Use of Short-Term CIDR-Based Protocols for Oestrus Synchronisation in Goats at Tropical and Subtropical Latitudes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Effectiveness of CIDR Compared with Intravaginal Progestogen-Impregnated Sponges
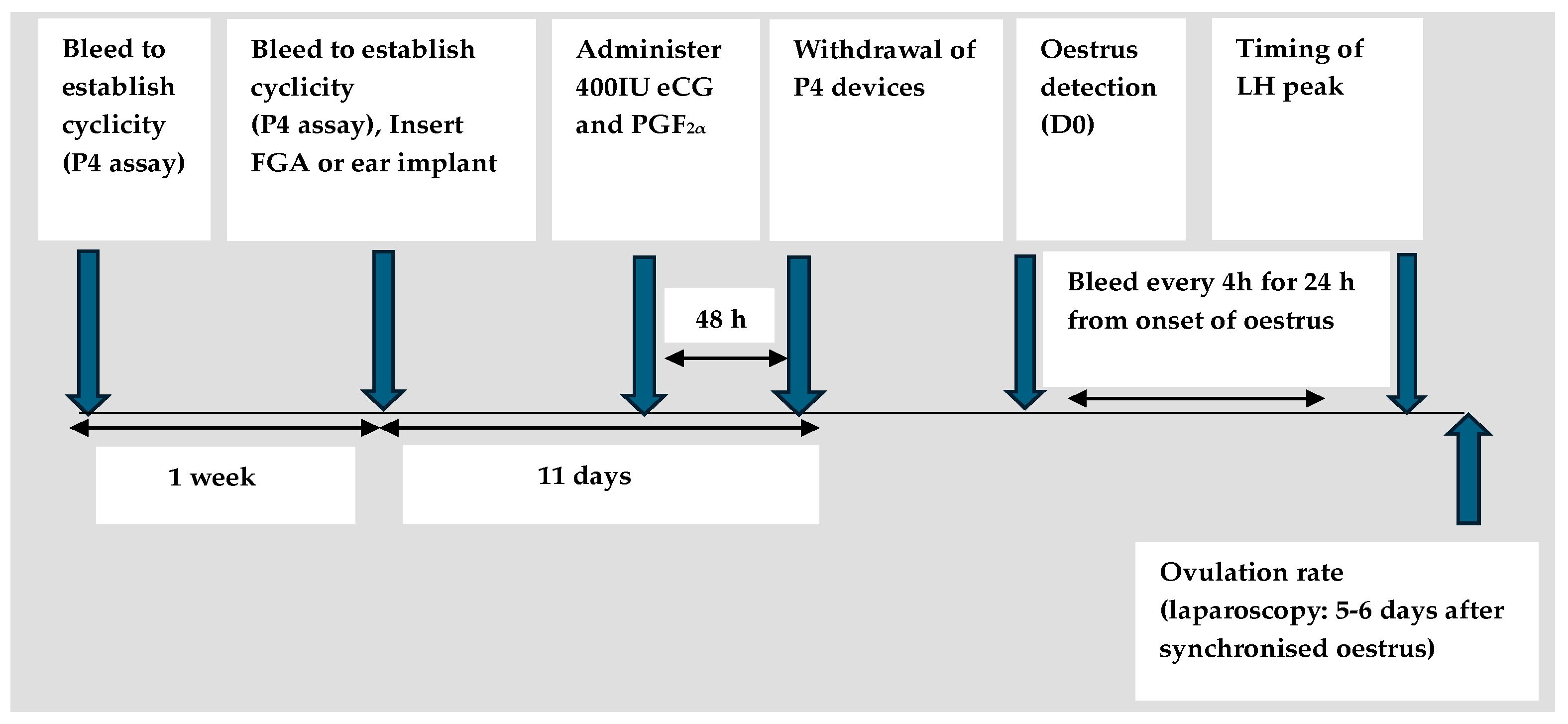
3. Effectiveness of Previously Used CIDR Devices
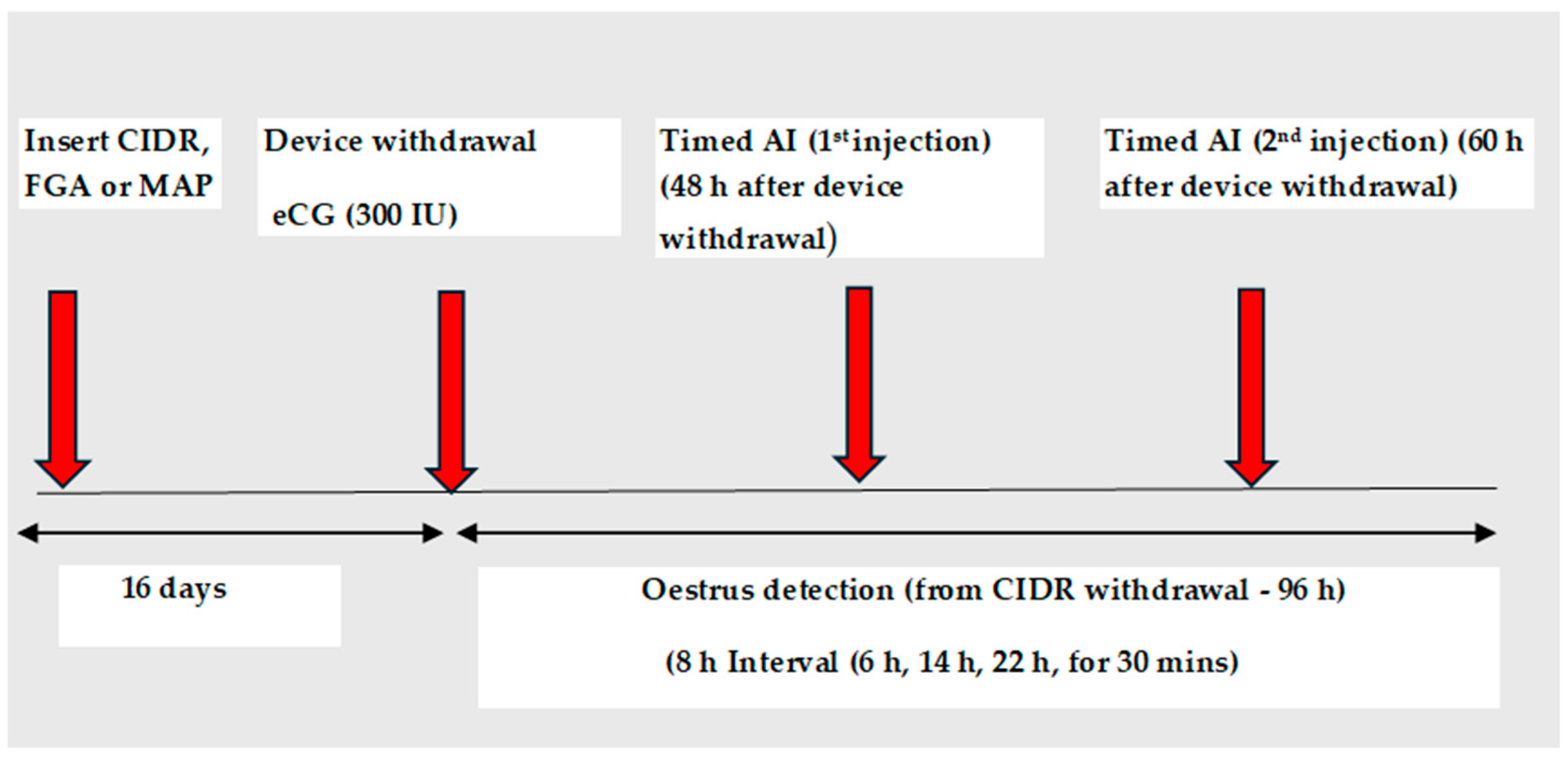
4. Effectiveness of Short-Term CIDR Protocols with or without eCG
5. Limitations of eCG Use and Alternatives and Challenges Associated with the Non-Breeding Season
5.1. Limitations of eCG Use
5.2. GnRH as an Alternative to eCG
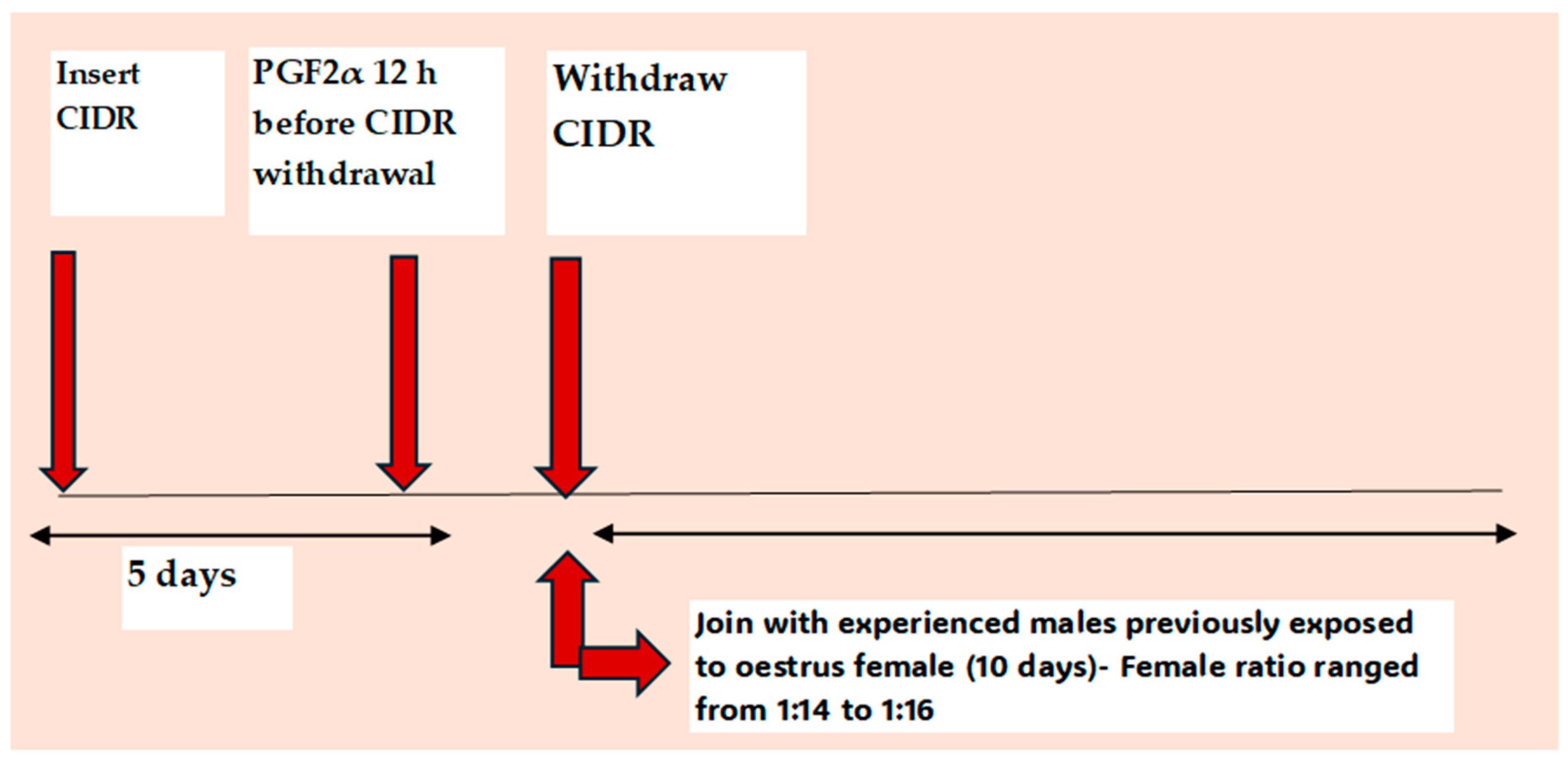
5.3. Male Effect as an Alternative to eCG
5.4. Use of Short-Term Nutritional Supplementation to Increase Ovulation Rate and Improve Embryo Quality and Fertility
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Livestock Primary (National-Global-Annual)-FAOSTAT—“FAO Catalog”. Available online: https://data.apps.fao.org/catalog/dataset/bd527657-ac64-4899-9f9b-12924f246bc0/resource/9bb4a69e-9d5a-401d-8dad-1f3931783235 (accessed on 28 March 2024).
- Escareño, L.; Salinas-Gonzalez, H.; Wurzinger, M.; Iñiguez, L.; Sölkner, J.; Meza-Herrera, C. Dairy goat production systems. Trop. Anim. Health Prod. 2012, 45, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Knights, M.; Garcia, G.W. The status and characteristics of the goat (Capra hircus) and its potential role as a significant milk producer in the tropics: A review. Small Rumin. Res. 1997, 26, 203–215. [Google Scholar] [CrossRef]
- Fatet, A.; Pellicer-Rubio, M.T.; Leboeuf, B. Reproductive cycle of goats. Anim. Reprod. Sci. 2011, 124, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Restall, B.J. Seasonal variation in reproductive activity in Australian goats. Anim. Reprod. Sci. 1992, 27, 305–318. [Google Scholar] [CrossRef]
