Transcriptome and Weighted Gene Co-Expression Network Analysis for Feather Follicle Density in a Chinese Indigenous Breed
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Feather Follicle Density Data Collection
2.3. RNA-seq Datasets
2.4. RNA-seq Data Analysis
2.5. Weighted Gene Co-Expression Network Analysis
2.6. Functional Enrichment Analysis of DEGs and Hub Genes
2.7. Real-Time Fluorescence Quantitative PCR
2.8. Hub Genes Expression Analysis
2.9. Statistical Analyses
3. Results
3.1. Feather Follicle Density
3.2. Sequencing Data Evaluation
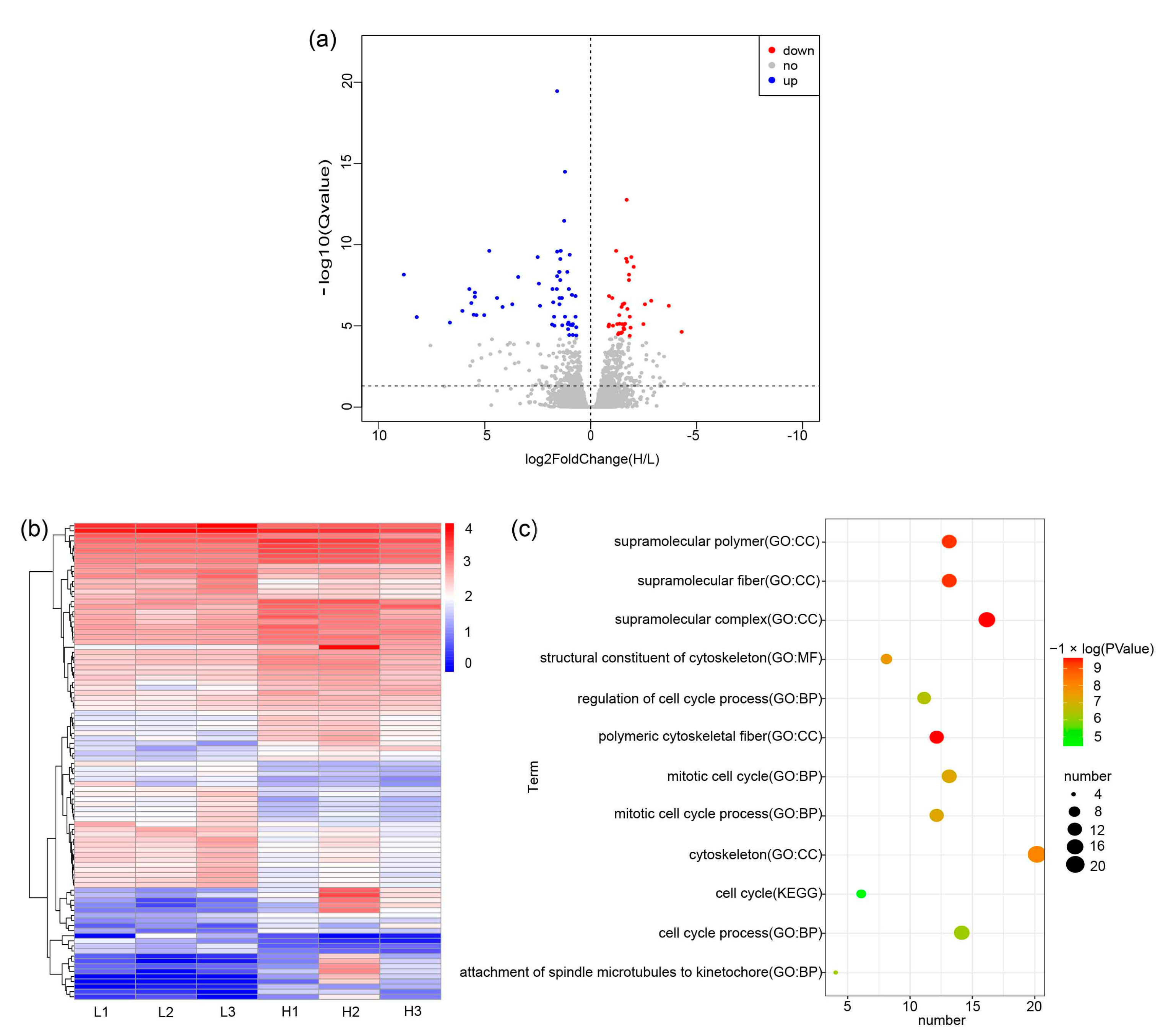
3.3. mRNA Expression Profiling of Feather Follicle Density
3.4. WGCNA for Feather Follicle Density
3.5. Hub Genes Related to Feather Follicle Density
3.6. Quantitative Real-Time PCR Validation
3.7. Hub Genes Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, C.G.; Gao, Y.; Dorshorst, B.; Song, C.; Gu, X.R.; Li, Q.Y.; Li, J.X.; Liu, T.X.; Rubin, C.J.; Zhao, Y.Q.; et al. A cis-regulatory mutation of PDSS2 causes silky-feather in chickens. PLoS Genet. 2014, 10, e1004576. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Holthaus, K.B.; Sukseree, S.; Hermann, M.; Tschachler, E.; Eckhart, L. Immunolocalization of a histidine-rich epidermal differentiation protein in the chicken supports the hypothesis of an evolutionary developmental link between the embryonic subperiderm and feather barbs and barbules. PLoS ONE 2016, 11, e0167789. [Google Scholar] [CrossRef]
- Lachner, J.; Ehrlich, F.; Mlitz, V.; Hermann, M.; Alibardi, L.; Tschachler, E.; Eckhart, L. Immunolocalization and phylogenetic profiling of the feather protein with the highest cysteine content. Protoplasma 2019, 256, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, F.; Lachner, J.; Hermann, M.; Tschachler, E.; Eckhart, L. Convergent evolution of cysteine-rich keratins in hard skin appendages of terrestrial vertebrates. Mol. Biol. Evol. 2020, 37, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Foley, J.; Tang, P.C.; Li, A.; Jiang, T.X.; Wu, P.; Widelitz, R.B.; Chuong, C.M. Development, regeneration, and evolution of feathers. Annu. Rev. Anim. Biosci. 2015, 3, 169–195. [Google Scholar] [CrossRef]
- Kondo, M.; Sekine, T.; Miyakoshi, T.; Kitajima, K.; Egawa, S.; Seki, R.; Abe, G.; Tamura, K. Flight feather development: Its early specialization during embryogenesis. Zool. Lett. 2018, 4, 2. [Google Scholar] [CrossRef]
- Ji, G.G.; Zhang, M.; Tu, Y.J.; Liu, Y.F.; Shan, Y.J.; Ju, X.J.; Zou, J.M.; Shu, J.T.; Sheng, Z.W.; Li, H. Molecular regulatory mechanisms in chicken feather follicle morphogenesis. Genes 2023, 14, 1646. [Google Scholar] [CrossRef]
- Clench, M.H. Variability in body pterylosis, with special reference to the genus passer. AUK 1970, 87, 650–691. [Google Scholar] [CrossRef]
