Density Mediates the Predator-Induced Growth and Metamorphic Plasticity of Chinhai Spiny Newt Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Maintenance
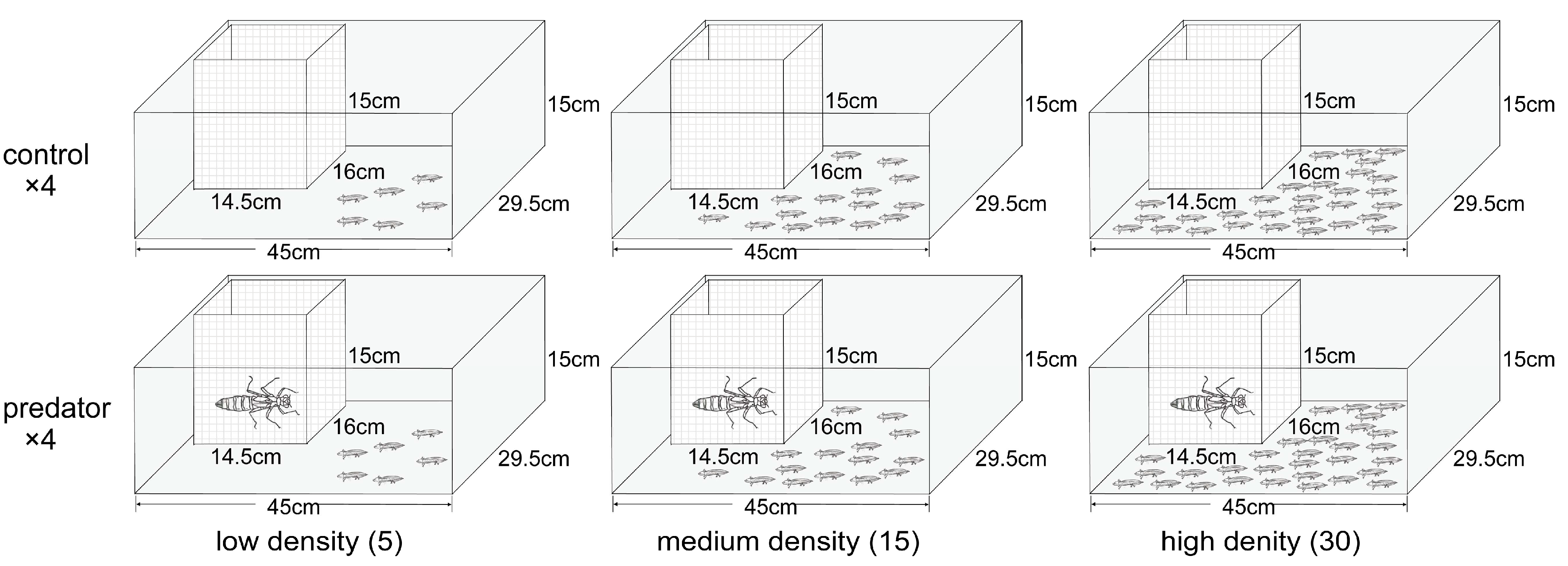
2.2. Experiment Design
2.3. Experiment Procedure
2.4. Statistical Analysis
3. Results
3.1. Growth Rate
3.2. Metamorphic Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benard, M.F. Predator-Induced Phenotypic Plasticity in Organisms with Complex Life Histories. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 651–673. [Google Scholar] [CrossRef]
- Relyea, R.A. Getting out Alive: How Predators Affect the Decision to Metamorphose. Oecologia 2007, 152, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.K.; Richardson, J.S. Predation Risk and Competition Effects on the Life-History Characteristics of Larval Oregon Spotted Frog and Larval Red-Legged Frog. Oecologia 2002, 132, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Sergio, C.; Luca, R.; Olivier, F. Plasticity and Flexibility in the Anti-Predator Responses of Treefrog Tadpoles. Behav. Ecol. Sociobiol. 2021, 75, 142. [Google Scholar] [CrossRef]
- Dehn, M.M. Vigilance for Predators: Detection and Dilution Effects. Behav. Ecol. Sociobiol. 1990, 26, 337–342. [Google Scholar]
- Lima, S.L.; Zollner, P.A. Anti-Predatory Vigilance and the Limits to Collective Detection: Visual and Spatial Separation between Foragers. Behav. Ecol. Sociobiol. 1996, 38, 355–363. [Google Scholar] [CrossRef]
- Spieler, M.; Linsenmair, K.E. Aggregation Behaviour of Bufo maculatus Tadpoles as an Antipredator Mechanism. Ethology 1999, 105, 665–686. [Google Scholar] [CrossRef]
- Browne, R.K.; Pomering, M.; Hamer, A.J. High Density Effects on the Growth, Development and Survival of Litoria aurea Tadpoles. Aquaculture 2003, 215, 109–121. [Google Scholar] [CrossRef]
- McCoy, M.W. Conspecific Density Determines the Magnitude and Character of Predator-Induced Phenotype. Oecologia 2007, 153, 871–878. [Google Scholar] [CrossRef]