- Balaro, M.F.A.; de Mello, S.G.V.; Santos, A.d.S.; Cavalcanti, L.M.; Almosny, N.R.P.; Fonseca, J.F.; Brandão, F.Z. Reproductive seasonality in Saanen goats kept under tropical conditions. Trop. Anim. Health Prod. 2019, 51, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.; Onder, H. The effect of estrus synchronization programmes on parturition time and some reproductive characteristics of Saanen goats. J. Appl. Anim. Res. 2016, 44, 376–379. [Google Scholar] [CrossRef]
- Brunet, A.G.; Santiago-Moreno, J.; Toledano-Diaz, A.; Sebastian, L. Reproductive seasonality and its control in Spanish sheep and goats. Trop. Subtrop. Agroecosyst. 2011, 15 (Suppl. S1), S47–S70. [Google Scholar]
- Abecia, J.A.; Forcada, F.; González-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Forcada, F.; González-Bulnes, A. Pharmaceutical control of reproduction in sheep and goats. Vet. Clin. Food Anim. Pract. 2011, 27, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sebastián, A.; Coloma, M.; Toledano, A.; Santiago-Moreno, J. Hormone-free Protocols for the Control of Reproduction and Artificial Insemination in Goats. Reprod. Domest. Anim. 2014, 49, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Menchaca, A.; Martin, G.B.; Martinez-Ros, P. Seventy years of progestagen treatments for management of the sheep oestrous cycle: Where we are and where we should go. Reprod. Fertil. Dev. 2020, 32, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Gamboa, D.; Zarco, L.; Ungerfeld, R. Response to the buck effect in goats primed with CIDRs, previously used CIDRs, or previously used autoclaved CIDRs during the non-breeding season. Livest. Sci. 2013, 155, 459–462. [Google Scholar] [CrossRef]
- Alvarez, L.; Gamboa, D.; Zarco, L.; Ungerfeld, R. Impact of short nutrient stimuli with different energy source on follicle dynamics and quality of oocyte from hormonally stimulated goats. Reprod. Domest. Anim. 2019, 54, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.P.M.; Fernandes, C.C.L.; Rossetto, R.; da Silva, C.P.; Galvão, I.T.O.M.; Bertolini, M.; Rondina, D. Hormonal treatments for the synchronisation of oestrus in dairy goats raised in the tropics. Reprod. Fertil. Dev. 2004, 16, 415–420. [Google Scholar] [CrossRef]
