Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 31 Warmblood Showjumpers in Full Work and Competing Regularly
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
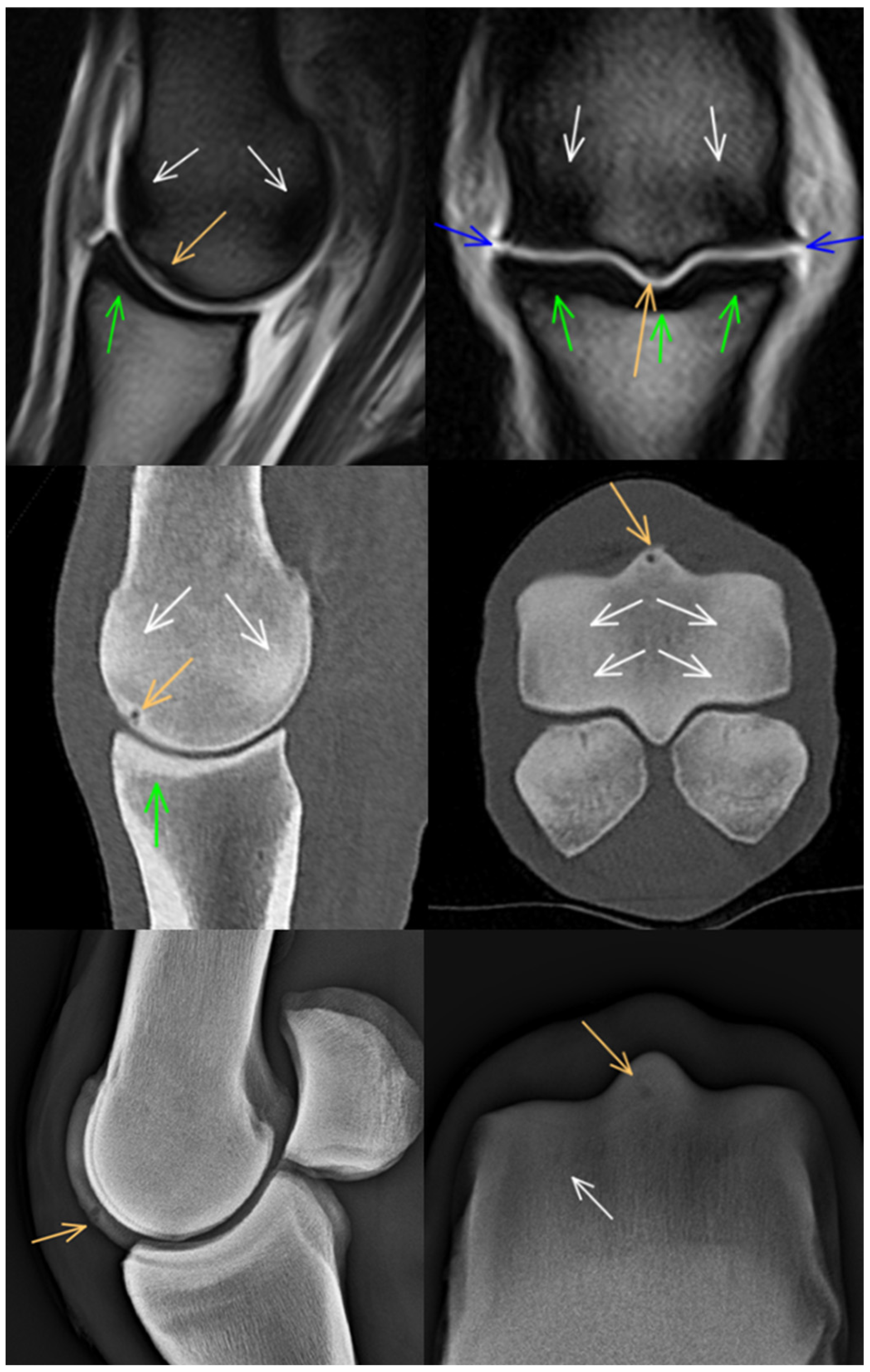
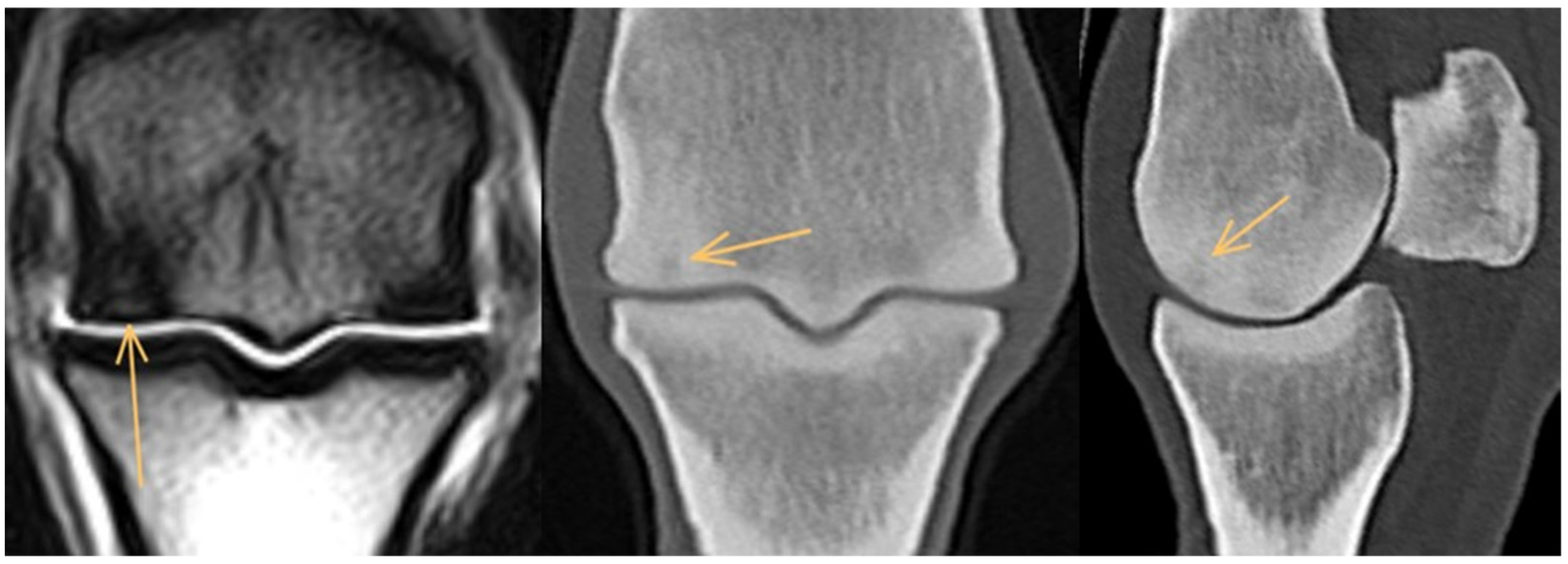
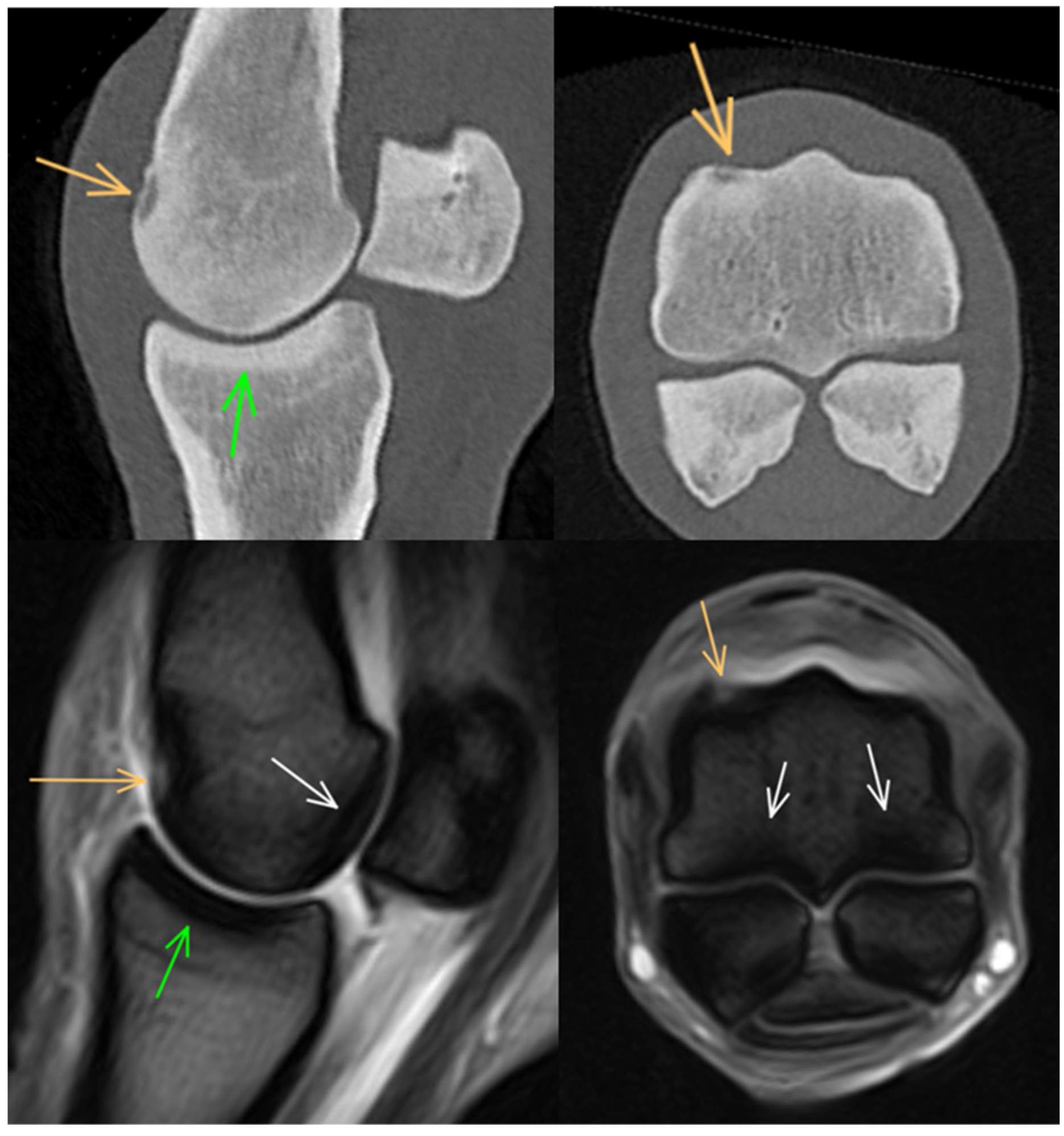
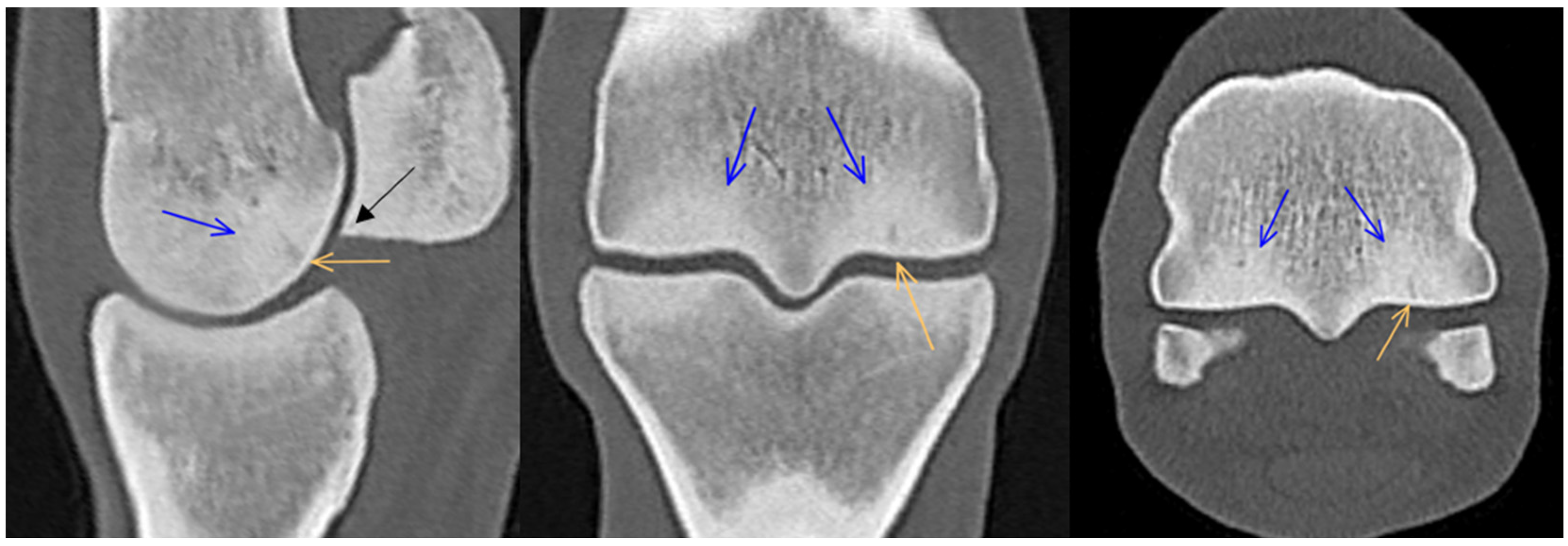
3. Results
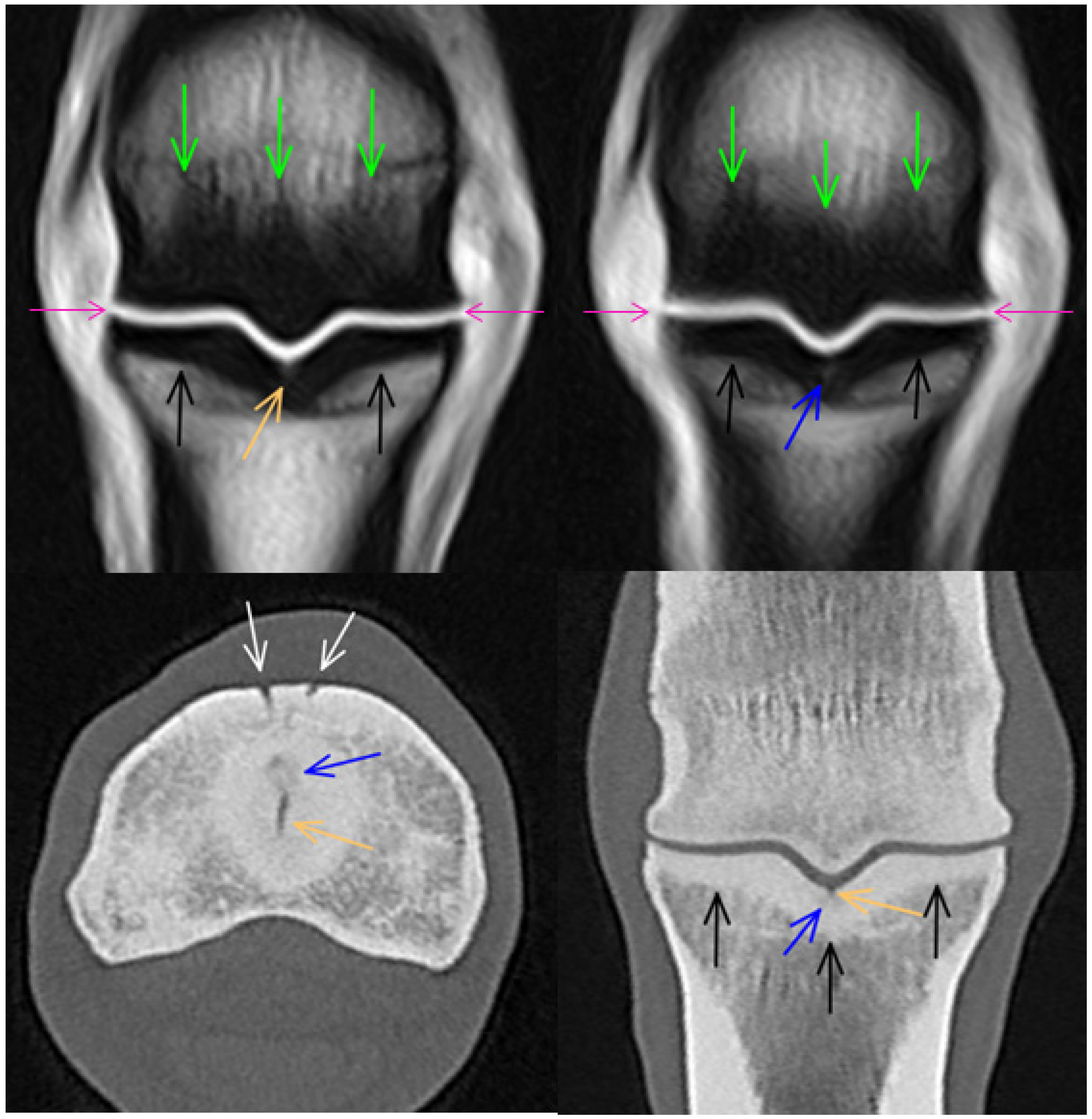
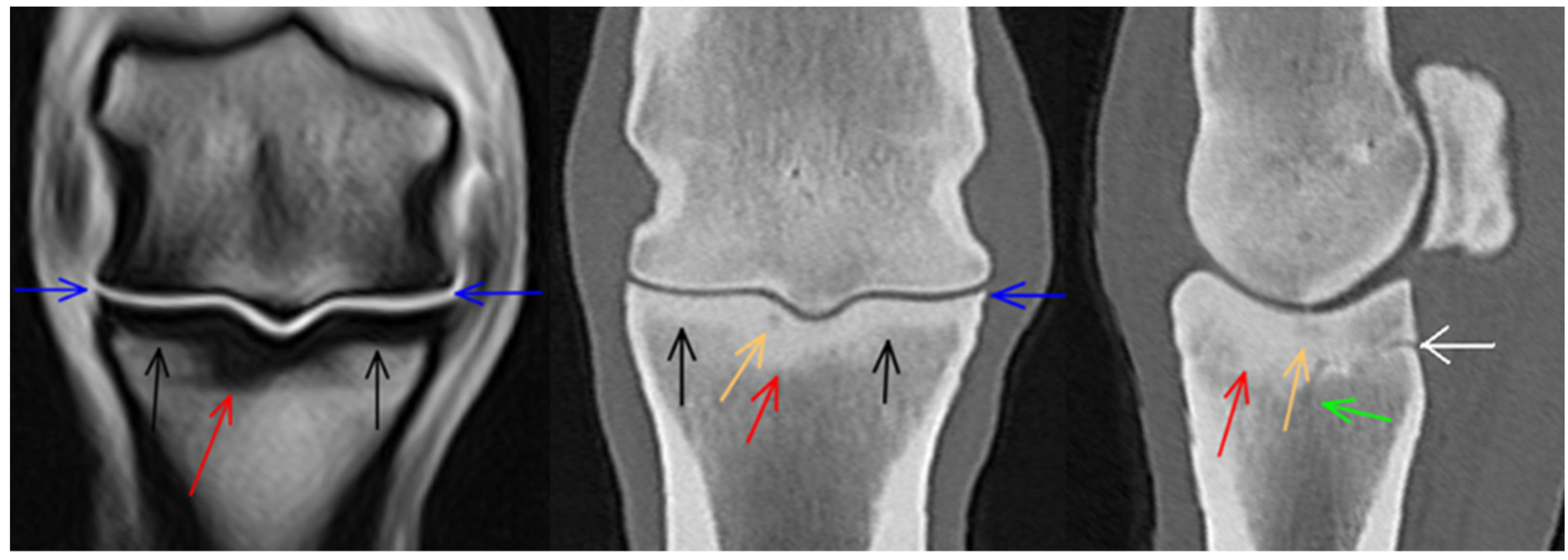
3.1. Third Metacarpal Bone
3.2. Proximal Phalanx
3.3. Proximal Sesamoid Bones
3.4. Soft Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyson, S.J.; Murray, R. Osseous trauma in the fetlock region of mature sports horses. Proc. Am. Assoc. Equine Pract. 2006, 52, 443–456. [Google Scholar]
- Gonzalez, L.M.; Schramme, M.C.; Robertson, I.D.; Thrall, D.E.; Redding, R.W. MRI features of metacarpo(tarso)phalangeal region lameness in 40 horses. Vet. Radiol. Ultrasound 2010, 51, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Nagy, A.; Murray, R. Clinical and diagnostic imaging findings in horses with subchondral bone trauma of the sagittal groove of the proximal phalanx. Vet. Radiol. Ultrasound 2011, 52, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.E. Low-field standing magnetic resonance imaging findings of the metacarpo/metatarsophalangeal joint of racing Thoroughbreds with lameness localised to the region: A retrospective study of 131 horses. Equine Vet. J. 2012, 44, 169–177. [Google Scholar] [CrossRef]
- King, J.N.; Zubrod, C.J.; Schneider, R.K.; Sampson, S.N.; Roberts, G. MRI findings in 232 horses with lameness localized to the metacarpo(tarso)phalangeal region and without a radiographic diagnosis. MRI findings in 232 horses with lameness localised to the metacarpo(tarso)phalangeal region and without a radiographic diagnosis. Vet. Radiol. Ultrasound 2013, 54, 36–47. [Google Scholar]
- Gold, S.J.; Werpy, N.M.; Gutierrez-Nibeyro, S.D. Injuries of the sagittal groove of the proximal phalanx in Warmblood horses detected with low-field magnetic resonance imaging: 19 cases (2007–2016). Vet. Radiol. Ultrasound 2017, 58, 344–353. [Google Scholar] [CrossRef]