- Peacor, S.D. Phenotypic Modifications to Conspecific Density Arising from Predation Risk Assessment. Oikos 2003, 100, 409–415. [Google Scholar] [CrossRef]
- Tollrian, R.; Duggen, S.; Weiss, L.C.; Laforsch, C.; Kopp, M. Density-Dependent Adjustment of Inducible Defenses. Sci. Rep. 2015, 5, 12736. [Google Scholar] [CrossRef] [PubMed]
- Relyea, R.A.; Auld, J.R. Having the Guts to Compete: How Intestinal Plasticity Explains Costs of Inducible Defences. Ecol. Lett. 2004, 7, 869–875. [Google Scholar] [CrossRef]
- Xie, F.; Gu, H. Echinotriton chinhaiensis, The IUCN Red List of Threatened Species. 2023, e.T59447A48311626. Available online: https://www.iucnredlist.org/species/59447/48311626 (accessed on 6 March 2024).
- Xu, A.; Jiang, Z.; Li, C. The Chinhai Spiny Newt Needs a Safer Habitat. Science 2021, 372, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Mathis, A. Friends in Low Places: Responses of a Benthic Stream Fish to Intra-Prey-Guild Alarm Cues. Ethology 2016, 122, 954–962. [Google Scholar] [CrossRef]
- Kłosiński, P.; Kobak, J.; Augustyniak, M.; Pawlak, R.; Jermacz, Ł.; Poznańska-Kakareko, M.; Kakareko, T. Behavioural Responses to Con- and Heterospecific Alarm Cues by an Alien and a Coexisting Native Fish. Hydrobiologia 2022, 849, 985–1000. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Ferrari, M.C.O.; Chivers, D.P.; Bisazza, A. Odour Recognition Learning of Multiple Predators by Amphibian Larvae. Anim. Behav. 2018, 140, 199–205. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kehr, A.I.; Schaefer, E.F.; Duré, M.I.; Gómez, V.I. Influence of Light Intensity, Water Volume and Density in Tadpoles Raised in Mesocosm Experiments. J. Zool. 2014, 293, 33–39. [Google Scholar] [CrossRef]
- Koprivnikar, J.; Forbes, M.R.; Baker, R.L. Larval Amphibian Growth and Development under Varying Density: Are Parasitized Individuals Poor Competitors? Oecologia 2008, 155, 641–649. [Google Scholar] [CrossRef]