- Knights, M.; Singh-Knights, D. Use of controlled internal drug releasing (CIDR) devices to control reproduction in goats: A review. Anim. Sci. J. 2016, 87, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, A.B.P.; Morais, M.C.C.; Rangel, P.S.C.; Oliveira, M.E.F.; Souza-Fabjan, J.M.G.; Fonseca, J.F. Effect of eCG in a short-term synchronization treatment on ovarian status, estrus synchrony, and ovulation in dairy goats managed under tropical conditions. Trop. Anim. Health Prod. 2021, 53, 246. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Kadokawa, H. “Clean, Green and Ethical” Animal Production. Case Study: Reproductive Efficiency in Small Ruminants. J. Reprod. Dev. 2006, 52, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Greyling, J.P.C.; Van Niekerk, C.H. Different synchronization techniques in Boer goat does outside the normal breeding season. Small Rumin. Res. 1991, 5, 233–243. [Google Scholar] [CrossRef]
- Wheaton, J.E.; Carlson, K.M.; Windels, H.F.; Johnston, L.J. CIDR: A new progesterone-releasing intravaginal device for induction of estrus and cycle control in sheep and goats. Anim. Reprod. Sci. 1993, 33, 127–141. [Google Scholar] [CrossRef]
- Romano, J.E. Synchronization of estrus using CIDR, FGA or MAP intravaginal pessaries during the breeding season in Nubian goats. Small Rumin. Res. 2004, 55, 15–19. [Google Scholar] [CrossRef]
- Menchaca, A.; Miller, V.; Salveraglio, V.; Rubianes, E. Endocrine, luteal and follicular responses after the use of the Short-Term Protocol to synchronize ovulation in goats. Anim. Reprod. Sci. 2007, 102, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Gore, D.L.M.; Mburu, J.N.; Okeno, T.O.; Muasya, T.K. Short-term oestrous synchronisation protocol following single fixed-time artificial insemination and natural mating as alternative to long-term protocol in dairy goats. Small Rumin. Res. 2020, 192, 106207. [Google Scholar] [CrossRef]
