Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization
Abstract
Simple Summary
Abstract
1. Introduction
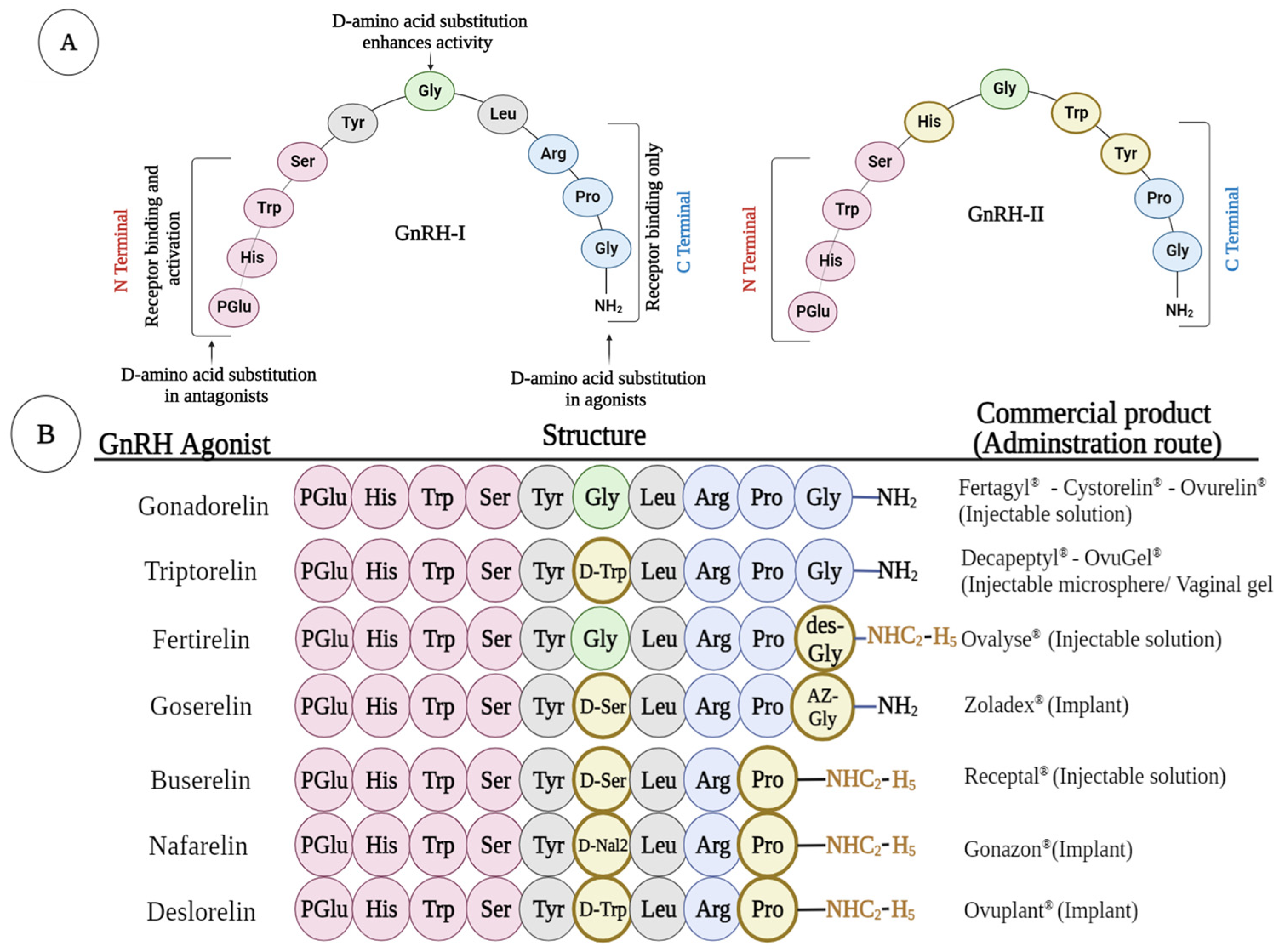
2. Structure of GnRH and Its Agonist
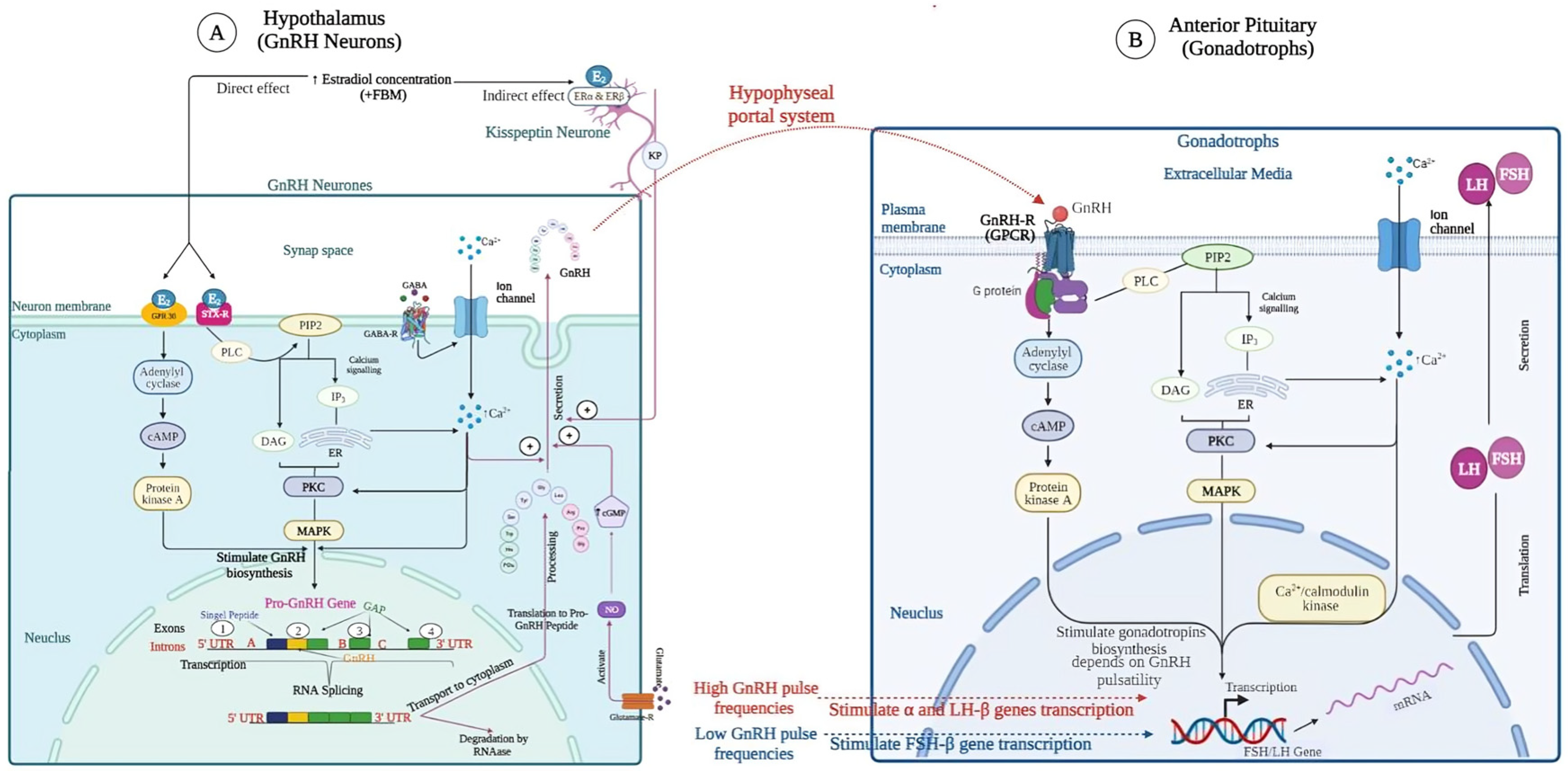
3. GnRH Gene Expression in Hypothalamus
4. Mechanism of Action of GnRH Analog
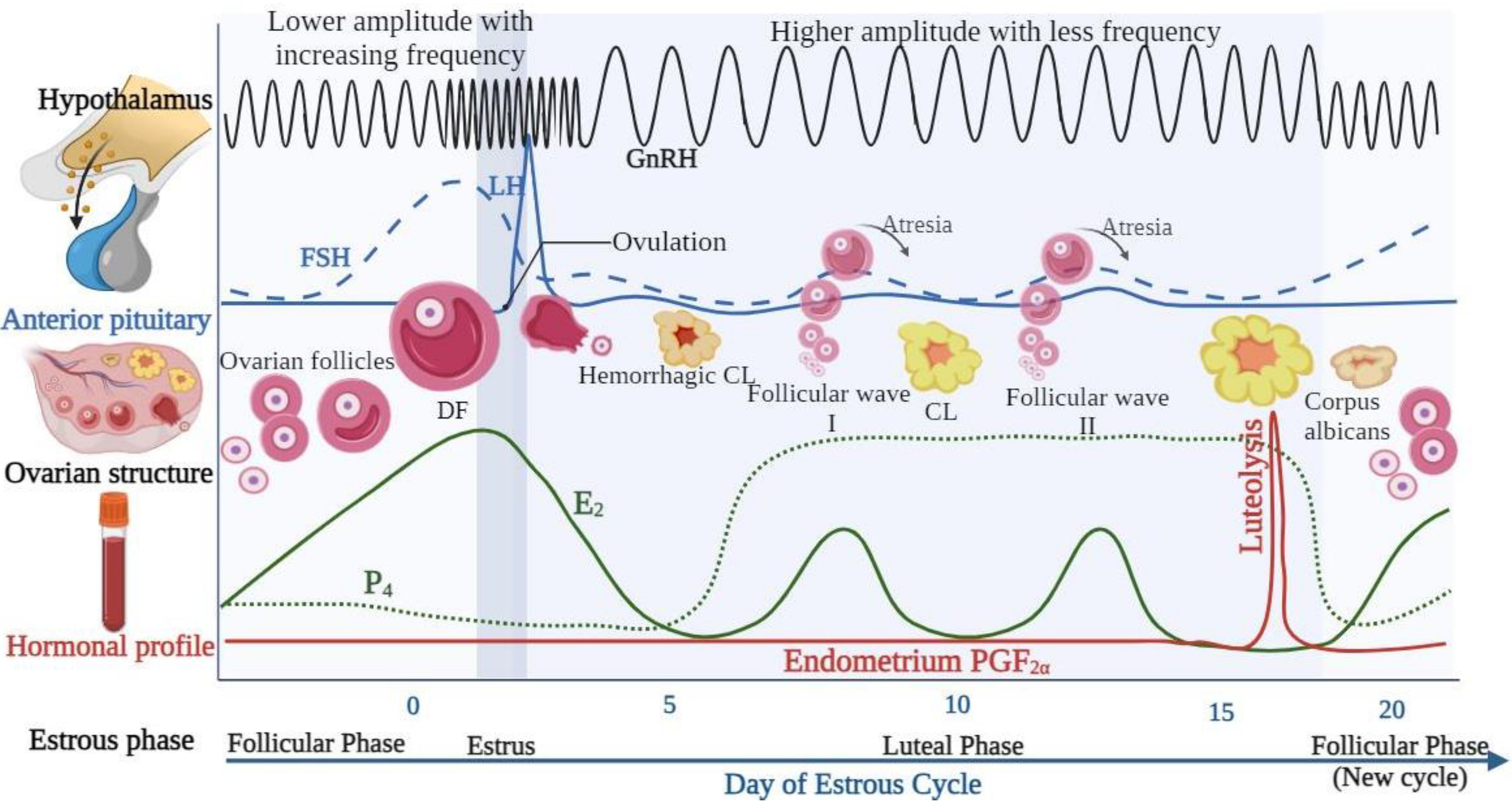
5. GnRH Regulation of the Female Estrous Cycle
6. Application of GnRH in the Reproduction of Dairy Cattle
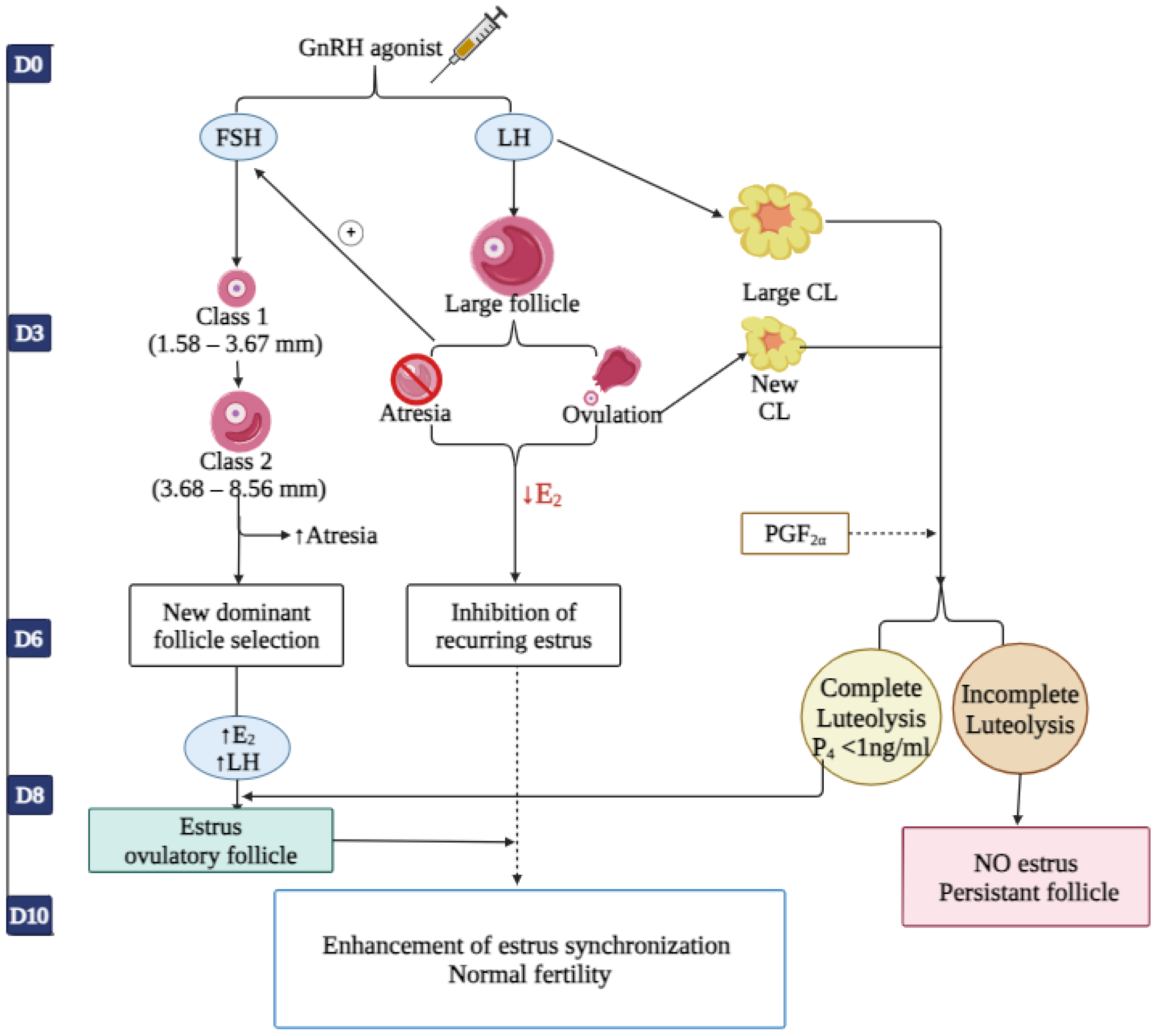
6.1. Synchronization of the Ovarian Follicular Wave Dynamics and Luteal Phase Support
6.2. Hormonal Control of the Timing of Behavioral Estrus among Cows Using GnRH and PGF2α
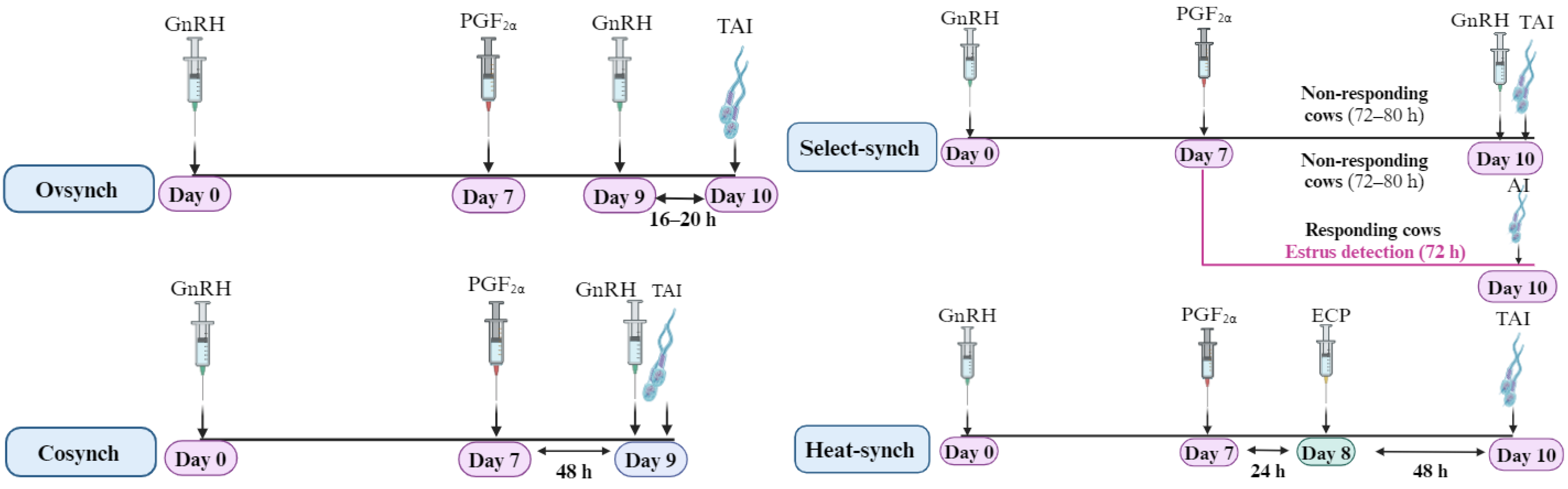
| Ref | Animal and Physiological Stage | Treatment Regimen | Modifications | Fertility Outcomes | Summary and Limitations | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days of TAI | Inseminated % | Ovulation % | Conception % | Pregnancy % | Embryo Loss % | |||||
| [78] | Ovular cows (n = 117) | Ovsynch | - | - | - | 97.0 | 32.0 | 32.0 | 14.0 | OV effectively synchronized ovulation in both groups but did not improve the reproductive performance of anovular cows. |
| Anovular cows (n = 33) | - | - | 94.0 | 9.0 | 9.0 | 0.0 | ||||
| [79] | Lactating dairy cows (58–78 DIM) | Ovsynch (n = 115) | - | 68 ± 1.1 | 100 | - | 35.6 | 35.6 | - | Pregnancy and Conception rates tended to be greater after OV because of poor expression of estrus. |
| Select-synch (n = 112) | AI 10–14 h after estrous detection | 73 ± 1.0 | 68.2 | - | 41.1 | 26.8 | - | |||
| [64] | Primi- and multiparous lactating dairy cows (50 d postpartum) | Ovsynch (n = 167) | - | 54 | - | - | - | 37.0 (d 60) 53.0 (d 100) | - | OV allowed effective management of AI without the need for estrous detection. |
| ED (n = 166) | Estrus detected using the a.m.–p.m. rule. | 83 | - | - | - | 5.0 (d 60) 35.0 (d 100) | - | |||
| [80] | Heifers (13 to 23 months) | Ovsynch (n = 77) | - | 9 ± 0 | - | - | - | 74.4 | - | Cows in the OV group that were >76 d postpartum had a greater pregnancy rate per AI than cows that were 60 to 75 d postpartum. |
| ED (n = 78) | - | 13 ± 11 | - | - | - | 35.1 | - | |||
| Multiparous dairy cows (60–289 d postpartum). | Ovsynch (n = 156) | - | 9 ± 0 | - | - | - | 37.8 | - | ||
| ED (n = 154) | - | 13 ± 11 | - | - | - | 38.9 | - | |||
| [81] | Holstein dairy cows (n = 40) with normal reproduction at 70–110 d postpartum. | Ovsynch full dose (n = 20) | 10.5 µg buserelin acetate | - | - | - | 85.0 | 50.0 | - | 5.25 µg buserelin is as effective as the full dose (10.5 µg) in the OV protocol of lactating dairy cows. |
| Ovsynch half dose (n = 20) | 5.25 µg buserelin acetate | - | - | - | 90.0 | 40.0 | - | |||
| [82] | Repeat breeding crossbred cows | Ovsynch (n = 6) | - | 50.0 | - | - | The incidence of accessory CL formation in treated cows after GnRH treatment on day 6 of the estrous cycle was high. | |||
| Control (n = 6) | Not treated | - | - | - | 0.0 | - | - | |||
| [83] | Primiparous and multiparous lactating dairy cows (n = 161) with a mature CL and a follicle with >10 mm. | Shortened Ovsynch (n = 22) | - | - | - | - | 36.4 | 33.3 | - | If a CL can be detected during reproductive examination in which the heat detection rate is poor, a shortened OV can be recommended. Shortened OV reduced the pregnancy rates for cows that ovulated late compared to the control group. |
| Control (n = 73) | One injection of PGF2α | - | - | - | 41.1 | 56.3 | - | |||
| [84] | Nonpregnant cows from three herds Eligible for reinsemination between 26–29 d after the 1st AI. | Shortened Ovsynch (n = 160) | - | 31 ± 1 | - | - | 23.3 | 85.6 | - | Conception and overall pregnancy rates did not differ significantly between groups. Shortened OV significantly reduced days to TAI. |
| Control (n = 189) | Not treated | 55 ± 1 | - | - | 22.8 | 75.9 | - | |||
| [85] | German Holsteins with ovarian cysts. On days 55 to 60 postpartum. | Ovsynch (n = 65) | M-OV: 1st GnRH + 1st PGF2α (d 0), 2nd PGF2α (d 14) and 2nd GnRH (d 16) | 74.8 ± 1.5 | - | - | 42.9 | 83.1 | - | OV can be used to treat ovarian cysts. The M-OV protocol led to a better cure rate and reproductive performance than the OV protocol. |
| Modified Ovsynch (n = 65) | 69.9 ± 1.5 | - | - | 27.3 | 60.0 | - | ||||
| [86] | Holstein crossbred cows from 5 herds. | Ovsynch (n = 851) | M-OV: Ovsynch with an additional PGF2α (d 8) | - | - | - | 42.0 | - | - | M-OV protocol increased conception rates and decreased P4 at insemination day compared with cows receiving OV protocol. |
| Modified Ovsynch (n = 852) | - | - | - | 49.0 | - | - | ||||
| [87] | Holstein dairy cows with CL and at least one follicle >10 mm in size | Ovsynch (n = 161) | M-OV: Injecting hCG instead of 1st GnRH in the Ovsynch | - | 59.6 | - | - | 48.5 | 0.05 | Administration hCG is not a suitable replacement for the 1st GnRH of OV due to its adverse impact. |
| Modified Ovsynch (n = 210) | - | 65.7 | - | - | 37.6 | 0.05 | ||||