- Beiswenger, R.E. Structure and Function in Aggregations of Tadpoles of the American Toad, Bufo Americanus. Herpetologica 1975, 31, 222–233. [Google Scholar]
- Chang, Y.-M.; Tseng, W.-H.; Chen, C.-C.; Huang, C.-H.; Chen, Y.-F.; Hatch, K.A. Winter Breeding and High Tadpole Densities May Benefit the Growth and Development of Tadpoles in a Subtropical Lowland Treefrog. J. Zool. 2014, 294, 154–160. [Google Scholar] [CrossRef]
- Babbitt, K.J. Behaviour and Growth of Southern Leopard Frog (Rana Sphenocephala) Tadpoles: Effects of Food and Predation Risk. Can. J. Zool. 2001, 79, 809–814. [Google Scholar] [CrossRef]
- Supekar, S.C.; Gramapurohit, N.P. Do Antipredator Responses of Euphlyctis cyanophlyctis Tadpoles Depend on the Intensity of Predation Risk? Aquat. Ecol. 2020, 54, 823–837. [Google Scholar] [CrossRef]
- Davenport, J.M.; Chalcraft, D.R. Increasing Conspecific Density Weakens the Ability of Intermediate Predators to Develop Induced Morphological Defences to Top Predators. Freshw. Biol. 2014, 59, 87–99. [Google Scholar] [CrossRef]
- Van Buskirk, J. Specific Induced Responses to Different Predator Species in Anuran Larvae. J. Evol. Biol. 2001, 14, 482–489. [Google Scholar] [CrossRef]
- Dijk, B.; Laurila, A.; Orizaola, G.; Johansson, F. Is One Defence Enough? Disentangling the Relative Importance of Morphological and Behavioural Predator-Induced Defences. Behav. Ecol. Sociobiol. 2016, 70, 237–246. [Google Scholar] [CrossRef]
- Laurila, A.; Kujasalo, J. Habitat Duration, Predation Risk and Phenotypic Plasticity in Common Frog (Rana temporaria) Tadpoles. J. Anim. Ecol. 1999, 68, 1123–1132. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. Damage, Digestion, and Defence: The Roles of Alarm Cues and Kairomones for Inducing Prey Defences: Damage, Digestion, and Defence. Ecol. Lett. 2005, 8, 505–512. [Google Scholar] [CrossRef]
- Joshi, A.M.; Wadekar, N.V.; Gramapurohit, N.P. Does Corticosterone Mediate Predator-Induced Responses of Larval Hylarana Indica? Gen. Comp. Endocrinol. 2017, 251, 30–37. [Google Scholar] [CrossRef]