- Vilariño, M.; Rubianes, E.; Menchaca, A. Re-use of intravaginal progesterone devices associated with the Short-term Protocol for timed artificial insemination in goats. Theriogenology 2011, 75, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Motlomelo, K.C.; Greyling, J.P.C.; Schwalbach, L.M.J. Synchronisation of oestrus in goats: The use of different progestagen treatments. Small Rumin. Res. 2002, 45, 45–49. [Google Scholar] [CrossRef]
- Greyling, J.P.C.; Brink, W.C.J. Synchronization of oestrus in sheep: The use of controlled internal drug release (CIDR) dispensers. S. Afr. J. Anim. Sci. 1987, 17, 128–132. [Google Scholar]
- Ungerfeld, R.; Rubianes, E. Short term primings with different progestogen intravaginal devices (MAP, FGA and CIDR) for eCG-estrous induction in anestrus ewes. Small Rumin. Res. 2002, 46, 63–66. [Google Scholar] [CrossRef]
- Gardón, J.C.; Simonetti, L. Residual levels on medroxyprogesterone acetate-impregnated sponges after estrus synchronization treatment and their relationship with fertility in cyclic goats. Braz. J. Vet. Res. Anim. Sci. São Paulo 1997, 34, 163–166. Available online: https://www.revistas.usp.br/bjvras/article/download/50287/54400/62190 (accessed on 12 May 2024). [CrossRef]
- Simonetti, L.; Gardón, J.C.; Ramos, G. Residual levels on medroxyprogesterone acetate (MAP) impregnated sponges after estrus synchronization treatment in cyclic ewes. Braz. J. Vet. Res. Anim. Sci. 1999, 36, 240–243. Available online: https://www.scielo.br/j/bjvras/a/bMCJkqDNzTJ8PHXZXGpGfWk/?lang=en (accessed on 12 May 2024). [CrossRef]
- Baril, G.; Saumande, J. Hormonal treatments to control time of ovulation and fertility of goats. In Proceedings of the 7th International Conference on Goats, Tours, France, 14 May 2000; pp. 15–21. Available online: https://www.researchgate.net/profile/Baril-Gerard-2/publication/263580415_Baril_G_Saumande_J_2000_Hormonal_treatments_to_control_time_of_ovulation_and_fertility_of_goats_7th_International_Conference_on_Goats_15-21_mai_2000_Tours_France_Vol1_p400-405/links/0a85e53b56ce88f219000000/Baril-G-Saumande-J-2000-Hormonal-treatments-to-control-time-of-ovulation-and-fertility-of-goats-7th-International-Conference-on-Goats-15-21-mai-2000-Tours-France-Vol1-p400-405 (accessed on 12 May 2024).