| [88] | Lactating Holstein cows with normal postpartum intervals to the first service | Ovsynch (n = 31) | - | 84 ± 10 | - | - | 29.0 | 93.5 | - | OV produced higher fertility, superior pregnancy rates and fewer days to FAI than AI at estrus detection in cows inseminated in early postpartum ≤ 100 DIM. |
| Select-synch (n = 42) | Monitored for estrus signs for 5 d and AI | 117 ± 7 | - | - | 26.2 | 85.7 | - | |||
| [89] | Nulliparous heifers and lactating cows Treated females exhibited extended intervals between AI (27 to 53 d since their previous AI). | Ovsynch (n = 224) | - | - | 82.0 | - | - | 37.0 | - | Pregnancy outcomes were similar between the OV and Heatsynch protocols. AI after detecting estrus before the scheduled TAI resulted in shorter days to conception and tended to increase conception rates, especially with the Heatsynch protocol. |
| Heat-synch (n = 230) | - | - | 62.0 | - | - | 29.0 | - | |||
| [69] | Holstein multiparous cows (n = 54) | Cosynch | - | - | - | 50.0 | - | 41.0 | - | Cosynch protocol is more effective in heifers than multiparous cows. Ovulations that occurred before AI could be the reason for the low conception rate of OV. |
| Heifers (n = 53). | - | - | - | 35.0 | - | 51.0 | - | |||
| Ref | Animal and Physiological Stage | Treatment Regimen | Modifications | Fertility Outcomes | Summary and Limitations | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days of TAI | Inseminated % | Ovulation % | Conception % | Pregnancy % | Embryo Loss % | |||||
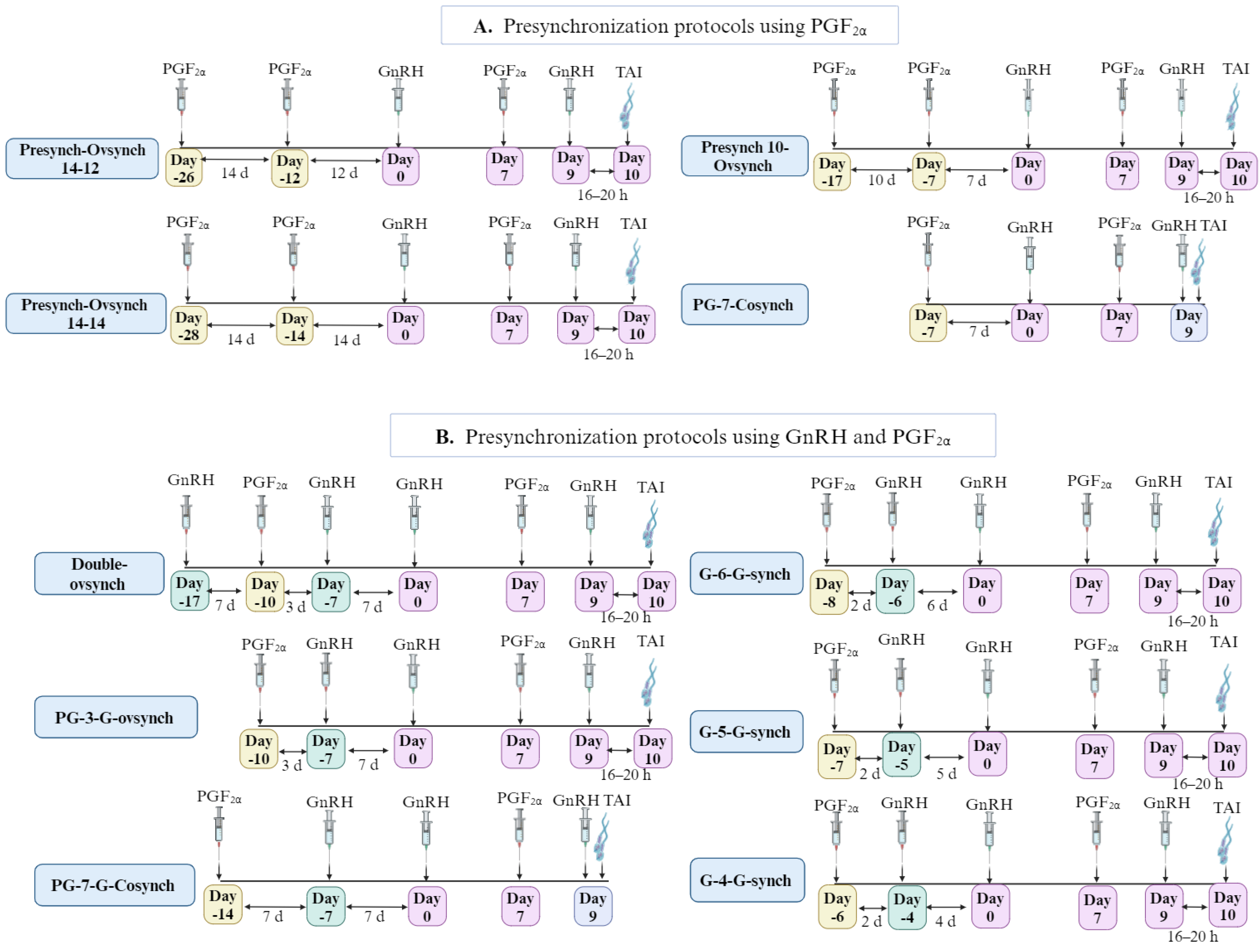
| [76] | Primiparous (P) and multiparous (M) lactating Holstein cows at ~60 to 70 DIM until dry-off. | Presynch-14–12- OV (n = 1566) | - | - | - | - | - | 40.5 (P) 31.2 (M) | 6.4 (P) 6.3 (M) | Extending the duration from 12 to 14 d apart from Presynch to OV decreased ovulatory response but did not reduce the fertility of cows that received TAI. |
| Presynch-14–14- OV (n = 1599) | - | - | - | - | - | 36.5 (P) 36.7 (M) | 5.2 (P) 7.1 (M) | |||
| [90] | Holstein dairy cows (60 DIM). Treatment at random stages of the estrous cycle. | Ovsynch (n = 134) | - | - | - | 69.6 | 37.3 | - | - | Presynchronization protocol increases the PR/AI of lactating dairy cows receiving TAI compared with OV. |
| Presynch (n = 135) | Ovsynch but with the addition of 2 PGF2α | - | - | 81.1 | 49.6 | - | - | |||
| [91] | Lactating dairy cows at 24 to 44 DIM All cows received Presynch- 14- 12- OV and then different Ovsynch. | G48-AI48 (n = 224) | GnRH + TAI at 48 h after PGF2α | - | - | - | - | 22.8 | 5.9 | GnRH and AI administration at 72 h after PGF2α in the Presynch 14 (G72-AI72 group) enhances pregnancy rates and reduces pregnancy loss compared to other groups. |
| G48-AI72 (n = 221) | GnRH at 48 h + TAI at 72 h after PGF2α | - | - | - | - | 23.5 | 13.3 | |||
| G72-AI72 (n = 220) | GnRH + TAI at 72 h after PGF2α | - | - | - | - | 31.4 | 1.6 | |||
| [92] | Lactating cows from 2 herds (59 to 79 DIM) | Presynch-14–12-OV (n = 318) | - | - | 68.0 | - | - | 46.8 | - | Presynch administration before OV protocol is recommended due to the increasing pregnancy rate in dairy cows. |
| Ovsynch (n = 312) | - | - | 73.0 | - | - | 37.5 | - | |||
| [93] | Lactating Holstein–Friesian dairy cows treated at 30–35 d postpartum | Presynch-14–12- OV (n = 100) | - | 64.2 ± 4.2 | - | - | 61.0 | - | - | Using PG + P-OV significantly reduces days to conception and NSC and improves P/AI. |
| PG + Presynch-14–12- OV (n = 41) | + PGF2α 15 d before applying Presynch. | 60.4 ± 3.7 | - | - | 87.8 | - | - | |||
| Control (n = 100) | Not treated | 79.1 ± 4.8 | - | - | 46.0 | - | - | |||
| [94] | Lactating Holstein cow treated at 30 days postpartum. | Presynch-14–12- OV (n = 446) | Primiparous | - | - | 31.8 | - | - | Primiparous cows responded more favorably to Presynch administration than multiparous cows by increasing conception rates and incidence of normal inter-estrus interval. | |
| Presynch-14–12- OV (n = 726) | Multiparous | 26.3 | ||||||||
| Ref | Animal and Physiological Stage | Treatment Regimen | Modifications | Fertility Outcomes | Summary and Limitations | ||||
|---|---|---|---|---|---|---|---|---|---|
| Synchronization % | Ovulation % | Conception % | Pregnancy % | Embryo loss % | |||||
| [95] | Primiparous lactating dairy cows (at 60 ± 3 DIM) during heat stress. | Presynch-Cosynch (n = 123) | - | - | 50.6 | - | 32.1 | 8.2 | Administration of GnRH before presynchronization increases P4 concentration (3.6 ± 0.3 ng/mL) compared to control (2.7 ± 0.4 ng/mL), improving fertility parameters under heat stress conditions. |
| GnRH- Presynch-Cosynch (n = 102) | + GnRH before applying presynch | - | 15.2 | - | 31.8 | 6.9 | |||
| [72] | Lactating Holstein cows at non-pregnancy diagnosis (d 0). | PG7-Cosynch (n = 967) + TAI | - | - | - | 17.2 | 11.5 | 23.5 | PG7-G7-Cosynch is an effective method to resynchronize cows, resulting in doubled P/AI. |
| PG7-G7-Cosynch (n = 962) + TAI | PGF2α on d0 and GnRH on d 7 | - | - | 28.0 | 21.2 | 16.4 | |||
| [74] | Postpartum lactating Holstein cows between 36 to 50 d DIM. | PG-3-G (n = 105) | - | - | 80.0 | - | 40.0 | 7.5 | The PG-3-G regimen improves ovulation rate and luteal function 7 d before OV, increasing follicular synchrony and P/AI in lactating dairy cows. |