- Ho, W.K.W.; Freem, L.; Zhao, D.B.; Painter, K.J.; Woolley, T.E.; Gaffney, E.A.; McGrew, M.J.; Tzika, A.; Milinkovitch, M.C.; Schneider, P.; et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 2019, 17, e3000132. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Jiang, Y.; Wang, Z.X.; Chen, G.H.; Bai, H.; Chang, G.B. Indigenous, yellow-feathered chickens body measurements, carcass traits, and meat quality depending on marketable age. Animals 2022, 12, 2422. [Google Scholar] [CrossRef]
- Ji, G.G.; Zhang, M.; Liu, Y.F.; Shan, Y.J.; Tu, Y.J.; Ju, X.J.; Zou, J.M.; Shu, J.T.; Wu, J.F.; Xie, J.F. A gene co-expression network analysis of the candidate genes and molecular pathways associated with feather follicle traits of chicken skin. J. Anim. Breed Genet. 2021, 138, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D.; Chunilall, V. Valorisation of chicken feathers: Characterisation of chemical properties. Waste Manag. 2017, 68, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Da Costa, M.J.; Oviedo-Rondón, E.O. Incubation temperature profiles affect broiler feathering. J. Appl. Poult. Res. 2015, 24, 49–57. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhang, K.Y.; Bai, S.P.; Ding, X.M.; Wang, J.P.; Peng, H.W.; Xuan, Y.; Su, Z.W.; Gang, T.; Zeng, Q.F. Dietary resistant potato starch improves growth performance and feather development in Pekin ducks fed a low phosphorus diet. Poult. Sci. 2021, 100, 100947. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Bai, H.C.; Li, L.; Zhang, W.; Jiang, R.S.; Geng, Z.Y. Follicle characteristics and follicle developmental related Wnt6 polymorphism in Chinese indigenous Wanxi-white goose. Mol. Biol. Rep. 2012, 39, 9843–9848. [Google Scholar] [CrossRef]
- Sun, H.Y.; Hu, Y.P.; Dou, T.C.; Qu, L.; Ma, M.; Lu, J.; Wang, X.G.; Shen, M.M.; Wang, K.H. Genetic architecture related to contour feathers density in an F (2) resource population via a genome-wide association study. 3 Biotech. 2019, 9, 400. [Google Scholar] [CrossRef]
- Li, L.; Abouelezz, K.F.M.; Gou, Z.; Lin, X.; Wang, Y.; Fan, Q.; Cheng, Z.; Ding, F.; Jiang, S.; Jiang, Z. Optimization of dietary zinc requirement for broiler breeder hens of chinese yellow-feathered chicken. Animals 2019, 9, 472. [Google Scholar] [CrossRef]
- Jin, S.H.; Yang, L.; Zang, H.; Xu, Y.; Chen, X.Z.; Chen, X.Y.; Liu, P.; Geng, Z.Y. Influence of free-range days on growth performance, carcass traits, meat quality, lymphoid organ indices, and blood biochemistry of Wannan Yellow chickens. Poult. Sci. 2019, 98, 6602–6610. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.X.; Chen, H.; Su, H.; Wang, Z.C.; Wan, Y.; Huang, Y.Y.; Jiang, R.S. Effects of exercise on carcass composition, meat quality, and mRNA expression profiles in breast muscle of a Chinese indigenous chicken breed. Poult. Sci. 2019, 98, 5241–5246. [Google Scholar] [CrossRef]