- Greyling, J.P.C.; Van der Nest, M. Synchronization of oestrus in goats: Dose effect of progestagen. Small Rumin. Res. 2000, 36, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.J.F.; Baril, G.; Saumande, J. Estrus synchronization in dairy goats: Use of fluorogestone acetate vaginal sponges or norgestomet ear implants. Anim. Reprod. Sci. 1997, 46, 237–244. [Google Scholar] [CrossRef]
- Simonetti, L.; Blanco, M.R.; Gardón, J.C. Estrus synchronization in ewes treated with sponges impregnated with different doses of medroxyprogesterone acetate. Small Rumin. Res. 2000, 38, 243–247. Available online: https://www.sciencedirect.com/science/article/pii/S0921448800001607 (accessed on 12 May 2024). [CrossRef]
- Mayorga, I.; Mourad, R.; Mara, L.; Gallus, M.; Ulutaş, Z.; Dattena, M. Organic breeding in Sarda ewes: Utilization of the ram effect in an artificial insemination program. Small Rumin. Res. 2019, 174, 131–134. [Google Scholar] [CrossRef]
- Oliveira, J.; Martins, G.; Esteves, L.; Penna, B.; Hamond, C.; Fonseca, J.; Rodrigues, A.; Brandão, F.; Lilenbaum, W. Changes in the vaginal flora of goats following a short-term protocol of oestrus induction and synchronisation with intravaginal sponges as well as their antimicrobial sensitivity. Small Rumin. Res. 2013, 113, 162–166. Available online: https://www.sciencedirect.com/science/article/pii/S0921448813000813 (accessed on 30 March 2024). [CrossRef]
- Penna, B.; Libonati, H.; Director, A.; Sarzedas, A.C.; Martins, G.; Brandão, F.Z.; Fonseca, J.; Lilenbaum, W. Progestin-impregnated intravaginal sponges for estrus induction and synchronization influences on goats vaginal flora and antimicrobial susceptibility. Anim. Reprod. Sci. 2013, 142, 71–74. Available online: https://www.sciencedirect.com/science/article/pii/S0378432013002704 (accessed on 30 March 2024). [CrossRef] [PubMed]
- Manes, J.; Ríos, G.; Fiorentino, M.A.; Ungerfeld, R. Vaginal mucus from ewes treated with progestogen sponges affects quality of ram spermatozoa. Theriogenology 2016, 85, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Casamassima, D.; D’Alessandro, A.G. Synchronization of oestrus in goats with Progestogen sponges and short term combined FGA, PGF2α protocols. Int. J. Anim. Vet. Sci. 2011, 5, 326–328. [Google Scholar]
- Fleisch, A.; Werne, S.; Heckendorn, F.; Hartnack, S.; Piechotta, M.; Bollwein, H.; Thun, R.; Janett, F. Comparison of 6-day progestagen treatment with Chronogest® CR and Eazi-breedTM CIDR® G intravaginal inserts for estrus synchronization in cyclic ewes. Small Rumin. Res. 2012, 107, 141–146. [Google Scholar] [CrossRef]
- Cox, J.; Allende, R.; Lara, E.; Leiva, A.; Díaz, T.; Dorado, J.; Saravia, F. Follicular Dynamics, Interval to Ovulation and Fertility After AI in Short-Term Progesterone and PGF2α Oestrous Synchronization Protocol in Sheep. Reprod. Domest. Anim. 2012, 47, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Letelier, C.; Mallo, F.; Encinas, T.; Ros, J.M.; Gonzalez-Bulnes, A. Glucogenic supply increases ovulation rate by modifying follicle recruitment and subsequent development of preovulatory follicles without effects on ghrelin secretion. Reproduction 2008, 136, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.A.; Saadeldin, I.M.; Moumen, A.F.; Ali, M.A.; Alowaimer, A.N. Efficacy of controlled internal drug release (CIDR) treatment durations on the reproductive performance, hormone profiles, and economic profit of Awassi ewes. Small Rumin. Res. 2018, 166, 47–52. [Google Scholar] [CrossRef]
- Souza, J.; Torres, C.; Maia, A.; Brandão, F.; Bruschi, J.; Viana, J.; Oba, E.; Fonseca, J. Autoclaved, previously used intravaginal progesterone devices induces estrus and ovulation in anestrous Toggenburg goats. Anim. Reprod. Sci. 2011, 129, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Neville, T.; Mercadante, V.; Waters, K.; Lamb, G.; Dahlen, C.; Redden, R. Efficacy of various five-day estrous synchronization protocols in sheep. Small Rumin. Res. 2014, 120, 100–107. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Gamboa, D.; Álvarez, L. Response of ewes primed with new CIDRs, previously used CIDRs, or previously used and autoclaved CIDRs to the ram effect during the non-breeding season. Anim. Reprod. 2018, 10, 704–707. [Google Scholar]
- Viñoles, C.; Forsberg, M.; Banchero, G.; Rubianes, E. Effect of long-term and short-term progestagen treatment on follicular development and pregnancy rate in cyclic ewes. Theriogenology 2001, 55, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.L.; Guido, S.I.; Lima, P.F. Comparison of different protocols used to induce and synchronize estrus cycle of Saanen goats. Small Rumin. Res. 2001, 40, 149–153. [Google Scholar] [CrossRef]