| Presynch-10 (n = 105) | - | - | 53.3 | - | 33.3 | 8.6 | |||
| [97] | Lactating Holstein dairy cows (n = 137) before 1st service between 62–70 DIM | G-4-G (n = 33) | - | 87.9 | 56.0 | - | 24.0 | - | G-6-G regimen before applying OV increases ovulatory and luteolytic response OV compared to other regimens |
| G-5-G (n = 31) | - | 62.9 | 66.7 | - | 34.0 | - | |||
| G-6-G (n = 32) | - | 92.7 | 84.6 | - | 50.0 | - | |||
| Ovsynch (n = 34) | - | 77.1 | 53.8 | - | 27.0 | - | |||
| [98] | Lactating dairy cows at 58 to 64 DIM (first service) and cows diagnosed as not pregnant 39 days after the previous AI (2nd service). | G-6-G (n = 116) | - | - | 67.0 | - | 57.0 (d 35) 54.0 (d 49) | - | However, the P/AI were similar between groups on d 35 and 49; the ovulation rate increased after G-6-G application due to increasing P4 concentrations at the time of PGF2α of OV (5.75 ng/mL vs. 4.64 ng/mL). |
| PG + G (n = 121) | PGF2α +GnRH 7d before OV. | - | 68.0 | - | 50.0 (d 35) 47.0 (d 49) | - | |||
| [101] | Noncycling lactating Holstein cows at 42 ± 3 DIM. | Double Ovsynch (n = 100) | - | - | 98.0 | - | - | - | Pre-synchronization using double OV induced ovulation in noncycling cows and appeared to increase synchronization features during the OV protocol. |
| Presynch-14–12-Ovsynch (n = 93) | - | - | 93.5 | - | - | - | |||
| [102] | Lactating Holstein cows (primiparous: P, multiparous: M) at 42 ± 3 DIM | D-OV (n = 157) | - | - | 71.8 | - | 65.2 (P) 37.5 (M) | - | The D-OV regimen increases P/AI only in primiparous and not in multiparous cows. The fertility in primiparous cows compared to P-OV is due to the induction of ovulation in noncycling cows and the improved synchronization of cycling cows. |
| Presynch-14–12-Ovsynch (n = 180) | - | - | 66.7 | - | 45.2 (P) 39.3 (M) | - | |||
| [103] | Primiparous (P) and multiparous (M) lactating dairy cows at 45 ± 3 DIM for the presynch group and 54 ± 3 DIM for the double Ovsynch group. | Double-Ovsynch (n = 837) | - | - | - | - | 52.5 (P) 40.3 (M) | - | D-OV improved fertility in dairy cows compared to the Presynch regimen, particularly benefiting primiparous cows. D-OV could be a beneficial reproductive management regimen for synchronizing the first service in dairy herds. |
| Presynch-14–12-Ovsynch (n = 850) | - | - | - | - | 42.3 (P) 34.3 (M) | - | |||
| [104] | Multiparous lactating Holstein cows during the heat-stress season | Double-Ovsynch (n = 486) | - | 26.6 | - | - | 23.2 | 6.1 | D-OV significantly increases the synchronization rate and P/AI in summer. Also, it increases the mean diameter of the ovulatory follicle at TAI by (0.5 mm) D-OV treatment regimen yields optimal reproductive performance in heat-stressed dairy cows. |
| Presynch-14-GnRH-Ovsynch (n = 453) | Additional GnRH 2 d after applying presynch | 21.4 | - | - | 16.7 | 6.6 | |||
| Presynch-14–14-Ovsynch (n = 435) | - | 17.2 | - | - | 12.4 | 7.4 | |||
| [105] | Lactating Holstein cows with VWP 60 ± 3 d: n = 458 and 88 ± 3 d: n = 462. | Double-Ovsynch (D-OV60, n = 458) | - | - | - | - | 43.3 | 5.9 | D-OV administration on d 60 and 88 of VWP, P/AI at 39 ± 3 days post-AI was similar among treatment groups. |
| Double-Ovsynch (D-OV88, n = 462) | - | - | - | - | 45.5 | 7.1 | |||
| [106] | Lactating primiparous and multiparous Holstein and Jersey crossbred | Double-Ovsynch (100, n = 24) | Using 100 or 200 μg of GnRH | - | - | - | - | - | D-OV with 200 μg of GnRH increased LH secretion instead of a 100 μg dose of GnRH, either in a high or low P4 concentration. |
| Double-Ovsynch (200, n = 22) | |||||||||
| [107] | Lactating primiparous cows (n = 165) between 60 and 172 d postpartum | Double-Ovsynch (n = 81) | - | - | - | - | 72.8 | - | D-OV regimen increases the pregnancy rates in postpartum primiparous cows compared to the OV regimen. |
| Ovsynch (n = 84) | - | - | - | - | 29.8 | - | |||
| [108] | Lactating Holstein cows at 42 DIM (41 ± 0.1 d). During the year’s warm (W) and cool (C)seasons. | PG-3-G (n = 1286) | - | - | - | - | 35.9 (W) 46.8 (C) | - | During the summer, PG-3-G enhanced P/AI compared to Presynch-10. However, no significant difference in P/AI between treatments during cold weather. |
| Presynch-10 (n = 1247) | - | - | - | - | 26.7 (W) 44.3 (C) | - | |||
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, F.; Tomek, W.; Gründker, C. Gonadotropin-releasing hormone (GnRH) and its natural analogues: A review. Theriogenology 2006, 66, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Sealfon, S.C.; Weinstein, H.; Millar, R.P. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr. Rev. 1997, 18, 180–205. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.G.; Cardoso, R.C. Neuroendocrine Control of Estrus and Ovulation. In Bovine Reproduction; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 269–292. ISBN 9781119602361. [Google Scholar]
- McArdle, C. Gonadotropin Releasing Hormone (GnRH) Receptor. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–12. ISBN 978-0-08-055232-3. [Google Scholar]