- Chi, Y.D.; Xu, Y.O.; Luo, F.; Lin, Y.Q.; Li, Z.X. Molecular cloning, expression profiles and associations of KLF6 gene with intramuscular fat in Tibetan chicken. Anim. Biotechnol. 2020, 31, 67–75. [Google Scholar] [CrossRef]
- Luo, N.; Shu, J.T.; Yuan, X.Y.; Jin, Y.X.; Cui, H.X.; Zhao, G.P.; Wen, J. Differential regulation of intramuscular fat and abdominal fat deposition in chickens. BMC Genom. 2022, 23, 308. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.X.; Yuan, P.T.; Li, S.H.; Li, H.T.; Zhai, B.; Li, Y.F.; Zhang, H.Y.; Gu, J.X.; Li, H.; Tian, Y.D.; et al. Genetic architecture and key regulatory genes of fatty acid composition in Gushi chicken breast muscle determined by GWAS and WGCNA. BMC Genom. 2023, 24, 434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; He, X.X.; Zhao, Y.C.; Wang, Y.; Wang, J.X.; Guo, X.; Jiang, R.S. Exercise profile and effect on growth traits, carcass yield, meat quality, and tibial strength in Chinese Wannan chickens. Poult. Sci. 2021, 100, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y. Btrim: A fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 2011, 98, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. Genome project data processing s: The sequence alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g: Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016, 44, W8. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Yue, Z.C.; Wu, P.; Wu, D.Y.; Mayer, J.A.; Medina, M.; Widelitz, R.B.; Jiang, T.X.; Chuong, C.M. The biology of feather follicles. Int. J. Dev. Biol. 2004, 48, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.W.; Huang, N.A.; Liu, I.H.; Wang, Y.H.; Wu, P.; Tseng, Y.T.; Hughes, M.W.; Jiang, T.X.; Tsai, M.H.; Chen, C.Y.; et al. Emergence of differentially regulated pathways associated with the development of regional specificity in chicken skin. BMC Genom. 2015, 16, 22. [Google Scholar] [CrossRef]
- Gong, H.; Wang, H.; Wang, Y.X.; Bai, X.; Liu, B.; He, J.F.; Wu, J.H.; Qi, W.M.; Zhang, W.G. Skin transcriptome reveals the dynamic changes in the Wnt pathway during integument morphogenesis of chick embryos. PLoS ONE. 2018, 13, e0190933. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, H.; Wang, H.; He, X.X.; Wang, J.X.; Wei, W.; Liu, M.; Xu, J.M.; Liu, Y.N.; Jiang, R.S. Identification of key modules and hub genes involved in regulating the color of chicken breast meat using WGCNA. Animals 2023, 13, 2356. [Google Scholar] [CrossRef]
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt pathway: From signaling mechanisms to synthetic modulators. Annu. Rev. Biochem. 2022, 91, 571–598. [Google Scholar] [CrossRef]