- Olive, J.; Serraud, N.; Vila, T.; Germain, J.-P. Metacarpophalangeal joint injury patterns on magnetic resonance imaging: A comparison in racing Standardbreds and Thoroughbreds. Vet. Radiol. Ultrasound 2017, 58, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Lipreri, G.; Bladon, B.M.; Giorio, M.E.; Singer, E.R. Conservative versus surgical treatment of 21 sports horses with osseous trauma in the proximal phalangeal sagittal groove diagnosed by low-field MRI. Vet. Surg. 2018, 47, 908–915. [Google Scholar] [CrossRef]
- Denoix, J.-M.; Coudry, V. Clinical insights: Imaging of the equine fetlock in Thoroughbred racehorses: Identification of imaging changes to predict catastrophic injury. Equine Vet. J. 2020, 52, 342–343. [Google Scholar] [CrossRef]
- Ammann, L.; Ohlerth, S.; Fürst, A.; Jackson, M.A. Differences of morphological attributes between 62 proximal and distal subchondral cystic lesions of the proximal phalanx as determined by radiography and computed tomography. Am. J. Vet. Res. 2022, 83, ajvr.22.04.0071. [Google Scholar] [CrossRef]
- Mageed, M. Standing computed tomography of the equine limb using a multi-slice helical scanner: Technique and feasibility study. Equine Vet. Educ. 2022, 34, 77–83. [Google Scholar] [CrossRef]
- Brounts, S.H.; Lund, J.R.; Whitton, R.C.; Ergun, D.L.; Muir, P. Use of a novel helical fan beam imaging system for computed tomography of the distal limb in sedated standing horses: 167 cases (2019–2020). J. Am. Vet. Med. Assoc. 2022, 22, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Mathee, N.; Robert, M.; Higgerty, S.H.; Fosgate, G.T.; Rogers, A.L.; d’Ablon, X.; Carstens, A. Computed Tomographic Evaluation of the Distal Limb in the Standing Sedated Horse: Technique, Imaging Diagnoses, Feasibility, and Artifacts. Vet. Radiol. Ultrasound 2022, 64, 243–252. [Google Scholar] [CrossRef]