- Krsmanovic, L.Z.; Martinez-Fuentes, A.J.; Arora, K.K.; Mores, N.; Tomić, M.; Stojilkovic, S.S.; Catt, K.J. Local regulation of gonadotroph function by pituitary gonadotropin-releasing hormone. Endocrinology 2000, 141, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Gault, P.M.; Maudsley, S.; Lincoln, G.A. Evidence that gonadotropin-releasing hormone II is not a physiological regulator of gonadotropin secretion in mammals. J. Neuroendocrinol. 2003, 15, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnappa, N.; Rajamahendran, R.; Lin, Y.-M.; Leung, P.C.K. GnRH in non-hypothalamic reproductive tissues. Anim. Reprod. Sci. 2005, 88, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Khazeni, S.; Varamini, P. Gonadotropin-Releasing-Hormone. In Reference Module in Biomedical Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–14. ISBN 9780128012383. [Google Scholar]
- Tsutsumi, R.; Webster, N.J.G. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 2009, 56, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Corradi, P.F.; Corradi, R.B.; Greene, L.W. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol. Clin. 2016, 43, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V. Use of GnRH in preference to LH-RH terminology in scientific papers. Hum. Reprod. 2000, 15, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Baba, Y.; Nair, R.M.G.; Arimura, A.; Schally, A. V Structure of the porcine LH-and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem. Biophys. Res. Commun. 1971, 43, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Burgus, R.; Butcher, M.; Amoss, M.; Ling, N.; Monahan, M.; Rivier, J.; Fellows, R.; Blackwell, R.; Vale, W.; Guillemin, R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF). Proc. Natl. Acad. Sci. USA 1972, 69, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Skorupskaite, K.; Rozario, K.S.; Anderson, R.A.; George, J.T. Physiology of GnRH and Gonadotropin Secretion. [Updated 2022 Jan 5]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Thatcher, W.W.; Drost, M.; Savio, J.D.; Macmillan, K.L.; Entwistle, K.W.; Schmitt, E.J.; De la Sota, R.L.; Morris, G.R. New clinical uses of GnRH and its analogues in cattle. Anim. Reprod. Sci. 1993, 33, 27–49. [Google Scholar] [CrossRef]
- Lamb, G.C.; Smith, M.F.; Perry, G.A.; Atkins, J.A.; Risley, M.E.; Busch, D.C.; Patterson, D.J. Reproductive Endocrinology and Hormonal Control of the Estrous Cycle. Bov. Pract. 2010, 44, 18–26. [Google Scholar] [CrossRef]
- Okubo, K.; Nagahama, Y. Structural and functional evolution of gonadotropin-releasing hormone in vertebrates. Acta Physiol. 2008, 193, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Arimura, A.; Kastin, A.J.; Matsuo, H.; Baba, Y.; Redding, T.W.; Nair, R.M.G.; Debeljuk, L.; White, W.F. Gonadotropin-releasing hormone: One polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 1971, 173, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Padula, A.M. GnRH analogues—Agonists and antagonists. Anim. Reprod. Sci. 2005, 88, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.S.; Jennes, L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995, 98, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P. GnRHs and GnRH receptors. Anim. Reprod. Sci. 2005, 88, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J. Control of GnRH secretion: One step back. Front. Neuroendocrinol. 2011, 32, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Baum, M.J. Neuroendocrine regulation of GnRH release in induced ovulators. Front. Neuroendocrinol. 2000, 21, 220–262. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Saito, H.; Sawada, T.; Yaegashi, T.; Yamashita, T.; Hirata, T.-I.; Sawai, K.; Hashizume, T. Characteristics of the stimulatory effect of kisspeptin-10 on the secretion of luteinizing hormone, follicle-stimulating hormone and growth hormone in prepubertal male and female cattle. J. Reprod. Dev. 2009, 55, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.S.; Wierman, M.E.; Nett, T.M.; Glode, L.M. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr. Relat. Cancer 2004, 11, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Kahnamouyi, S.; Nouri, M.; Farzadi, L.; Darabi, M.; Hosseini, V.; Mehdizadeh, A. The role of mitogen-activated protein kinase–extracellular receptor kinase pathway in female fertility outcomes: A focus on pituitary gonadotropins regulation. Ther. Adv. Endocrinol. Metab. 2018, 9, 209–215. [Google Scholar] [CrossRef]
- Perrett, R.M.; McArdle, C.A. Molecular mechanisms of gonadotropin-releasing hormone signaling: Integrating cyclic nucleotides into the network. Front. Endocrinol. 2013, 4, 180. [Google Scholar] [CrossRef] [PubMed]
- Karges, B.; Karges, W.; de Roux, N. Clinical and molecular genetics of the human GnRH receptor. Hum. Reprod. Update 2003, 9, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Claudia, A.; Rangel De Abreu, M.; Busato, M.-C.; Gomes Bergstein-Galan, T.; An-Drea, M.; Bertol, F.; Weiss, R.R. Bovine Reproductive Physiology and Endocrinology. In Reproduction Biotechnology in Farm Animals; AvidScience: Hyderabad, India, 2017. [Google Scholar]
