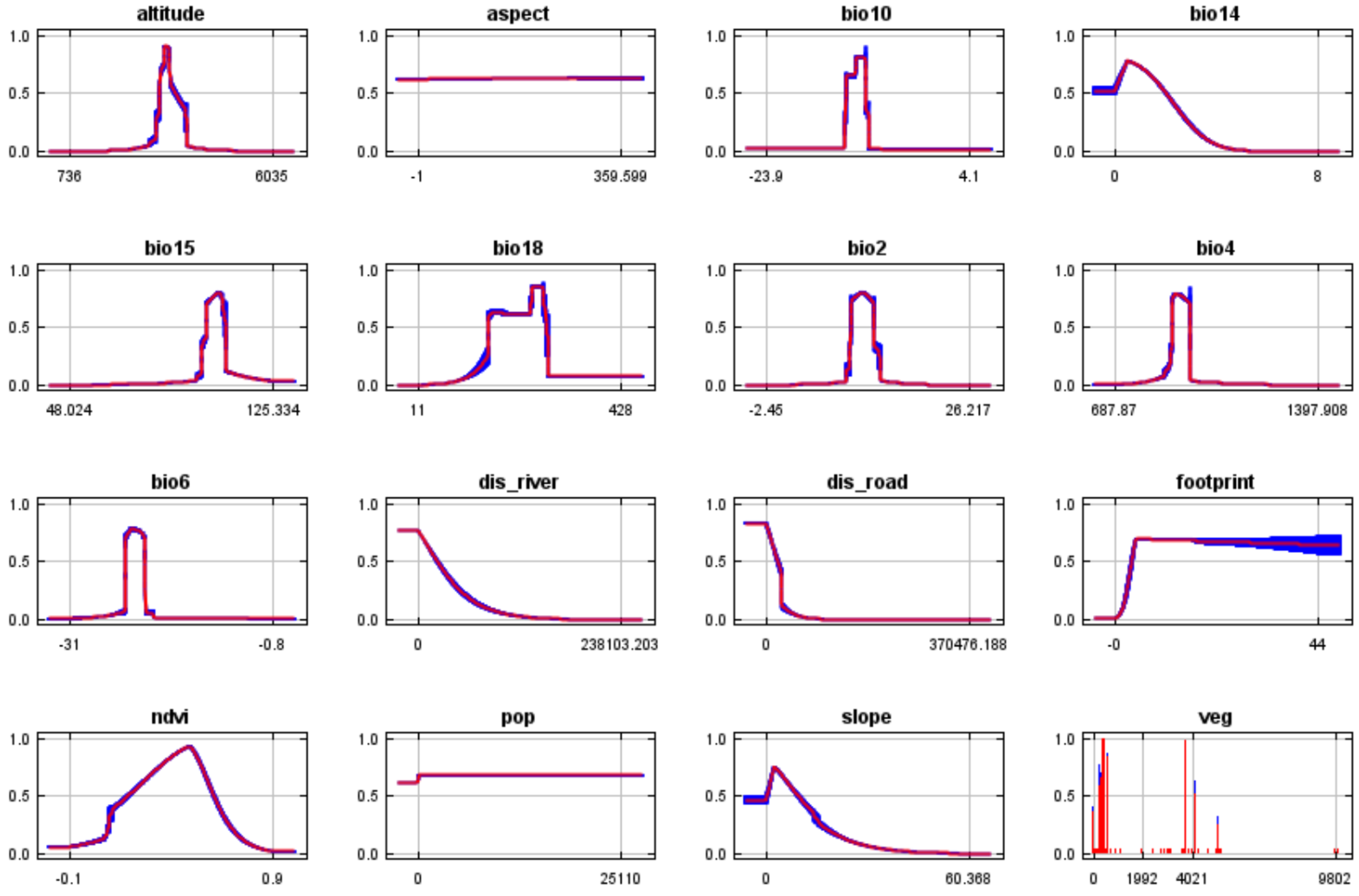
3.2. Main Environmental Variables Affecting the Distribution of Przewalski’s Gazelle
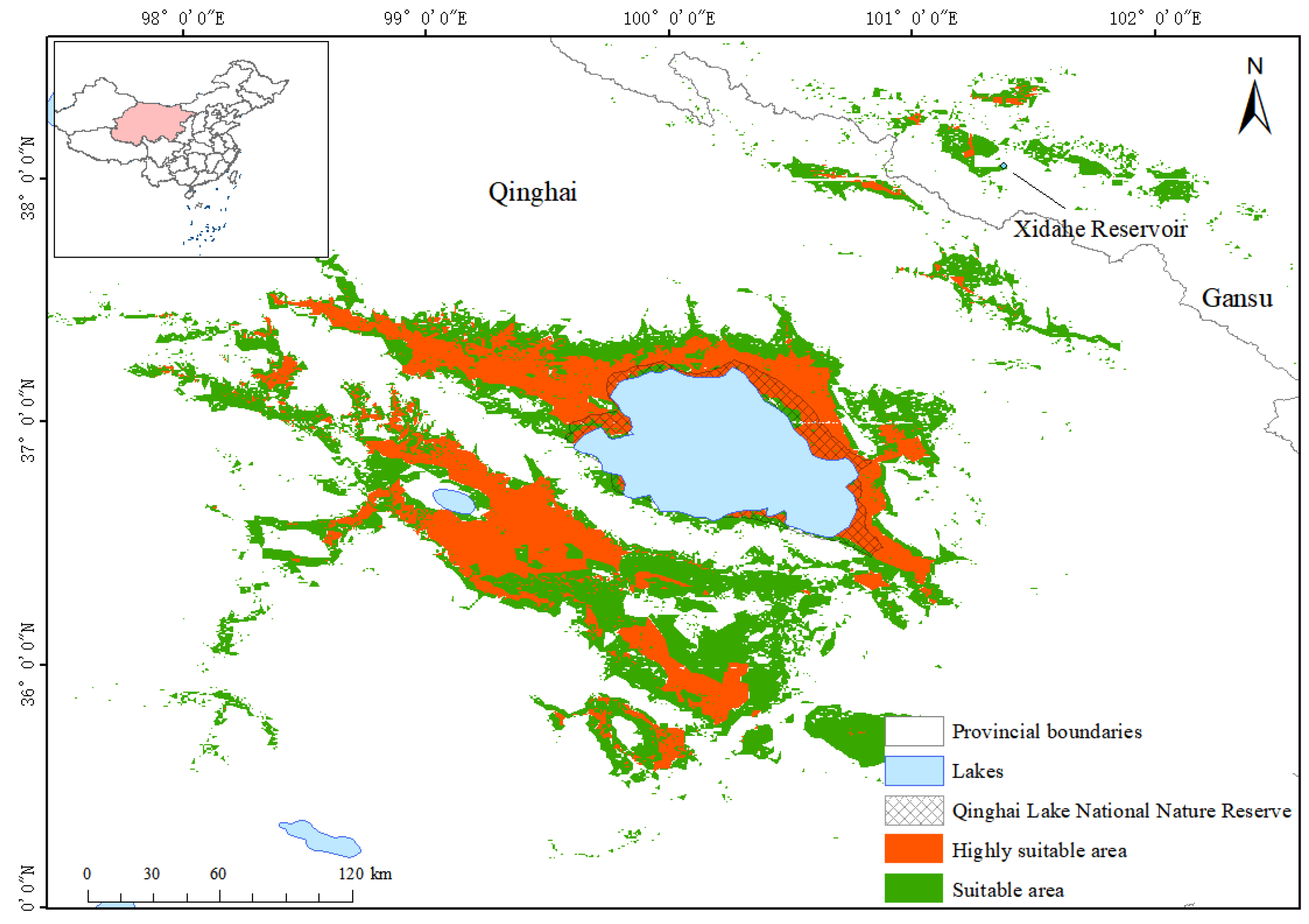
3.3. Potential Suitable Distribution Area of Przewalski’s Gazelle
3.4. GAP Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, M.C.; Chen, X.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jiang, L.; et al. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Allen, G.M. The Mammals of China and Mongolia. Part 2. In Journal of Mammalogy; American Museum of Natural History: New York, NY, USA, 1940; pp. 1216–1222. [Google Scholar]
- Zhao, K. Ungulates from Inner Mongolia. J. Inn. Mong. Univ. 1960, 1, 53–60. [Google Scholar]
- Jiang, Z.; Feng, Z.; Wang, Z.; Chen, L.; Cai, P.; Li, Y. Historical and current distribution of Przewalski’s gazelle. Acta Theriol. Sin. 1995, 15, 241–245. [Google Scholar]
- Jiang, Z.; Li, D.; Wang, Z.; Zhu, S.; Wei, W. Population structure of Przewalski’s gazelle in Qinghai Lake area. Acta Zool. Sin. 2001, 47, 158–162. [Google Scholar]
- Liang, D.; Liu, Y.; Ping, X.; Jiang, Z.; Li, C. Ensuring recovery for the Przewalski’s gazelle. Science 2021, 374, 163. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.; Li, C.; Li, C.; Tang, S.; Fang, H.; Cui, S.; Chen, J.; Wang, E.; He, Y.; Cai, P.; et al. The distribution, population and conservation status of Przewalski’s gazelle (Procapra przewalskii). Biodiversity 2018, 26, 177–184. [Google Scholar]
- Li, Z.X.J. The Impact of Climate Change on the Breeding Population and Habitat Quality of the Spotted Goose in Qinghai Lake. Master’s Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar]
- Hu, J.; Jiang, Z. Climate change hastens the conservation urgency of an endangered ungulate. PLoS ONE 2011, 6, e22873. [Google Scholar] [CrossRef]
- You, Z.; Jiang, Z.; Li, C.; Mallon, D. Impacts of grassland fence on the behavior and distribution range of the critically endangered Przewalski’s gazelle on the Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2013, 58, 2262–2268. [Google Scholar] [CrossRef]
- Li, C.; Jiang, Z.; Feng, Z.; Yang, X.; Yang, J.; Chen, L. Effects of highway traffic on diurnal activity of the critically endangered Przewalski’s gazelle. Wildl. Res. 2009, 36, 379–385. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Shi, M.; Chen, J. Prediction of potential geographical distribution patterns of Salix tetrasperma Roxb. in Asia under different climate scenario. Acta Ecol. Sin. 2019, 39, 3224–3234. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Machine Learning, Proceedings of the Twenty-first International Conference (ICML 2004), Banff, AB, Canada, 4–8 July 2004; Elsevier: Amsterdam, The Netherlands, 2004; pp. 83–88. [Google Scholar]