- Faulkner, J.E.; Joostens, Z.; Broeckx, B.J.G.; Hauspie, S.; Mariën, T.; Vanderperren, K. Follow-up magnetic resonance imaging of sagittal groove disease of the equine proximal phalanx using a classification system in 29 non-racing sports horses. Animals 2024, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Auth, A.; Hinnigan, G.; Smith, M.A.; Owen, K.R. Low-Field magnetic resonance imaging findings of the fetlock region of nonracehorses. J. Equine Vet. Sci. 2024, 132, 104938. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Bolas, N.M.; Sargan, D.R.; Peter, V.G.; Murray, R.C. Comparison of standing cone-beam computed tomography and low-field magnetic resonance imaging findings in the equine metacarpo- or metatarsophalangeal region of standing sedated horses. Equine Vet. Educ. 2024. [Google Scholar] [CrossRef]
- Faulkner, J.E.; Joostens, Z.; Broeckx, B.J.G.; Hauspie, S.; Mariën, T.; Vanderperren, K. Low-field magnetic resonance imaging of sagittal groove disease of the proximal phalanx in non-racing sport horses. Equine Vet. J. 2024. [Google Scholar] [CrossRef]
- Murray, R.C.; Schramme, M.C.; Dyson, S.J.; Branch, M.V.; Blunden, T.S. Magnetic resonance imaging characteristics of the foot in horses with palmar foot pain and control horses. Vet. Radiol. Ultrasound 2006, 47, 1–16. [Google Scholar] [CrossRef]
- Nagy, A.; Dyson, S. Magnetic resonance imaging and histological findings in the proximal aspect of the suspensory ligament of forelimbs in nonlame horses. Equine Vet. J. 2012, 44, 43–50. [Google Scholar] [CrossRef]
- Nagy, A.; Dyson, S. Anatomical, magnetic resonance imaging and histological findings in the accessory ligament of the deep digital flexor tendon of forelimbs in nonlame horses. Equine Vet. J. 2011, 43, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Dyson, S. Magnetic resonance findings in the carpus and proximal metacarpal region of non-lame horses. Proc. Am. Assoc. Equine Prac. 2009, 55, 408–417. [Google Scholar]
- Likon, I.; Dyson, S.; Nagy, A. Magnetic Resonance Imaging Measurements of the Proximal Palmar Cortex of the Third Metacarpal Bone and the Suspensory Ligament in Non-Lame Endurance Horses before and after Six Months of Training. Animals 2023, 13, 1106. [Google Scholar] [CrossRef]