- Souza-Fabjan, J.M.G.; Torres, C.A.A.; Maia, A.L.R.S.; Brandão, F.Z.; Oba, E.; Bertoldo, M.J.; Fonseca, J.F. Re-used progesterone devices efficiently synchronise oestrus and ovulation after autoclaving process in Toggenburg goats during the breeding season. Anim. Prod. Sci. 2014, 55, 818–822. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Clemente, N.; Orihuela, A.; Ungerfeld, R.; Clemente, N.; Orihuela, A. Treatments with eCG and courtship behaviour in rams during the breeding and the non-breeding seasons. Anim. Prod. Sci. 2018, 59, 865–869. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Farmer, S.W.; Papkoff, H.; Stewart, F.; Allen, W.R. Purification and Characterization of the Gonadotropin Secreted by Cultured Horse Trophoblast Cells. Endocrinology 1980, 106, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.D.; Martinuk, S.D. Equine chorionic gonadotropin. Endocr. Rev. 1991, 12, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Bister, J.L.; Noël, B.; Perrad, B.; Mandiki, S.N.M.; Mbayahaga, J.; Paquay, R. Control of ovarian follicles activity in the ewe. Domest. Anim. Endocrinol. 1999, 17, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ayres, H.; Oliveira, L.; Barros, F.; Oba, E.; Bicudo, S.; Bartlewski, P.; Fonseca, J.; Vicente, W. Effects of season and ovarian status on the outcome of long-term progesterone-based estrus synchronization protocols and ovulatory follicle development in Santa Inês ewes under subtropical conditions. Theriogenology 2016, 85, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zarkawi, M.; Al-Merestani, M.R.; Wardeh, M.F. Induction of synchronized oestrous in indigenous Damascus goats outside the breeding season. Small Rumin. Res. 1999, 33, 193–197. [Google Scholar] [CrossRef]
- Fonseca, J.F.; Souza-Fabjan, J.M.; Oliveira, M.E.F.; Cruz, R.C.; Esteves, L.V.; de Paiva, M.P.S.M.; Brandão, F.Z.; Mancio, A.B. Evaluation of cervical mucus and reproductive efficiency of seasonally anovular dairy goats after short-term progestagen-based estrous induction protocols with different gonadotropins. Reprod. Biol. 2017, 17, 363–369. [Google Scholar] [CrossRef] [PubMed]
- de Bulnes, A.G.; Moreno, J.S.; Gomez-Brunet, A.; Inskeep, E.K.; Townsend, E.C.; Lopez-Sebastian, A. Follicular dynamics during the oestrous cycle in dairy goats. Anim. Sci. 1999, 68, 547–554. [Google Scholar] [CrossRef]
- Menchaca, A.; Cuadro, F.; dos Santos-Neto, P.C.; Bosolasco, D.; Barrera, N.; de Brun, V.; Crispo, M. Oocyte developmental competence is improved by relatively greater circulating progesterone concentrations during preovulatory follicular growth. Anim. Reprod. Sci. 2018, 195, 321–328. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Gonzalez-Bulnes, A.; Tresguerres, J.A.F.; Dominguez, V.; Ariznavarreta, C.; Cocero, M.J. Causes, characteristics and consequences of anovulatory follicles in superovulated sheep. Domest. Anim. Endocrinol. 2006, 30, 76–87. Available online: https://www.sciencedirect.com/science/article/pii/S0739724005001335 (accessed on 12 May 2024). [CrossRef] [PubMed]
- Martinez-Ros, P.; Rios-Abellan, A.; Gonzalez-Bulnes, A. Influence of progesterone-treatment length and eCG administration on appearance of estrous behavior, ovulatory success and fertility in sheep. Animals 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ros, P.; Gonzalez-Bulnes, A. Efficiency of CIDR-Based Protocols Including GnRH Instead of eCG for Estrus Synchronization in Sheep. Animals 2019, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.A.; Austin, E.J.; Crowe, M.A. Oestrous synchronisation in cattle—Current options following the EU regulations restricting use of oestrogenic compounds in food-producing animals: A review. Anim. Reprod. Sci. 2008, 109, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bodin, L.; Drion, P.V.; Remy, B.; Brice, G.; Cognié, Y.; Beckers, J.F. Anti-PMSG antibody levels in sheep subjected annually to oestrus synchronisation. Reprod. Nutr. Dev. 1997, 37, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Drion, P.V.; Furtoss, V.; Baril, G.; Manfredi, E.; Bouvier, F.; Pougnard, J.-L.; Bernelas, D.; Caugnon, P.; McNamara, E.M.; Remy, B.; et al. Four years of induction/synchronization of estrus in dairy goats: Effect on the evolution of eCG binding rate in relation with the parameters of reproduction. Reprod. Nutr. Dev. 2001, 41, 401–412. [Google Scholar] [CrossRef]
- Anel, L.; Kaabi, M.; Abroug, B.; Alvarez, M.; Anel, E.; Boixo, J.; de la Fuente, L.; de Paz, P. Factors influencing the success of vaginal and laparoscopic artificial insemination in churra ewes: A field assay. Theriogenology 2005, 63, 1235–1247. [Google Scholar] [CrossRef]