- Anand, V.; Oinam, B.; Singh, I.H. Predicting the current and future potential spatial distribution of endangered Rucervus eldii eldii (Sangai) using MaxEnt model. Environ. Monit. Assess. 2021, 193, 147. [Google Scholar] [CrossRef]
- Pant, G.; Maraseni, T.; Apan, A.; Allen, B.J. Predicted declines in suitable habitat for greater one-horned rhinoceros (Rhinoceros unicornis) under future climate and land use change scenarios. Ecol. Evol. 2021, 11, 18288–18304. [Google Scholar] [CrossRef]
- Gull, E.; Fareen, A.; Mahmood, T.; Bodlah, I.; Rashid, A.; Khalid, A.; Mahmood, S. Modeling potential distribution of newly recorded ant, Brachyponera nigrita using MaxEnt under climate change in Pothwar region, Pakistan. PLoS ONE 2022, 17, e0262451. [Google Scholar]
- Scott, J.M.; Davis, F.W.; Csuti, B.A.; Noss, R.F.; Groves, C.R.; Anderson, H.N.; Caicco, S.L.; Edwards, T.C.; Ulliman, J.J.; Wright, R.G. Gap analysis: A geographic approach to protection of biological diversity. Wildl. Monogr. 1993, 123, 3–41. [Google Scholar]
- Jennings, M.D. Gap analysis: Concepts, methods, and recent results. Landsc. Ecol. 2000, 15, 5–20. [Google Scholar] [CrossRef]
- Stoms, D.M. Gap management status is and regional indicators of threats to diversity. Landsc. Ecol. 2000, 15, 21–33. [Google Scholar] [CrossRef]
- Maiorano, L.; Falcucci, A.; Boitani, L. Gap analysis of terrestrial vertebrates in Italy: Priorities for conservation planning in a human dominated landscape. Biol. Conserv. 2006, 133, 455–473. [Google Scholar] [CrossRef]
- van Breugel, P.; Kindt, R.; Barnekow Lillesø, J.P.; van Breugel, M. Environmental Gap analysis to prioritize conservation efforts in eastern Africa. PLoS ONE 2015, 10, e0121444. [Google Scholar] [CrossRef]
- Feng, L.; Duan, W.; Gao, W.; Run, S. Atlas of Rare and Endangered Animals in Mongolia; China Agricultural Science and Technology Press: Beijing, China, 1991; pp. 182–185. [Google Scholar]