- Steiner, U.K. Linking Antipredator Behaviour, Ingestion, Gut Evacuation and Costs of Predator-Induced Responses in Tadpoles. Anim. Behav. 2007, 74, 1473–1479. [Google Scholar] [CrossRef]
- Relyea, R.A.; Rosenberger, D. Predator Effects on Metamorphosis: The Effects of Scaring versus Thinning at High Prey Densities. Copeia 2018, 106, 457–467. [Google Scholar] [CrossRef]
- Spieler, M. Can Aggregation Behaviour of Phrynomantis microps Tadpoles Reduce Predation Risk? Herpetol. J. 2005, 15, 153–157. [Google Scholar]
- Üveges, B.; Basson, A.C.; Móricz, Á.M.; Bókony, V.; Hettyey, A. Chemical Defence Effective against Multiple Enemies: Does the Response to Conspecifics Alleviate the Response to Predators? Funct. Ecol. 2021, 35, 2294–2304. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Ferrari, M.; Kueng, D.; Näpflin, K.; Ritter, N. Prey Risk Assessment Depends on Conspecific Density. Oikos 2011, 120, 1235–1239. [Google Scholar] [CrossRef]
- Groner, M.L.; Rollins-Smith, L.A.; Reinert, L.K.; Hempel, J.; Bier, M.E.; Relyea, R.A. Interactive Effects of Competition and Predator Cues on Immune Responses of Leopard Frogs at Metamorphosis. J. Exp. Biol. 2013, 217, 351–358. [Google Scholar] [CrossRef]
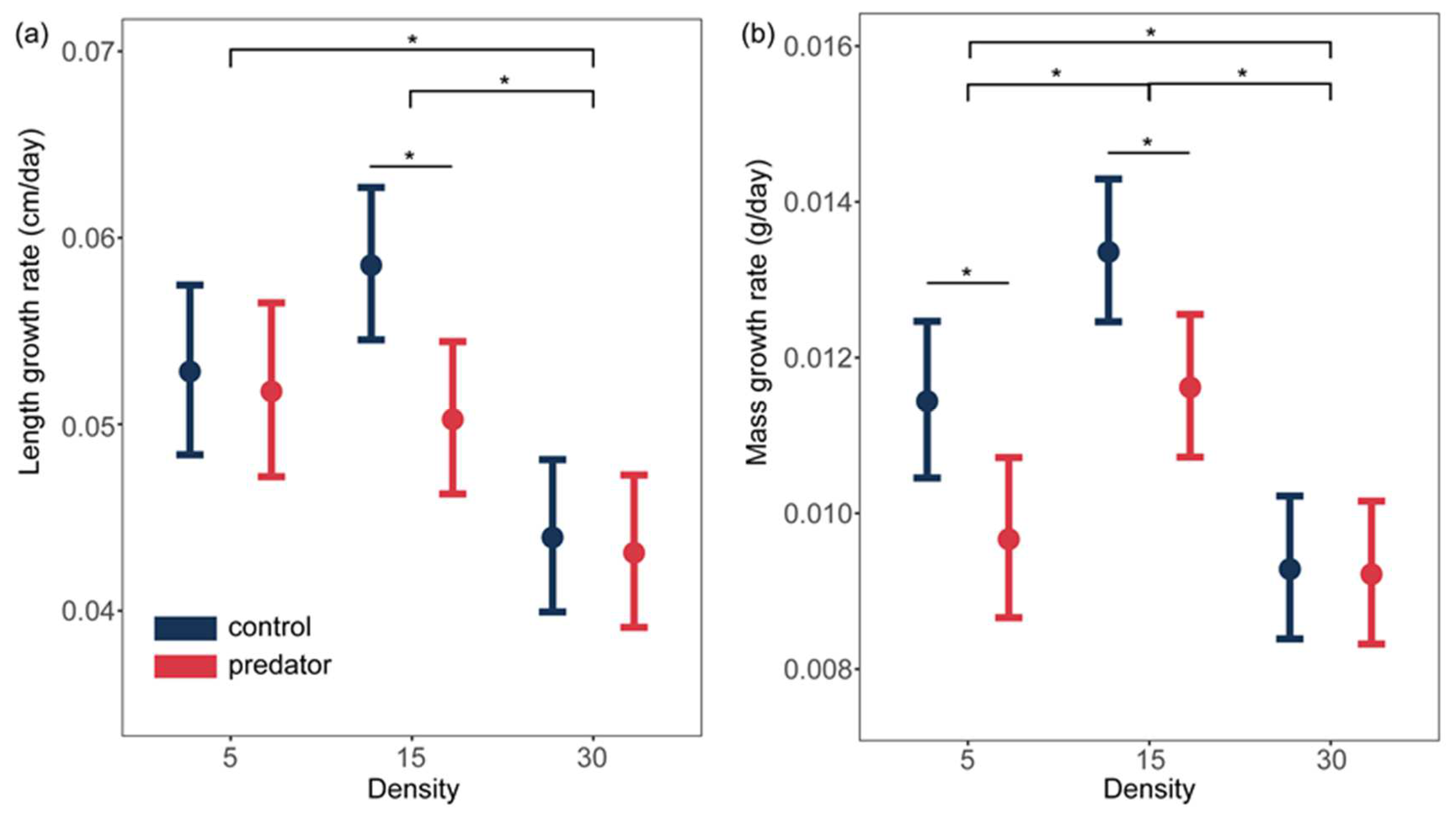
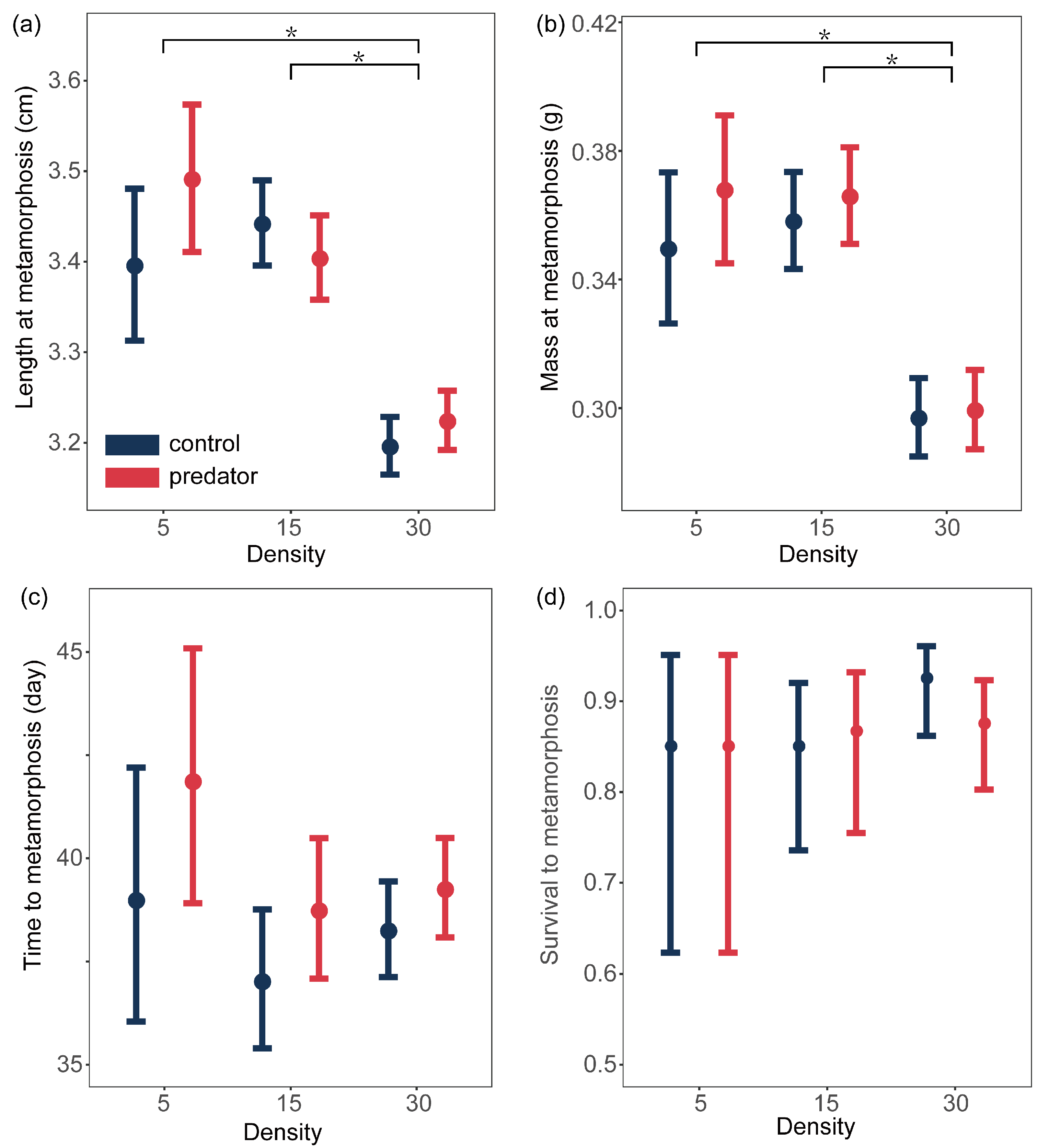
| Response | Fixed Effect | X2 | df | P |
|---|---|---|---|---|
| Length growth rate | Density treatments | 30.453 | 2 | <0.001 |
| Predator treatments | 4.125 | 1 | 0.042 | |
| Density treatments × Predator treatments | 4.027 | 2 | 0.134 | |
| Mass growth rate | Density treatments | 48.946 | 2 | <0.001 |
| Predator treatments | 8.661 | 1 | 0.003 | |
| Density treatments × Predator treatments | 4.252 | 2 | 0.119 | |
| Length at metamorphosis | Density treatments | 136.897 | 2 | <0.001 |
| Predator treatments | 0.678 | 1 | 0.410 | |
| Density treatments × Predator treatments | 4.649 | 2 | 0.098 | |
| Mass at metamorphosis | Density treatments | 98.809 | 2 | <0.001 |
| Predator treatments | 1.052 | 1 | 0.305 | |