- Xie, W.Y.; Chen, M.J.; Jiang, S.G.; Yan, H.C.; Wang, X.Q.; Gao, C.Q. Investigation of feather follicle morphogenesis and the expression of the Wnt/β-catenin signaling pathway in yellow-feathered broiler chick embryos. Br. Poult. Sci. 2020, 61, 557–565. [Google Scholar] [CrossRef]
- Xie, W.Y.; Chen, M.J.; Jiang, S.G.; Yan, H.C.; Wang, X.Q.; Gao, C.Q. The Wnt/β-catenin signaling pathway is involved in regulating feather growth of embryonic chicks. Poult. Sci. 2020, 99, 2315–2323. [Google Scholar] [CrossRef]
- Wang, C.L.; Wen, M.Y.; Xu, J.L.; Gao, P.N.; Liu, S.L.; Liu, J.Y.; Chen, Y.; Zhou, L. GTSE1 promotes the growth of NSCLC by regulating microtubule-associated proteins through the ERK/MAPK pathway. Thorac. Cancer 2023, 14, 1624–1634. [Google Scholar] [CrossRef]
- Guo, W.T.; Zhu, J.; Zhu, Y.; Wang, K. G2 and S phase-expressed-1 acts as a putative tumor promoter in cervical cancer by enhancing Wnt/β-catenin signaling via modulation of GSK-3β. Environ. Toxicol. 2021, 36, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.M.; Graves, L.M. Enigmatic MELK: The controversy surrounding its complex role in cancer. J. Biol. Chem. 2020, 295, 8195–8203. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.H.Y.; Bin, X.; Zhou, J.; Tang, Z.G. Ectopic expression of MELK in oral squamous cell carcinoma and its correlation with epithelial mesenchymal transition. Aging 2021, 13, 13048–13060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Bode, A.M.; Zhang, T.S. Targeting CDK1 in cancer: Mechanisms and implications. NPJ Precis. Oncol. 2023, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.X.; Li, B.K.; Zhao, Z.W. GRIK3 deficiency promotes non-small cell lung cancer progression by the regulation of the UBE2C/CDK1/Wnt signaling pathway. Am. J. Cancer Res. 2023, 13, 2066–2075. [Google Scholar] [PubMed]
- Li, L.; Liu, P.W.; He, C.Y.; Xu, C.F. miRNA-223-3p regulates ECT2 to promote proliferation, invasion, and metastasis of gastric cancer through the Wnt/β-catenin signaling pathway. J. Cancer Res. Clin. Oncol. 2023, 149, 121–134. [Google Scholar] [CrossRef]
- Xu, Q.; Xi, Y.; Ma, S.; Wang, J.; Li, J.; Han, C.; Li, L.; Wang, J.; Liu, H. Transcriptome profiling of morphogenetic differences between contour and flight feathers in duck. Br. Poult. Sci. 2022, 63, 597–604. [Google Scholar] [CrossRef]
- Feng, X.L.; Guo, J.J.; An, G.; Wu, Y.B.W.; Liu, Z.H.; Meng, B.; He, N.H.; Zhao, X.Y.; Chen, S.L.; Zhu, Y.H.; et al. Genetic aberrations and interaction of NEK2 and tp53 accelerate aggressiveness of multiple myeloma. Adv. Sci. 2022, 9, e2104491. [Google Scholar] [CrossRef]
- Xu, T.; Zeng, Y.L.; Shi, L.L.; Yang, Q.; Chen, Y.S.; Wu, G.; Li, G.L.; Xu, S.B. Targeting NEK2 impairs oncogenesis and radioresistance via inhibiting the Wnt1/β-catenin signaling pathway in cervical cancer. J. Exp. Clin. Cancer Res. 2020, 39, 183. [Google Scholar] [CrossRef]