- Fails, A.D.; Magee, C. Anatomy and Physiology of Farm Animals; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 1119239710. [Google Scholar]
- Crowe, M.A.; Mullen, M.P. Regulation and Function of Gonadotropins Throughout the Bovine Oestrous Cycle. In Gonadotropin; IntechOpen: London, UK, 2013; pp. 143–154. [Google Scholar]
- Gvozdić, D.; Dovenski, T.; Stančić, I.; Stančić, B.; Božić, A.; Jovanović, I.; Atanasov, B.; Šuluburić, A. Hormonal methods for estrous cycle manipulation in dairy cows. Contemp. Agric. 2013, 62, 319–332. [Google Scholar]
- Forde, N.; Beltman, M.E.; Lonergan, P.; Diskin, M.; Roche, J.F.; Crowe, M.A. Oestrous cycles in Bos taurus cattle. Anim. Reprod. Sci. 2011, 124, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Singh, J. Ovarian Follicular and Luteal Dynamics in Cattle. In Bovine Reproduction; Hopper, R.M., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 292–323. [Google Scholar]
- Kenealy, B.P.; Terasawa, E. Rapid direct action of estradiol in GnRH neurons: Findings and implications. Front. Endocrinol. 2012, 3, 106. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Beg, M.A.; Bergfelt, D.R.; Donadeu, F.X.; Kot, K. Follicle selection in monovular species. Biol. Reprod. 2001, 65, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Sartori, R.; Herlihy, M.M.; Vasconcelos, J.L.M.; Nascimento, A.B.; Souza, A.H.; Ayres, H.; Cunha, A.P.; Keskin, A.; Guenther, J.N.; et al. Managing the dominant follicle in lactating dairy cows. Theriogenology 2011, 76, 1568–1582. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.R.; Pimentel, M.G.; Lamming, G.E. Hormone responses to exogenous GnRH pulses in postpartum dairy cows. J. Reprod. Fertil. 1985, 75, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.J.; Fortin, J.; Wang, Y.; Lamba, P. Mechanisms of FSH synthesis: What we know, what we don’t, and why you should care. Fertil. Steril. 2010, 93, 2465–2485. [Google Scholar] [CrossRef] [PubMed]
- Savio, J.D.; Thatcher, W.W.; Badinga, L.; De La Sota, R.L.; Wolfenson, D. Regulation of dominant follicle turnover during the oestrous cycle in cows. Reproduction 1993, 97, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Glencross, R.G. Effect of pulsatile infusion of gonadotrophin-releasing hormone on plasma oestradiol-17β concentrations and follicular development during naturally and artificially maintained high levels of plasma progesterone in heifers. J. Endocrinol. 1987, 112, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chenault, J.R.; Kratzer, D.D.; Rzepkowski, R.A.; Goodwin, M.C. LH and FSH response of Holstein heifers to fertirelin acetate, gonadorelin and buserelin. Theriogenology 1990, 34, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Kastelic, J.P.; Knopf, L. Composition and characteristics of follicular waves during the bovine estrous cycle. Anim. Reprod. Sci. 1989, 20, 187–200. [Google Scholar] [CrossRef]
- Savio, J.D.; Keenan, L.; Boland, M.P.; Roche, J.F. Pattern of growth of dominant follicles during the oestrous cycle of heifers. Reproduction 1988, 83, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, K.L.; Thatcher, W.W. Effects of an agonist of gonadotropin-releasing hormone on ovarian follicles in cattle. Biol. Reprod. 1991, 45, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, L.A.; Lussier, J.G.; Grasso, F.; Matton, P. Influence of a GnRH analogue on follicular dynamics in cows pretreated or not with FSH-P. Theriogenology 1990, 33, 240. [Google Scholar] [CrossRef]
- Rettmer, I.; Stevenson, J.S.; Corah, L.R. Endocrine responses and ovarian changes in inseminated dairy heifers after an injection of a GnRH agonist 11 to 13 days after estrus. J. Anim. Sci. 1992, 70, 508–517. [Google Scholar] [CrossRef]
- Lucy, M.C.; Stevenson, J.S. Gonadotropin-releasing hormone at estrus: Luteinizing hormone, estradiol, and progesterone during the periestrual and postinsemination periods in dairy cattle. Biol. Reprod. 1986, 35, 300–311. [Google Scholar] [CrossRef]
- Rosenberg, M.; Chun, S.Y.; Kaim, M.; Herz, Z.; Folman, Y. The effect of GnRH administered to dairy cows during oestrus on plasma LH and conception in relation to the time of treatment and insemination. Anim. Reprod. Sci. 1991, 24, 13–24. [Google Scholar] [CrossRef]
- Grieger, D.M.; Roberts, A.J.; Reeves, J.J. The association of the pre-ovulatory surge of LH and conception in beef cattle. Anim. Reprod. Sci. 1991, 24, 205–209. [Google Scholar] [CrossRef]
- Laurinčík, J.; Picha, J.; Pichova, D.; Pivko, J. Effect of LH-RH on meiotic maturation of pre-ovulatory oocytes in superovulated heifers. Anim. Reprod. Sci. 1991, 25, 189–197. [Google Scholar] [CrossRef]