- Dyson, S.; Blunden, A.; Murray, R. Magnetic resonance imaging and gross post mortem and histological findings of the soft tissues of the plantar aspect of the tarsus and proximal metatarsal region in non-lame horses. Vet. Radiol. Ultrasound 2017, 58, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Biggi, M.; Dyson, S.J. Use of high-field and low-field magnetic resonance imaging to describe the anatomy of the proximal portion of the tarsal region of nonlame horses. Am. J. Vet. Res. 2018, 79, 299–310. [Google Scholar] [CrossRef]
- De Guio, C.; Ségard-Weisse, E.; Thomas-Cancian, A.; Schramme, M. Bone marrow lesions of the distal condyles of the third metacarpal bone are common and not always related to lameness in sports and pleasure horses. Vet. Radiol. Ultrasound 2019, 60, 167–175. [Google Scholar] [CrossRef] [PubMed]
- van Veggel, E.; Selberg, K.; van der Velde-Hoogelander, B.; Bolas, N.; Vanderperren, K.; Bergman, H.J. Magnetic Resonance Imaging Findings of the Proximal Metacarpal Region in Warmblood Horses: 36 Lame and 26 Control Limbs (2015–2021). Front. Vet. Sci. 2021, 8, 714423. [Google Scholar] [CrossRef]
- Olive, J.; D’anjou, M.A.; Alexander, K.; Laverty, S.; Theoret, C. Comparison of magnetic resonance imaging, computed tomography, and radiography for assessment of noncartilaginous changes in equine metacarpophalangeal osteoarthritis. Vet. Radiol. Ultrasound 2010, 51, 267–279. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Baker, T.A.; Brounts, S.H.; Sample, S.J.; Markel, M.D.; Scollay, M.C.; Marquis, P.; Muir, P. Detection of articular pathology of the distal aspect of the third metacarpal bone in thoroughbred racehorses: Comparison of radiography, computed tomography and magnetic resonance imaging. Vet. Surg. 2011, 40, 942–951. [Google Scholar] [CrossRef]
- Hontoir, F.; Nisolle, J.F.; Meurisse, H.; Simon, V.; Tallier, M.; Vanderstricht, R.; Antoine, N.; Piret, J.; Clegg, P.; Vandeweerd, J.M. A comparison of 3-T magnetic resonance imaging and computed tomography arthrography to identify structural cartilage defects of the fetlock joint in the horse. Vet. J. 2014, 199, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.C.A.; Ahern, B.J.; Palmieri, C.; Young, A.C. Imaging and Gross Pathological Appearance of Changes in the Parasagittal Grooves of Thoroughbred Racehorses. Animals. 