- Bi, J.; Feng, L. Investigation on the historical distribution and current status of Przewalski’s gazelle in Inner Mongolia. In Chinese Animal Science Research, Zoological Society of China Edition; Proceedings of the 14th Member Congress of the Zoological Society of China and the 65th Annual Conference of the Zoological Society of China; China Forestry Publishing House: Beijing, China, 1999; pp. 311–314. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Xu, X. Spatial Distribution Dataset of Monthly Normalized Difference Vegetation Index (NDVI) in China; Data Registration and Publishing System of Resource and Environmental Science Data Center; Chinese Academy of Sciences: Beijing, China, 2018. [Google Scholar]
- Li, H.; Niu, C.; Yuan, X.; Huo, A.; Shang, W.; Liu, J.; Han, P.; Zhang, Y.; Jian, N. Predicting the Potential Distribution Area of Moschus moschiferus Using the MaxEnt Model in the Huanglong Mountains, Shaanxi Province. J. Ecol. Rural Environ. 2021, 37, 1616–1622. [Google Scholar]
- Abolmaali, M.R.S.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Zhu, G.; Qiao, H. Effect of the MaxEnt model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Zou, H. Optimizing MaxEnt model in the prediction of species distribution. Chin. J. Appl. Ecol. 2019, 30, 2116–2128. [Google Scholar]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in MaxEnt: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for MaxEnt ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Z.; Peng, Y.; Wang, Y.; Wang, P.; Wang, S.; Cui, G. Prediction of potential suitable areas of Actinidia chinensis based on MaxEnt model. Acta Ecol. Sin. 2020, 40, 4921–4928. [Google Scholar]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either—Or presence-absence. Acta Oecol. 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Kaushalendra, K.J.; Radhika, J. Study of Vulture Habitat Suitability and Impact of Climate Change in Central India Using MaxEnt. J. Resour. Ecol. 2021, 12, 30–42. [Google Scholar]
- Helenbrook, W.D.; Valdez, J.W. Species distribution and conservation assessment of the black-headed night monkey (Aotus nigriceps): A species of Least Concern that faces widespread anthropogenic threats. Primates 2021, 62, 817–825. [Google Scholar] [CrossRef]
- Moreno, E.; Sane, A.; Benzal, J.; Ibáñez, B.; Sanz-Zuasti, J.; Espeso, G. Changes in habitat structure may explain decrease in reintroduced mohor gazelle population in the Guembeul Fauna Reserve, Senegal. Animals 2012, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Makki, T.; Fakheran, S.; Moradi, H.; Iravani, M.; Senn, J. Landscape-scale impacts of transportation infrastructure on spatial dynamics of two vulnerable ungulate species in Ghamishloo Wildlife Refuge, Iran. Ecol. Indic. 2013, 31, 6–14. [Google Scholar] [CrossRef]