- Twagiramungu, H.; Guilbault, L.A.; Dufour, J.J. Synchronization of ovarian follicular waves with a gonadotropin-releasing hormone agonist to increase the precision of estrus in cattle: A review. J. Anim. Sci. 1995, 73, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.E.; Bergfeld, E.G.; Cupp, A.S.; Kojima, F.N.; Mariscal, V.; Sanchez, T.; Wehrman, M.E.; Grotjan, H.E.; Hamernik, D.L.; Kinder, J.E. Luteinizing Hormone Has a Role in Development of Fully Functional Corpora Lutea (CL) But Is Not Required to Maintain CL Function in Heifers. Biol. Reprod. 1994, 51, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Chiang, C.-F.; Ho, C.-T.; Chan, J.P.-W. Effect of GnRH on ovulatory response after luteolysis induced by two low doses of PGF2α in lactating dairy cows. Theriogenology 2018, 105, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Twagiramungu, H.; Guilbault, L.A.; Proulx, J.G.; Dufour, J.J. Buserelin alters the development of the corpora lutea in cyclic and early postpartum cows. J. Anim. Sci. 1995, 73, 805–811. [Google Scholar] [CrossRef]
- Mee, M.O.; Stevenson, J.S.; Alexander, B.M.; Sasser, R.G. Administration of GnRH at estrus influences pregnancy rates, serum concentrations of LH, FSH, estradiol-17 beta, pregnancy-specific protein B, and progesterone, proportion of luteal cell types, and in vitro production of progesterone in dairy cows. J. Anim. Sci. 1993, 71, 185–198. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, K.L.; Day, A.M.; Taufa, V.K.; Gibb, M.; Pearce, M.G. Effects of an agonist of gonadotrophin releasing hormone in cattle. I. Hormone concentrations and oestrous cycle length. Anim. Reprod. Sci. 1985, 8, 203–212. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Phatak, A.P.; Rettmer, I.; Stewart, R.E. Postinsemination Administration of Receptal: Follicular Dynamics, Duration of Cycle, Hormonal Responses, and Pregnancy Rates. J. Dairy Sci. 1993, 76, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Rodger, L.D.; Stormshak, F. Gonadotropin-releasing hormone-induced alteration of bovine corpus luteum function. Biol. Reprod. 1986, 35, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Lei, Z.M.; Rao, C.V.; Hansel, W. Cellular distribution and cycle phase dependency of gonadotropin and eicosanoid binding sites in bovine corpora lutea. Biol. Reprod. 1991, 45, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Thompson, K.E.; Forbes, W.L.; Lamb, G.C.; Grieger, D.M.; Corah, L.R. Synchronizing estrus and(or) ovulation in beef cows after combinations of GnRH, norgestomet, and prostaglandin F2α with or without timed insemination. J. Anim. Sci. 2000, 78, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Call, E.P.; Scoby, R.K.; Phatak, A.P. Double Insemination and Gonadotropin-Releasing Hormone Treatment of Repeat-Breeding Dairy Cattle. J. Dairy Sci. 1990, 73, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Szenci, O.; Szelényi, Z.; Lénárt, L.; Buják, D.; Kovács, L.; Kézér, L.F.; Han, B.; Horváth, A. Importance of monitoring the peripartal period to increase reproductive performance in dairy cattle. Vet. Stanica 2018, 49, 297–307. [Google Scholar]
- Pursley, J.R.; Kosorok, M.R.; Wiltbank, M.C. Reproductive Management of Lactating Dairy Cows Using Synchronization of Ovulation. J. Dairy Sci. 1997, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, A.; Barański, W.; Baryczka, A.; Janowski, T. OvSynch protocol and its modifications in the reproduction management of dairy cattle herds -an update. J. Vet. Res. 2017, 61, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Pursley, J.R. The cow as an induced ovulator: Timed AI after synchronization of ovulation. Theriogenology 2014, 81, 170–185. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Britt, J.H. A 100-Year Review: Practical female reproductive management. J. Dairy Sci. 2017, 100, 10292–10313. [Google Scholar] [CrossRef] [PubMed]
- De Jarnette, M. Ovsynch, Co-Synch, Presynch and Kitchensynch: How Did Breeding Cows Get So Complicated? Select Sires Inc.: Plain City, OH, USA, 2015. [Google Scholar]
- Demiral, O.; Ün, M.; Abay, M.; Bekyürek, T.; Öztürk, A. The effectiveness of Cosynch protocol in dairy heifers and multiparous cows. Turk. J. Vet. Anim. Sci. 2006, 30, 213–217. [Google Scholar]
- Lucy, M.C.; McDougall, S.; Nation, D.P. The use of hormonal treatments to improve the reproductive performance of lactating dairy cows in feedlot or pasture-based management systems. Anim. Reprod. Sci. 2004, 82–83, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Yoisungnern, T.; Bunaparte, N. Hormonal treatment and estrus synchronization in cows: A mini-review. J. Adv. Vet. Anim. Res. 2015, 2, 10–17. [Google Scholar] [CrossRef]
- Mendonça, L.G.D.; Rocha, L.S.; Voelz, B.E.; Lima, G.T.; Scanavez, A.L.A.; Stevenson, J.S. Presynchronization strategy using prostaglandin F2α, gonadotropin-releasing hormone, and detection of estrus to improve fertility in a resynchronization program for dairy cows. Theriogenology 2019, 124, 39–47. [Google Scholar] [CrossRef] [PubMed]