2021, 11, 3366. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Peter, V.G.; Schiavo, S.; Pokora, R.; Patrick, H.; Bolas, N.; Foote, A.K.; Sargan, D.R.; Murray, R.C. Identification of heterotropic mineralisation and adjacent pathology in the equine fetlock region by low-field magnetic resonance imaging, cone-beam and fan-beam computed tomography. J. Equine Vet. Sci. 2023, 126, 104252. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Bolas, N.M.; Peter, V.G.; Pokora, R.; Patrick, H.; Foote, A.K.; Sargan, D.R.; Murray, R.C. Comparison of cone-beam and fan-beam computed tomography and low-field magnetic resonance imaging for detection of palmar/plantar osteochondral disease in Thoroughbred horses. Equine Vet. J. 2024, 56, 484–493. [Google Scholar] [CrossRef]
- Lin, S.T.; Foote, A.K.; Bolas, N.M.; Peter, V.G.; Pokora, R.; Patrick, H.; Sargan, D.R.; Murray, R.C. Three-dimensional imaging and histopathological features of third metacarpal/tarsal parasagittal groove and proximal phalanx sagittal groove fissures in Thoroughbred horses. Animals 2023, 13, 2912. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Boros, K.; Dyson, S. Magnetic resonance imaging, computed tomographic and radiographic findings in the metacarpophalangeal joints of 40 non-lame Thoroughbred Yearlings. Animals 2023, 13, 3466. [Google Scholar] [CrossRef]
- Dyson, S. Can lameness be graded reliably? Equine Vet. J. 2011, 43, 379–382. [Google Scholar] [CrossRef]
- Contino, E.; Daglish, J.; Kawcak, C. The prevalence of lameness in FEI athletes and its correlation to performance. Proc. Am. Assoc. Equine Pract. 2023, 69, 369–370. [Google Scholar]
- Scheidegger, M.D.; Gerber, V.; Dolf, G.; Burger, D.; Flammer, A.; Ramseyer, A. Quantitative Gait Analysis before and after a Cross-country Test in a Population of Elite Eventing Horses. J. Equine Vet. Sci. 2022, 117, 104077. [Google Scholar] [CrossRef]