| Density treatments × Predator treatments | 0.726 | 2 | 0.696 | |
| Time to metamorphosis | Density treatments | 4.411 | 2 | 0.110 |
| Predator treatments | 4.378 | 1 | 0.036 | |
| Density treatments × Predator treatments | 0.708 | 2 | 0.702 | |
| Survival to metamorphosis | Density treatments | 1.742 | 2 | 0.419 |
| Predator treatments | 0.569 | 1 | 0.451 | |
| Density treatments × Predator treatments | 1.134 | 2 | 0.567 |
| Predator Treatments | Contrast | Estimate | SE | t/z Value | p Value |
|---|---|---|---|---|---|
| Length growth rate | |||||
| Averaged | 5 vs. 15 | 0.002 | 0.002 | 0.951 | 0.353 |
| 5 vs. 30 | −0.009 | 0.002 | −3.975 | 0.001 | |
| 15 vs. 30 | −0.011 | 0.002 | −5.25 | <0.001 | |
| Control | 5 vs. 15 | 0.006 | 0.003 | 1.839 | 0.081 |
| 5 vs. 30 | −0.009 | 0.003 | −2.87 | 0.014 | |
| 15 vs. 30 | −0.015 | 0.003 | −4.983 | <0.001 | |
| Predator | 5 vs. 15 | −0.002 | 0.003 | −0.478 | 0.638 |
| 5 vs. 30 | −0.009 | 0.003 | −2.753 | 0.036 | |
| 15 vs. 30 | −0.007 | 0.003 | −2.442 | 0.04 | |
| Mass growth rate | |||||
| Averaged | 5 vs. 15 | 0.002 | 0 | 3.937 | 0.001 |
| 5 vs. 30 | −0.001 | 0 | −2.653 | 0.015 | |
| 15 vs. 30 | −0.003 | 0 | −6.96 | <0.001 | |
| Control | 5 vs. 15 | 0.002 | 0.001 | 2.777 | 0.012 |
| 5 vs. 30 | −0.002 | 0.001 | −3.125 | 0.008 | |
| 15 vs. 30 | −0.004 | 0.001 | −6.194 | <0.001 | |
| Predator | 5 vs. 15 | 0.002 | 0.001 | 2.79 | 0.017 |
| 5 vs. 30 | 0.001 | 0.001 | −0.643 | 0.528 | |
| 15 vs. 30 | −0.002 | 0.001 | −3.649 | 0.007 | |
| Length at metamorphosis | |||||
| Averaged | 5 vs. 15 | −0.021 | 0.034 | −0.606 | 0.546 |
| 5 vs. 30 | −0.234 | 0.032 | −7.32 | <0.001 | |
| 15 vs. 30 | −0.213 | 0.02 | −10.417 | <0.001 | |
| Control | 5 vs. 15 | 0.046 | 0.049 | 0.943 | 0.348 |
| 5 vs. 30 | −0.2 | 0.046 | −4.381 | <0.001 | |
| 15 vs. 30 | −0.246 | 0.029 | −8.521 | <0.001 | |
| Predator | 5 vs. 15 | −0.088 | 0.048 | −1.835 | 0.069 |
| 5 vs. 30 | −0.267 | 0.045 | −5.991 | <0.001 | |
| 15 vs. 30 | −0.18 | 0.029 | −6.214 | <0.001 | |