- Guo, D.; Yao, W.N.; Du, X.Y.; Dong, J.; Zhang, X.Y.; Shen, W.B.; Zhu, S.C. NEK2 promotes esophageal squamous cell carcinoma cell proliferation, migration and invasion through the Wnt/β-catenin signaling pathway. Discov. Oncol. 2023, 14, 80. [Google Scholar] [CrossRef]
- Tyson, J.J.; Novak, B. Regulation of the eukaryotic cell cycle: Molecular antagonism, hysteresis, and irreversible transitions. J. Theor. Biol. 2001, 210, 249–263. [Google Scholar] [CrossRef]
- Tyson, J.J.; Novak, B. Temporal organization of the cell cycle. Curr. Biol. 2008, 18, R759–R768. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Wang, J. Landscape and flux reveal a new global view and physical quantification of mammalian cell cycle. Proc. Natl. Acad. Sci. USA 2014, 111, 14130–14135. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.L.; Hu, H. The influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef] [PubMed]
- Purba, T.S.; Brunken, L.; Hawkshaw, N.J.; Peake, M.; Hardman, J.; Paus, R. A primer for studying cell cycle dynamics of the human hair follicle. Exp. Dermatol. 2016, 25, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Dawn, B.; Xuan, Y.T. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc. Med. 2003, 13, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Harel, S.; Higgins, C.A.; Cerise, J.E.; Dai, Z.P.; Chen, J.C.; Clynes, R.; Christiano, A.M. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 2015, 1, e1500973. [Google Scholar] [CrossRef]
- Kim, I.M.; Zhou, Y.; Ramakrishna, S.; Hughes, D.E.; Solway, J.; Costa, R.H.; Kalinichenko, V.V. Functional characterization of evolutionarily conserved DNA regions in forkhead box f1 gene locus. J. Biol. Chem. 2005, 280, 37908–37916. [Google Scholar] [CrossRef]
- Zhu, G.D.; Yang, Y.P.; Jiang, Z.J. Knockdown CRNDE alleviates LPS-induced inflammation injury via FOXM1 in WI-38 cells. Biomed. Pharmacother. 2018, 103, 1678–1687. [Google Scholar]
- Zeng, R.M.; Lu, X.H.; Lin, J.; Ron, Z.J.; Fang, J.H.; Liu, Z.W.; Zeng, W.T. FOXM1 activates JAK1/STAT3 pathway in human osteoarthritis cartilage cell inflammatory reaction. Exp. Biol. Med. 2021, 246, 644–653. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, F.R.; Liu, X.; Hengst, J.; Fox, T.; Calvert, V.; Petricoin, E.F., III; Yun, J.; Feith, D.J.; Loughran, T.P., Jr. Sphingosine kinase inhibitors decrease viability and induce cell death in natural killer-large granular lymphocyte leukemia. Cancer Biol. Ther. 2015, 16, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Sun, Y.B.; Ye, F.; Li, W.; Kharbuja, P.; Gao, L.; Zhang, D.Y.; Suo, J. Anti-tumor activity of the X-linked inhibitor of apoptosis (XIAP) inhibitor embelin in gastric cancer cells. Mol. Cell. Biochem. 