- Keegan, K. Use of Equinosis Q with Lameness Locator to evaluate lameness in horses. Proc. Am. Assoc. Equine Pract. 2022, 69, 335–344. [Google Scholar]
- Leclerq, A.; Persson-Sjödin, E.; Ask, K.; Zetterburg, E.; Hernlund, E.; Andersen, P.H.; Rhodin, M. Perceived and correlation to vertical motion asymmetry in young warmblood horses. PLoS ONE 2023, 18, e0288043. [Google Scholar]
- Greve, L.; Dyson, S. What can we learn from visual and objective assessment of non-lame and lame horses in straight lines, on the lunge and ridden? Equine Vet. Educ. 2020, 32, 479–491. [Google Scholar] [CrossRef]
- Firth, E.C. The Response of Bone, Articular Cartilage and Tendon to Exercise in the Horse. J. Anat. 2006, 208, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Kawcak, C.E.; McIlwraith, C.W.; Firth, E.C. Effects of early exercise on metacarpophalangeal joints in horses. Am. J. Vet. Res. 2010, 71, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Dykgraaf, S.; Firth, E.C.; Rogers, C.W.; Kawcak, C.E. Effects of exercise on chondrocyte viability and subchondral bone sclerosis in the distal third metacarpal and metatarsal bone of young horses. Vet. J. 2008, 178, 53–61. [Google Scholar] [CrossRef]
- Brama, P.A.J.; Tekoppele, J.M.; Bank, R.A.; Karssenberg, D.; Barneveld, A.; van Weeren, P.R. Topographical mapping of biomechanical properties of articular cartilage in the equine fetlock joint. Equine Vet. J. 2000, 23, 19–26. [Google Scholar] [CrossRef]
- Brama, P.A.J.; Karssenberg, D.; Barneveld, A.; van Weeren, P.R. Contact areas and pressure distribution on the proximal articular surface of the proximal phalanx under sagittal plane loading. Equine Vet. J. 2001, 33, 26–32. [Google Scholar] [CrossRef]
- Singer, E.; Garcia, T.; Stover, S. How does bone strain vary between the third metacarpal and the proximal phalangeal bones of the equine distal limb? J. Biomech. 2021, 123, 110455. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Kawcak, C.E. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front. Vet. Sci. 2018, 5, 178. [Google Scholar] [CrossRef]