| Mass at metamorphosis | |||||
| Averaged | 5 vs. 15 | 0.003 | 0.01 | 0.33 | 0.743 |
| 5 vs. 30 | −0.061 | 0.009 | −6.407 | <0.001 | |
| 15 vs. 30 | −0.064 | 0.007 | −9.145 | <0.001 | |
| Control | 5 vs. 15 | 0.009 | 0.014 | 0.602 | 0.55 |
| 5 vs. 30 | −0.053 | 0.013 | −3.91 | <0.001 | |
| 15 vs. 30 | −0.061 | 0.01 | −6.199 | <0.001 | |
| Predator | 5 vs. 15 | −0.002 | 0.014 | −0.141 | 0.888 |
| 5 vs. 30 | −0.069 | 0.013 | −5.159 | <0.001 | |
| 15 vs. 30 | −0.067 | 0.01 | −6.734 | <0.001 | |
| Time to metamorphosis | |||||
| Averaged | 5 vs. 15 | −0.065 | 0.032 | −2.036 | 0.125 |
| 5 vs. 30 | −0.042 | 0.03 | −1.416 | 0.235 | |
| 15 vs. 30 | 0.023 | 0.019 | 1.179 | 0.239 | |
| Control | 5 vs. 15 | −0.052 | 0.046 | −1.117 | 0.396 |
| 5 vs. 30 | −0.019 | 0.043 | −0.446 | 0.656 | |
| 15 vs. 30 | 0.033 | 0.028 | 1.174 | 0.396 | |
| Predator | 5 vs. 15 | −0.078 | 0.044 | −1.783 | 0.168 |
| 5 vs. 30 | −0.065 | 0.041 | −1.589 | 0.168 | |
| 15 vs. 30 | 0.013 | 0.027 | 0.487 | 0.626 | |
| Survival to metamorphosis | |||||
| Averaged | 5 vs. 15 | 0.069 | 0.515 | 0.133 | 0.894 |
| 5 vs. 30 | 0.495 | 0.495 | 0.999 | 0.477 | |
| 15 vs. 30 | 0.426 | 0.343 | 1.241 | 0.477 | |
| Control | 5 vs. 15 | 0 | 0.723 | 0 | 1 |
| 5 vs. 30 | 0.778 | 0.716 | 1.087 | 0.416 | |
| 15 vs. 30 | 0.778 | 0.501 | 1.553 | 0.361 | |
| Predator | 5 vs. 15 | 0.137 | 0.732 | 0.187 | 0.875 |
| 5 vs. 30 | 0.211 | 0.684 | 0.309 | 0.875 | |
| 15 vs. 30 | 0.074 | 0.469 | 0.158 | 0.875 |
| Density Treatments | Estimate | SE | t/z Value | p Value |
|---|---|---|---|---|
| Length growth rate | ||||
| 5 | 0.001 | 0.003 | 0.322 | 0.75 |
| 15 | 0.008 | 0.003 | 2.823 | 0.012 |
| 30 | 0.001 | 0.003 | 0.282 | 0.782 |
| Mass growth rate | ||||
| 5 | 0.002 | 0.001 | 2.429 | 0.023 |
| 15 | 0.002 | 0.001 | 2.644 | 0.018 |
| 30 | 0 | 0.001 | 0.099 | 0.922 |
| Length at metamorphosis | ||||
| 5 | −0.095 | 0.059 | −1.606 | 0.11 |
| 15 | 0.038 | 0.034 | 1.135 | 0.265 |
| 30 | −0.028 | 0.023 | −1.207 | 0.264 |