2014, 386, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.H.; Ma, X.B.; Tang, X.Q.; Xing, J.; Sheng, X.Z.; Chi, H.; Zhan, W.B. Genome characterization of Hirame novirhabdovirus (HIRRV) isolate CNPo2015 and transcriptome analysis of Hirame natural embryo (HINAE) cells infected with CNPo2015. Fish Shellfish. Immunol. 2023, 132, 108493. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Widelitz, R.B.; Jiang, T.; Noveen, A.; Chen, C.J.; Chuong, C. FGF induces new feather buds from developing avian skin. J. Investig. Dermatol. 1996, 107, 797–803. [Google Scholar] [CrossRef]
- Yue, Z.C.; Jiang, T.X.; Wu, P.; Widelitz, R.B.; Chuong, C.M. Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Dev. Biol. 2012, 372, 45–54. [Google Scholar] [CrossRef]
- Padrissa-Altés, S.; Bachofner, M.; Bogorad, R.L.; Pohlmeier, L.; Rossolini, T.; Böhm, F.; Liebisch, G.; Hellerbrand, C.; Koteliansky, V.; Speicher, T.; et al. Control of hepatocyte proliferation and survival by Fgf receptors is essential for liver regeneration in mice. Gut 2015, 64, 1444–1453. [Google Scholar] [CrossRef]
- Yang, Y.N.; Zhu, X.L.; Jia, X.; Hou, W.W.; Zhou, G.Q.; Ma, Z.J.; Yu, B.; Pi, Y.; Zhang, X.M.; Wang, J.Q.; et al. Phosphorylation of Msx1 promotes cell proliferation through the Fgf9/18-MAPK signaling pathway during embryonic limb development. Nucleic Acids Res. 2020, 48, 11452–11467. [Google Scholar] [CrossRef]



| Group (n = 30) | Mean |
|---|---|
| H (piece/cm2) | 4.95 ± 0.19 A |
| L (piece/cm2) | 3.27 ± 0.04 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wei, W.; Xing, C.; Wang, H.; Liu, M.; Xu, J.; He, X.; Liu, Y.; Guo, X.; Jiang, R. Transcriptome and Weighted Gene Co-Expression Network Analysis for Feather Follicle Density in a Chinese Indigenous Breed. Animals 2024, 14, 173. https://doi.org/10.3390/ani14010173
Wang J, Wei W, Xing C, Wang H, Liu M, Xu J, He X, Liu Y, Guo X, Jiang R. Transcriptome and Weighted Gene Co-Expression Network Analysis for Feather Follicle Density in a Chinese Indigenous Breed. Animals. 2024; 14(1):173. https://doi.org/10.3390/ani14010173
Chicago/Turabian StyleWang, Jiangxian, Wei Wei, Chaohui Xing, Hao Wang, Meng Liu, Jinmei Xu, Xinxin He, Yanan Liu, Xing Guo, and Runshen Jiang. 2024. "Transcriptome and Weighted Gene Co-Expression Network Analysis for Feather Follicle Density in a Chinese Indigenous Breed" Animals 14, no. 1: 173. https://doi.org/10.3390/ani14010173
APA StyleWang, J., Wei, W., Xing, C., Wang, H., Liu, M., Xu, J., He, X., Liu, Y., Guo, X., & Jiang, R. (2024). Transcriptome and Weighted Gene Co-Expression Network Analysis for Feather Follicle Density in a Chinese Indigenous Breed. Animals, 14(1), 173. https://doi.org/10.3390/ani14010173