- Brommer, H.; van Weeren, P.R.; Brama, P.A.J.; Barneveld, A. Quantification and age-related distribution of articular cartilage degeneration in the equine fetlock joint. Equine Vet. J. 2003, 35, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.M.; Minshall, G.J. Identification and treatment of osteochondritis dissecans of the distal sagittal ridge of the third metacarpal bone. Equine Vet. J. 2014, 46, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Riggs, C.M.; Boyde, A. Effect of exercise on bone density in distal regions of the equine third metacarpal bone in 2-year-old thoroughbreds. Equine Vet. J. 1999, 31 (Suppl. S30), 555–560. [Google Scholar] [CrossRef] [PubMed]
- Firth, E.C.; Rogers, C.W.; Jopson, N. Effects of racetrack exercise on third metacarpal and carpal bone of New Zealand thoroughbred horses. J. Muscoskelet. Neuron. Interact. 2000, 1, 145–147. [Google Scholar]
- Firth, E.C.; Rogers, C.W.; van Weeren, P.R.; Barneveld, A.; McIlwraith, C.W.; Kawcak, C.E.; Goodship, A.E.; Smith, R.K. Mild exercise early in life produces changes in bone size and strength but not density in proximal phalangeal, third metacarpal and third carpal bones of foals. Vet. J. 2011, 190, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ciamillo, S.A.; Wulster, K.B.; Gassert, T.M.; Richardson, D.W.; Brown, K.A.; Stefanovski, D.; Ortved, K.F. Prospective, longitudinal assessment of subchondral bone morphology and pathology using standing, cone-beam computed tomography in fetlock joints of 2-year-old Thoroughbred racehorses in their first year of training. Equine Vet. J. 2024, 1–14, published online. [Google Scholar] [CrossRef]
- van Weeren, P.R.; Barneveld, A. The effect of exercise on the distribution and manifestation of osteochondrotic lesions in the Warmblood foal. Equine Vet. J. 1999, 31, 16–25. [Google Scholar] [CrossRef]
- Barneveld, A.; van Weeren, P.R. Conclusions regarding the influence of exercise on the development of the equine musculoskeletal system with special reference to osteochondrosis. Equine Vet. J. 1999, 31 (Suppl. S31), 112–119. [Google Scholar] [CrossRef]