- El-Zarkouny, S.Z.; Cartmill, J.A.; Richardson, A.M.; Medina-Britos, M.A. Presynchronization of estrous cycles in dairy cows before ovsynch + CIDR and resynchronization of repeat estrus using the CIDR. Kansas Agric. Exp. Stn. Res. Rep. 2001, 2, 52–54. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Pulley, S.L.; Mellieon, J.I. Prostaglandin F 2α and gonadotropin-releasing hormone administration improve progesterone status, luteal number, and proportion of ovular and anovular dairy cows with corpora lutea before a timed artificial insemination program. J. Dairy Sci. 2012, 95, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.F.; Kastelic, J.P.; Adams, G.P.; Cook, B.; Olson, W.O.; Mapletoft, R.J. The use of progestins in regimens for fixed-time artificial insemination in beef cattle. Theriogenology 2002, 57, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.O.; Thomas, M.J.; Catucuamba, G.; Curler, M.D.; Wijma, R.; Stangaferro, M.L.; Masello, M. Effect of extending the interval from Presynch to initiation of Ovsynch in a Presynch-Ovsynch protocol on fertility of timed artificial insemination services in lactating dairy cows. J. Dairy Sci. 2016, 99, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S. Synchronization and AI Strategies in Dairy Herds. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Gümen, A.; Guenther, J.N.; Wiltbank, M.C. Follicular size and response to Ovsynch versus detection of estrus in anovular and ovular lactating dairy cows. J. Dairy Sci. 2003, 86, 3184–3194. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Kobayashi, Y.; Thompson, K.E. Reproductive performance of dairy cows in various programmed breeding systems including OvSynch and combinations of gonadotropin-releasing hormone and prostaglandin F2α. J. Dairy Sci. 1999, 82, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Pursley, J.R.; Wiltbank, M.C.; Stevenson, J.S.; Ottobre, J.S.; Garverick, H.A.; Anderson, L.L. Pregnancy Rates Per Artificial Insemination for Cows and Heifers Inseminated at a Synchronized Ovulation or Synchronized Estrus. J. Dairy Sci. 1997, 80, 295–300. [Google Scholar] [CrossRef]
- Karaca, F.; Dogruer, G.; Saribay, M.K.; Ergun, Y.; Ates, C.T. The Effect of the Reduced Dose of GnRH on Conception, Ovulation and Ovarian Structures in Ovsynch Program of Lactating Dairy Cows. Anim. Rev. 2016, 3, 66–72. [Google Scholar] [CrossRef]
- Ahmed, N.; Kathiresan, D.; Ahmed, F.A.; Lalrintluanga, K.; Mayengbam, P.; Gali, J.M. Pattern of induced estrus and conception rate following Ovsynch and Ovsynch based gonadotropin-releasing hormone treatments initiated on day 6 of estrous cycle in repeat breeding crossbred cows. Vet. World 2016, 9, 342–345. [Google Scholar] [CrossRef]
- Répási, A.; Szelényi, Z.; De Sousa, N.M.; Beckers, J.F.; Nagy, K.; Szenci, O. Effect of ovulation rate and timing of ovulation after different hormone treatments on pregnancy rate in dairy cows. Pol. J. Vet. Sci. 2019, 22, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Cartmill, J.A.; Hensley, B.A.; El-Zarkouny, S.Z. Conception rates of dairy cows following early not-pregnant diagnosis by ultrasonography and subsequent treatments with shortened Ovsynch protocol. Theriogenology 2003, 60, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Gundling, N.; Drews, S.; Hoedemaker, M. Comparison of Two Different Programmes of Ovulation Synchronization in the Treatment of Ovarian Cysts in Dairy Cows. Reprod. Domest. Anim. 2015, 50, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Rheinberger, J.M.; Colson, D.D.; Beggs, D.S.; Mansell, P.D.; Stevenson, M.A.; Rheinberger, R.J.; Pyman, M.F. Effect of a second treatment of prostaglandin F2α during the Ovsynch program on fixed-time artificial insemination conception rates and luteolysis in split-calving, pasture-fed dairy cows. Aust. Vet. J. 2020, 98, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.; Yilmazbas-Mecitoglu, G.; Gumen, A.; Karakaya, E.; Darici, R.; Okut, H. Effect of hCG vs. GnRH at the beginning of the Ovsynch on first ovulation and conception rates in cyclic lactating dairy cows. Theriogenology 2010, 74, 602–607. [Google Scholar] [CrossRef] [PubMed]
- El-Zarkouny, S.Z.; Shaaban, M.M.; Stevenson, J.S. Blood metabolites and hormone-based programmed breeding treatments in anovular lactating dairy cows. J. Dairy Sci. 2011, 94, 6001–6010. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Tiffany, S.M. Resynchronizing estrus and ovulation after not-pregnant diagnosis and various ovarian states including cysts. J. Dairy Sci. 2004, 87, 3658–3664. [Google Scholar] [CrossRef] [PubMed]
- Navanukraw, C.; Redmer, D.A.; Reynolds, L.P.; Kirsch, J.D.; Grazul-Bilska, A.T.; Fricke, P.M. A modified presynchronization protocol improves fertility to timed artificial insemination in lactating dairy cows. J. Dairy Sci. 2004, 87, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Portaluppi, M.A.; Stevenson, J.S. Pregnancy rates in lactating dairy cows after presynchronization of estrous cycles and variations of the Ovsynch protocol. J. Dairy Sci. 2005, 88, 914–921. [Google Scholar] [CrossRef] [PubMed]