- Wright, P.; Coomber, F.; Bellamy, C.; Perkins, S.E.; Mathews, F. Predicting hedgehog mortality risks on British roads using habitat suitability modelling. PeerJ 2020, 7, e8154. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wu, X.; Zhang, H. Distribution of ungulate in different grades of highway and influential factors concerned in Qinling Mountains. Shaanxi For. Sci. Technol. 2019, 47, 1–6. [Google Scholar]
- Khadka, K.K. Habitat Ecology, Trophic Interactions, and Distribution of Endangered Himalayan Musk Deer in the Nepal Himalaya. Graduate Thesis, University of Arkansas, Fayetteville, AR, USA, 2017. [Google Scholar]
- Liu, Y.; Niu, Y.; Zhou, S.; Zhang, Z.; Liang, Z.; Yang, J.; Ju, D. Winter moving, bedding habitat selection and evaluation of roe deer in southern Laoyeling, Heilongjiang Province. Acta Ecol. Sin. 2021, 41, 6913–6923. [Google Scholar]
- Shams-Esfandabad, B.; Ahmadi, A.; Yusefi, T. Seasonal changes in distribution of suitable habitats for Persian goitered gazelle (Gazella subgutturosa) in Isfahan province. J. Wildl. Biodivers. 2019, 3, 58–65. [Google Scholar]
- Wei, F.; Ping, X.; Hu, Y.; Nie, Y.; Zeng, Y.; Huang, G. Main Achievements, Challenges, and Recommendations of Biodiversity Conservation in China. Bull. Chin. Acad. Sci. 2021, 36, 375–383. [Google Scholar]
- Hellmann, J.J. Translocation as a conservation strategy. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 236–240. [Google Scholar]
- Lloyd, N.A.; Keating, L.M.; Friesen, A.J.; Cole, D.M.; McPherson, J.M.; Akçakaya, H.R.; Moehrenschlager, A. Prioritizing species conservation programs based on IUCN green status and estimates of cost-sharing potential. Conserv. Biol. 2023, 37, e14051. [Google Scholar] [CrossRef]
- Ellerman, J.R.; Morrison-Scott, T.C.S. Checklist of Palaearctic and Indian Mammals-New Name 1758–1946; The British Museum: London, UK, 1951. [Google Scholar]
- Corbet, G.B. The Mammals of the Palaearctic Regioni a Taxonomic Review; Cornell University Press: London, UK, 1978. [Google Scholar]
- Chen, P.; He, D. Research on key and difficult problems in the establishment of Qinghai Lake National Park. J. Natl. Acad. For. Grassl. Adm. 2023, 22, 29–39. [Google Scholar]


| The Number of Records of Przewalski’s Gazelle’s Presence | Source | Years |
|---|---|---|
| 13 | GBIF | 2016–2021 |
| 1 | [7] | 2018 |
| 180 | This field survey | 2019 |
| Types | Variables | Description | Units |
|---|---|---|---|
| Climatic factors | Bio1 | Annual Mean Temperature | °C |
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp-min temp)) | °C | |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | % | |
| Bio4 | Temperature Seasonality (standard deviation × 100) | % | |
| Bio5 | Max Temperature of Warmest Month | °C | |
| Bio6 | Min Temperature of Coldest Month | °C | |
| Bio7 | Temperature Annual Range (BIO5-BIO6) | °C | |
| Bio8 | Mean Temperature of Wettest Quarter | °C | |
| Bio9 | Mean Temperature of Driest Quarter | °C | |
| Bio10 | Mean Temperature of Warmest Quarter | °C | |
| Bio11 | Mean Temperature of Coldest Quarter | °C | |
| Bio12 | Annual Precipitation | mm | |
| Bio13 | Precipitation in Wettest Month | mm | |
| Bio14 | Precipitation in Driest Month | mm | |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | % | |
| Bio16 | Precipitation in Wettest Quarter | mm | |
| Bio17 | Precipitation in Driest Quarter | mm | |
| Bio18 | Precipitation in Warmest Quarter | mm | |
| Bio19 | Precipitation in Coldest Quarter | mm | |
| Vegetation factor | NDVI | Normalized Difference Vegetation Index | - |
| Veg | Vegetation Type | ||
| Geographical factors | Altitude | Altitude | m |
| Dis_river | Distance from river | m | |
| Slope | Slope Degree | ° | |
| Aspect | Slope Aspect | - | |
| Anthropogenic factor | Dis_road | Distance from Road | m |
| Footprint | Human Footprint Index | - | |
| Pop | Population density | people/km2 |
| bio19 | bio1 | bio2 | bio3 | bio4 | bio5 | bio6 | bio7 | bio8 | bio9 | bio10 | bio11 | bio12 | bio13 | bio14 | bio15 | bio16 | bio17 | bio18 | |
| bio19 | 0 | 0.59 | 0.71 | 0.12 | 0.45 | 0.29 | 0.69 | 0.57 | 0.26 | 0.66 | 0.40 | 0.66 | 0.84 | 0.71 | 0.97 | 0.60 | 0.73 | 0.98 | 0.63 |
| bio1 | 0 | 0.00 | 0.59 | 0.15 | 0.30 | 0.81 | 0.91 | 0.42 | 0.84 | 0.88 | 0.89 | 0.91 | 0.64 | 0.57 | 0.62 | 0.53 | 0.58 | 0.62 | 0.55 |
| bio2 | 0 | 0 | 0 | 0.29 | 0.47 | 0.23 | 0.74 | 0.67 | 0.33 | 0.57 | 0.40 | 0.67 | 0.85 | 0.80 | 0.76 | 0.46 | 0.81 | 0.75 | 0.78 |
| bio3 | 0 | 0 | 0 | 0 | 0.67 | 0.51 | 0.10 | 0.49 | 0.48 | 0.21 | 0.49 | 0.16 | 0.05 | 0.08 | 0.18 | 0.09 | 0.04 | 0.16 | 0.02 |
| bio4 | 0 | 0 | 0 | 0 | 0 | 0.30 | 0.66 | 0.97 | 0.22 | 0.64 | 0.17 | 0.66 | 0.55 | 0.46 | 0.43 | 0.30 | 0.51 | 0.44 | 0.50 |
| bio5 | 0 | 0 | 0 | 0 | 0 | 0 | 0.50 | 0.18 | 0.96 | 0.50 | 0.98 | 0.51 | 0.24 | 0.21 | 0.33 | 0.36 | 0.19 | 0.32 | 0.16 |
| bio6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.76 | 0.56 | 0.95 | 0.63 | 0.99 | 0.77 | 0.68 | 0.72 | 0.56 | 0.71 | 0.72 | 0.67 |
| bio7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.09 | 0.70 | 0.02 | 0.75 | 0.70 | 0.61 | 0.57 | 0.37 | 0.66 | 0.57 | 0.64 |