| Mass at metamorphosis | ||||
| 5 | −0.018 | 0.017 | −1.092 | 0.278 |
| 15 | −0.008 | 0.011 | −0.715 | 0.483 |
| 30 | −0.002 | 0.009 | −0.27 | 0.793 |
| Time to metamorphosis | ||||
| 5 | −0.071 | 0.055 | −1.3 | 0.194 |
| 15 | −0.045 | 0.032 | −1.41 | 0.159 |
| 30 | −0.026 | 0.022 | −1.186 | 0.236 |
| Survival to metamorphosis | ||||
| 5 | 0 | 0.886 | 0 | 1 |
| 15 | −0.137 | 0.524 | −0.262 | 0.794 |
| 30 | 0.566 | 0.443 | 1.278 | 0.201 |
| Predator Treatments | Density Treatments | Length Growth Rate (cm/Day) | Mass Growth Rate (g/Day) | Length at Metamorphosis (cm) | Mass at Metamorphosis (g) | Time to Metamorphosis (Day) | Metamorphosis/Total |
|---|---|---|---|---|---|---|---|
| Control | 5 | 0.053 ± 0.01 | 0.011 ± 0.002 | 3.397 ± 0.193 | 0.350 ± 0.043 | 39 ± 2 | N = 16/20 (80%) |
| 15 | 0.059 ± 0.006 | 0.013 ± 0.002 | 3.443 ± 0.141 | 0.358 ± 0.041 | 37 ± 2 | N = 51/60 (88%) | |
| 30 | 0.044 ± 0.005 | 0.009 ± 0.001 | 3.197 ± 0.179 | 0.297 ± 0.045 | 38 ± 3 | N = 111/120 (92.5%) | |
| Predator | 5 | 0.051 ± 0.007 | 0.010 ± 0.002 | 3.492 ± 0.2 | 0.367 ± 0.055 | 42 ± 4 | N = 17/20 (85%) |
| 15 | 0.050 ± 0.008 | 0.012 ± 0.001 | 3.405 ± 0.157 | 0.366 ± 0.044 | 39 ± 2 | N = 52/60 (86.67%) | |
| 30 | 0.043 ± 0.005 | 0.009 ± 0.001 | 3.225 ± 0.173 | 0.299 ± 0.045 | 39 ± 3 | N = 105/120 (87.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Qiu, X.; Li, W.; Feng, S.; Xu, A. Density Mediates the Predator-Induced Growth and Metamorphic Plasticity of Chinhai Spiny Newt Larvae. Animals 2024, 14, 1510. https://doi.org/10.3390/ani14101510
Zhu X, Qiu X, Li W, Feng S, Xu A. Density Mediates the Predator-Induced Growth and Metamorphic Plasticity of Chinhai Spiny Newt Larvae. Animals. 2024; 14(10):1510. https://doi.org/10.3390/ani14101510
Chicago/Turabian StyleZhu, Xihong, Xia Qiu, Wei Li, Shiyan Feng, and Aichun Xu. 2024. "Density Mediates the Predator-Induced Growth and Metamorphic Plasticity of Chinhai Spiny Newt Larvae" Animals 14, no. 10: 1510. https://doi.org/10.3390/ani14101510