- El-Zarkouny, S.Z.; Cartmill, J.A.; Hensley, B.A.; Stevenson, J.S. Pregnancy in dairy cows after synchronized ovulation regimens with or without presynchronization and progesterone. J. Dairy Sci. 2004, 87, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Akbarabadi, M.A.; Shabankareh, H.K.; Abdolmohammadi, A.; Shahsavari, M.H. Effect of PGF2a and GnRH on the reproductive performance of postpartum dairy cows subjected to synchronization of ovulation and timed artificial insemination during the warm or cold periods of the year. Theriogenology 2014, 82, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, R.L.; Abdel-Wahab, A. Reproductive responses of primiparous and multiparous Holstein cows submitted to presynch-ovsynch protocol. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 149–153. [Google Scholar] [CrossRef]
- Voelz, B.E.; Rocha, L.; Scortegagna, F.; Stevenson, J.S.; Mendonça, L.G.D. Treatment of lactating dairy cows with gonadotropin-releasing hormone before first insemination during summer heat stress. J. Dairy Sci. 2016, 99, 7612–7623. [Google Scholar] [CrossRef]
- Mendonça, L.G.D.; Dewey, S.T.; Lopes, G.; Rivera, F.A.; Guagnini, F.S.; Fetrow, J.P.; Bilby, T.R.; Chebel, R.C. Effects of resynchronization strategies for lactating Holstein cows on pattern of reinsemination, fertility, and economic outcome. Theriogenology 2012, 77, 1151–1158. [Google Scholar] [CrossRef]
- Bello, N.M.; Steibel, J.P.; Pursley, J.R. Optimizing ovulation to first GnRH improved outcomes to each hormonal injection of ovsynch in lactating dairy cows. J. Dairy Sci. 2006, 89, 3413–3424. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.R.; Martins, J.P.N.; Ahmad, N.; Nobis, K.; Pursley, J.R. Presynchronization of lactating dairy cows with PGF2α and GnRH simultaneously, 7 days before Ovsynch have similar outcomes compared to G6G. Theriogenology 2016, 86, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- DeJarnette, J.M.; Marshall, C.E. Effects of pre-synchronization using combinations PGF2α and (or) GnRH on pregnancy rates of Ovsynch- and Cosynch-treated lactating Holstein cows. Anim. Reprod. Sci. 2003, 76, 51–60. [Google Scholar] [CrossRef]
- Colazo, M.G.; Ambrose, D.J. Neither duration of progesterone insert nor initial GnRH treatment affected pregnancy per timed-insemination in dairy heifers subjected to a Co-synch protocol. Theriogenology 2011, 76, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Ayres, H.; Ferreira, R.M.; Cunha, A.P.; Araújo, R.R.; Wiltbank, M.C. Double-Ovsynch in high-producing dairy cows: Effects on progesterone concentrations and ovulation to GnRH treatments. Theriogenology 2013, 79, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.H.; Ayres, H.; Ferreira, R.M.; Wiltbank, M.C. A new presynchronization system (Double-Ovsynch) increases fertility at first postpartum timed AI in lactating dairy cows. Theriogenology 2008, 70, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, M.M.; Giordano, J.O.; Souza, A.H.; Ayres, H.; Ferreira, R.M.; Keskin, A.; Nascimento, A.B.; Guenther, J.N.; Gaska, J.M.; Kacuba, S.J.; et al. Presynchronization with Double-Ovsynch improves fertility at first postpartum artificial insemination in lactating dairy cows. J. Dairy Sci. 2012, 95, 7003–7014. [Google Scholar] [CrossRef] [PubMed]
- Dirandeh, E.; Roodbari, A.R.; Colazo, M.G. Double-Ovsynch, compared with presynch with or without GnRH, improves fertility in heat-stressed lactating dairy cows. Theriogenology 2015, 83, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Stangaferro, M.L.; Wijma, R.; Masello, M.; Giordano, J.O. Reproductive performance and herd exit dynamics of lactating dairy cows managed for first service with the Presynch-Ovsynch or Double-Ovsynch protocol and different duration of the voluntary waiting period. J. Dairy Sci. 2018, 101, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.O.; Fricke, P.M.; Guenther, J.N.; Lopes, G.; Herlihy, M.M.; Nascimento, A.B.; Wiltbank, M.C. Effect of progesterone on magnitude of the luteinizing hormone surge induced by two different doses of gonadotropin-releasing hormone in lactating dairy cows. J. Dairy Sci. 2012, 95, 3781–3793. [Google Scholar] [CrossRef]
- Öztürk, Ö.A.; Cirit, Ü.; Baran, A.; Ak, K. Is Doublesynch protocol a new alternative for timed artificial insemination in anestrous dairy cows. Theriogenology 2010, 73, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Pulley, S.L. Pregnancy per artificial insemination after presynchronizing estrous cycles with the Presynch-10 protocol or prostaglandin F2α injection followed by gonadotropin-releasing hormone before Ovsynch-56 in 4 dairy herds of lactating dairy cows. J. Dairy Sci. 2012, 95, 6513–6522. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanein, E.M.; Szelényi, Z.; Szenci, O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals 2024, 14, 1473. https://doi.org/10.3390/ani14101473
Hassanein EM, Szelényi Z, Szenci O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals. 2024; 14(10):1473. https://doi.org/10.3390/ani14101473
Chicago/Turabian StyleHassanein, Eman M., Zoltán Szelényi, and Ottó Szenci. 2024. "Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization" Animals 14, no. 10: 1473. https://doi.org/10.3390/ani14101473
APA StyleHassanein, E. M., Szelényi, Z., & Szenci, O. (2024). Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals, 14(10), 1473. https://doi.org/10.3390/ani14101473