- Bramlage, L. Operative orthopedics of the fetlock joint of the horse: Traumatic and developmental diseases of the equine fetlock joint. Proc. Am. Assoc. Equine Pract. 2009, 96–143. [Google Scholar]
- Ammann, L.; Fürst, A.E.; Jackson, M.A. Complete fractures through osseous cyst-like lesions of the proximal phalanx in three horses. Equine Vet. Educ. 2024. [Google Scholar] [CrossRef]
- O’Brien, E.J.O.; Smith, R.K.W. Mineralization can be an incidental ultrasonographic finding in equine tendons and ligaments. Vet. Radiol. Ultrasound 2018, 59, 613–623. [Google Scholar] [CrossRef] [PubMed]

| Pulse Sequence | TE (ms) | TR (ms) | Flip Angle (°) | Slice Thickness (mm) | Slice Gap (mm) | Matrix Size (FE × PE) | FOV (mm) |
|---|---|---|---|---|---|---|---|
| Pilot | 7 | 66 | 45 | 7 | 0 | 150 × 120 | 220 |
| Pilot of a pilot | 7 | 66 | 45 | 7 | 0 | 150 × 120 | 220 |
| STIR TEST | 22 | 2100 | 50/60/85/110 | 4 | 0.8 | 256 × 144 | 200 |
| T1W GRE MI | 8 | 50 | 55 | 5 | 1 | 170 × 130 | 170 |
| T2*W GRE MI | 13 | 68 | 25 | 5 | 1 | 340 × 160 | 170 |
| T2W FSE FAST | 88 | 1544 | 90 | 5 | 1 | 168 × 168 | 170 |
| STIR FSE FAST | 22 | 2336 | 95 | 5 | 1 | 168 × 168 | 170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, A.; Dyson, S. Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 31 Warmblood Showjumpers in Full Work and Competing Regularly. Animals 2024, 14, 1417. https://doi.org/10.3390/ani14101417
Nagy A, Dyson S. Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 31 Warmblood Showjumpers in Full Work and Competing Regularly. Animals. 2024; 14(10):1417. https://doi.org/10.3390/ani14101417
Chicago/Turabian StyleNagy, Annamaria, and Sue Dyson. 2024. "Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 31 Warmblood Showjumpers in Full Work and Competing Regularly" Animals 14, no. 10: 1417. https://doi.org/10.3390/ani14101417
APA StyleNagy, A., & Dyson, S. (2024). Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 31 Warmblood Showjumpers in Full Work and Competing Regularly. Animals, 14(10), 1417. https://doi.org/10.3390/ani14101417