- Hameed, N.; Khan, M.I.-U.; Ahmad, W.; Abbas, M.; Murtaza, A.; Shahzad, M.; Ahmad, N. Follicular dynamics, estrous response and pregnancy rate following GnRH and progesterone priming with or without eCG during non-breeding season in anestrous Beetal goats. Small Rumin. Res. 2020, 182, 73–77. [Google Scholar] [CrossRef]
- Año-Perello, A.; Santos-Jimenez, Z.; Encinas, T.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Use of GnRH for Synchronization of the Follicular Wave in Assisted Reproductive Technologies in Sheep: A Preliminary Study. Animals 2020, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Santos-Jimenez, Z.; Martinez-Herrero, C.; Encinas, T.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Comparative efficiency of oestrus synchronization in sheep with progesterone/eCG and progesterone/GnRH during breeding and non-breeding season. Reprod. Domest. Anim. 2020, 55, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Sallam, S.M. Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus. Domest. Anim. Endocrinol. 2020, 71, 106390. Available online: https://www.sciencedirect.com/science/article/pii/S0739724019300682 (accessed on 5 February 2024). [CrossRef] [PubMed]
- Hashem, N.M.; EL-Sherbiny, H.R.; Fathi, M.; Abdelnaby, E.A. Nanodelivery System for Ovsynch Protocol Improves Ovarian Response, Ovarian Blood Flow Doppler Velocities, and Hormonal Profile of Goats. Animals 2022, 12, 1442. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Hawy, A.S.; El-Bassiony, M.F.; El-Hamid, I.S.A.; Gonzalez-Bulnes, A.; Martinez-Ros, P. Use of GnRH-Encapsulated Chitosan Nanoparticles as an Alternative to eCG for Induction of Estrus and Ovulation during Non-Breeding Season in Sheep. Biology 2023, 12, 351. [Google Scholar] [CrossRef]
- Zarazaga, L.A.; Gatica, M.C.; Gallego-Calvo, L.; Celi, I.; Guzmán, J.L. The timing of oestrus, the preovulatory LH surge and ovulation in Blanca Andaluza goats synchronised by intravaginal progestagen sponge treatment is modified by season but not by body condition score. Anim. Reprod. Sci. 2014, 146, 170–175. [Google Scholar] [CrossRef]
- Nogueira, D.M.; Cavalieri, J.; Fitzpatrick, L.A.; Gummow, B.; Blache, D.; Parker, A.J. Effect of hormonal synchronisation and/or short-term supplementation with maize on follicular dynamics and hormone profiles in goats during the non-breeding season. Anim. Reprod. Sci. 2016, 171, 87–97. [Google Scholar] [CrossRef]
- Greyling, J.P.C.; Erasmus, J.A.; Taylor, G.J.; Van der Merwe, S. Synchronization of estrus in sheep using progestagen and inseminating with chilled semen during the breeding season. Small Rumin. Res. 1997, 26, 137–143. [Google Scholar] [CrossRef]
- Delgadillo, J.A.; Gelez, H.; Ungerfeld, R.; Hawken, P.A.R.; Martin, G.B. The ‘male effect’ in sheep and goats—Revisiting the dogmas. Behav. Brain Res. 2009, 200, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.A.; Vélez, L.I.; Flores, J.A. Continuous light after a long-day treatment is equivalent to melatonin implants to stimulate testosterone secretion in Alpine male goats. Animal 2016, 10, 649–654. [Google Scholar] [CrossRef]
- Netto, M.M.; Balaro, M.F.A.; Cosentino, I.O.; Santo, C.G.D.E.; de Oliveira, R.V.; Souza-Fabjan, J.M.G.; Brandao, F.Z.; Fonseca, J.F. Use of two cloprostenol administrations 11.5 days apart efficiently synchronizes oestrus in photostimulated multiparous dairy goats in the non-breeding season. Reprod. Domest. Anim. 2020, 55, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; D’Alessandro, A.G. Synchronization of oestrus and ovulation by short time combined FGA, PGF2α, GnRH, eCG treatments for natural service or AI fixed-time. Anim. Reprod. Sci. 2011, 123, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zarazaga, L.Á.; Gatica, M.C.; Gallego-Calvo, M.L.; Guzmán, J.L. When using photostimulated bucks to induce the male effect in female goats living at Mediterranean latitudes, a male: Female ratio of 1:20 is optimum. J. Appl. Anim. Res. 2018, 46, 883–887. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.; Ángel-García, O.; Guillén-Muñoz, J.M.; Robles-Trillo, P.A.; Santiago-Miramontes, M.d.L.A.D.; Meza-Herrera, C.A.; Mellado, M.; Véliz, F.G. Estrus induction in anestrous mixed-breed goats using the “female-to-female effect”. Trop. Anim. Health Prod. 2013, 45, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.; Bollwein, H.; Piechotta, M.; Janett, F. Reproductive performance of Lacaune dairy sheep exposed to artificial long days followed by natural photoperiod without and with additional progestagen treatment during the nonbreeding season. Theriogenology 2015, 83, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Rubio, M.T.; Boissard, K.; Forgerit, Y.; Pougnard, J.L.; Bonné, J.L.; Leboeuf, B. Evaluation of hormone-free protocols based on the “male effect” for artificial insemination in lactating goats during seasonal anestrus. Theriogenology 2016, 85, 960–969. [Google Scholar] [CrossRef]