| bio8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.52 | 0.97 | 0.56 | 0.32 | 0.33 | 0.33 | 0.29 | 0.31 | 0.32 | 0.30 |
| bio9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.60 | 0.97 | 0.66 | 0.55 | 0.65 | 0.58 | 0.58 | 0.65 | 0.53 |
| bio10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.63 | 0.40 | 0.37 | 0.44 | 0.40 | 0.36 | 0.44 | 0.33 |
| bio11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.74 | 0.65 | 0.68 | 0.54 | 0.68 | 0.68 | 0.64 |
| bio12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.95 | 0.86 | 0.43 | 0.97 | 0.86 | 0.94 |
| bio13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.73 | 0.21 | 0.99 | 0.73 | 0.98 |
| bio14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.60 | 0.75 | 0.99 | 0.66 |
| bio15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.27 | 0.61 | 0.20 |
| bio16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.75 | 0.98 |
| bio17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.66 |
| bio18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
 corresponds to r ≤ 0.10,
corresponds to r ≤ 0.10,  corresponds to 0.10 < r ≤ 0.20,
corresponds to 0.10 < r ≤ 0.20,  corresponds to 0.20 < r ≤ 0.30,
corresponds to 0.20 < r ≤ 0.30,  corresponds to 0.30 < r ≤ 0.40,
corresponds to 0.30 < r ≤ 0.40,  corresponds to 0.40 < r ≤ 0.50,
corresponds to 0.40 < r ≤ 0.50,  corresponds to 0.50 < r ≤ 0.60,
corresponds to 0.50 < r ≤ 0.60,  corresponds to 0.60 < r ≤ 0.70,
corresponds to 0.60 < r ≤ 0.70,  corresponds to 0.70 < r ≤ 0.80,
corresponds to 0.70 < r ≤ 0.80,  corresponds to 0.80 < r ≤ 0.90, and
corresponds to 0.80 < r ≤ 0.90, and  corresponds to r > 0.90.
corresponds to r > 0.90.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Li, C. Habitat Suitability, Distribution Modelling and GAP Analysis of Przewalski’s Gazelle Conservation. Animals 2024, 14, 149. https://doi.org/10.3390/ani14010149
Liang D, Li C. Habitat Suitability, Distribution Modelling and GAP Analysis of Przewalski’s Gazelle Conservation. Animals. 2024; 14(1):149. https://doi.org/10.3390/ani14010149
Chicago/Turabian StyleLiang, Dongni, and Chunwang Li. 2024. "Habitat Suitability, Distribution Modelling and GAP Analysis of Przewalski’s Gazelle Conservation" Animals 14, no. 1: 149. https://doi.org/10.3390/ani14010149
APA StyleLiang, D., & Li, C. (2024). Habitat Suitability, Distribution Modelling and GAP Analysis of Przewalski’s Gazelle Conservation. Animals, 14(1), 149. https://doi.org/10.3390/ani14010149