- Habibizad, J.; Riasi, A.; Kohram, H.; Rahmani, H.R. Effect of long-term or short-term supplementation of high energy or high energy-protein diets on ovarian follicles and blood metabolites and hormones in ewes. Small Rumin. Res. 2015, 132, 37–43. [Google Scholar] [CrossRef]
- Berlinguer, F.; Gonzalez-Bulnes, A.; Contreras-Solis, I.; Spezzigu, A.; Torres-Rovira, L.; Succu, S.; Naitana, S.; Leoni, G.G. Glucogenic supply increases oocyte developmental competence in sheep. Reprod. Fertil. Dev. 2012, 24, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Porcu, C.; Sotgiu, F.D.; Pasciu, V.; Cappai, M.G.; Barbero-Fernandez, A.; Gonzalez-Bulnes, A.; Dattena, M.; Gallus, M.; Molle, G.; Berlinguer, F. Administration of glycerol-based formulations in sheep results in similar ovulation rate to eCG but red blood cell indices may be affected. BMC Vet. Res. 2020, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Rodríguez, G.; De Santiago-Miramontes, M.A.; Scaramuzzi, R.J.; Malpaux, B.; Delgadillo, J.A. Nutritional supplementation improves ovulation and pregnancy rates in female goats managed under natural grazing conditions and exposed to the male effect. Anim. Reprod. Sci. 2009, 116, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Vargas-Beltran, F.; Vergara-Hernandez, H.P.; Macias-Cruz, U.; Avendaño-Reyes, L.; Rodriguez-Martinez, R.; Arellano-Rodriguez, G.; Veliz-Deras, F.G. Betacarotene supplementation increases ovulation rate without an increment in LH secretion in cyclic goats. Reprod. Biol. 2013, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gore, D.L.M.; Lehloenya, K.C. β-carotene supplementation increases progesterone concentration and glutathione peroxidase activity following alternative progesterone primed oestrous synchronization protocol in goats. Am. J. Anim. Veter Sci. 2020, 15, 211–219. [Google Scholar] [CrossRef]
- Mahla, A.S.; Chaudhari, R.K.; Verma, A.K.; Singh, A.K.; Singh, S.K.; Singh, G.; Sarkar, M.; Dutta, N.; Kumar, H.; Krishnaswamy, N. Effect of dietary supplementation of omega-3 polyunsaturated fatty acid (PUFA) rich fish oil on reproductive performance of the goat (Capra hircus). Theriogenology 2017, 99, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Garza, D.; Gutiérrez-Zamora, B.; Rodríguez-Ramírez, H.; Méndez-Zamora, G.; Kawas, J.R. Superovulatory response and embryo quality in Boer does following dietary supplementation with different sources of omega-3 and omega-6 fatty acids during the breeding season. Anim. Reprod. Sci. 2021, 227, 106718. [Google Scholar] [CrossRef]
- Viñoles, C.; Forsberg, M.; Martin, G.B.; Cajarville, C.; Repetto, J.; Meikle, A. Short-term nutritional supplementation of ewes in low body condition affects follicle development due to an increase in glucose and metabolic hormones. Reproduction 2005, 129, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gutiérrez, M.; Blache, D.; Martin, G.B.; Scaramuzzi, R.J. Folliculogenesis and ovarian expression of mRNA encoding aromatase in anoestrous sheep after 5 days of glucose or glucosamine infusion or supplementary lupin feeding. Reprod. Camb. 2002, 124, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Scaramuzzi, R.J. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem. J. 2016, 473, 1483–1501. [Google Scholar] [CrossRef] [PubMed]
- RScaramuzzi, J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.U.; McKelvey, W.A.C.; Watson, E.D. Effect of undernutrition on gonadotrophin profiles in non-pregnant, cycling goats. Anim. Reprod. Sci. 1996, 43, 25–33. [Google Scholar] [CrossRef]
- Viñoles, C.; Harris, L.J.; Forsberg, M.; Banchero, G.; Rubianes, E. Ovarian follicular dynamics and endocrine profiles in Polwarth ewes with high and low body condition. Anim. Sci. 2002, 74, 539–545. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakafeero, A.; Gonzalez-Bulnes, A.; Martinez-Ros, P. Use of Short-Term CIDR-Based Protocols for Oestrus Synchronisation in Goats at Tropical and Subtropical Latitudes. Animals 2024, 14, 1560. https://doi.org/10.3390/ani14111560
Nakafeero A, Gonzalez-Bulnes A, Martinez-Ros P. Use of Short-Term CIDR-Based Protocols for Oestrus Synchronisation in Goats at Tropical and Subtropical Latitudes. Animals. 2024; 14(11):1560. https://doi.org/10.3390/ani14111560
Chicago/Turabian StyleNakafeero, Angella, Antonio Gonzalez-Bulnes, and Paula Martinez-Ros. 2024. "Use of Short-Term CIDR-Based Protocols for Oestrus Synchronisation in Goats at Tropical and Subtropical Latitudes" Animals 14, no. 11: 1560. https://doi.org/10.3390/ani14111560




