Potential of Circulating miRNA Biomarkers and Exosomes for Early Pregnancy Diagnoses in Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
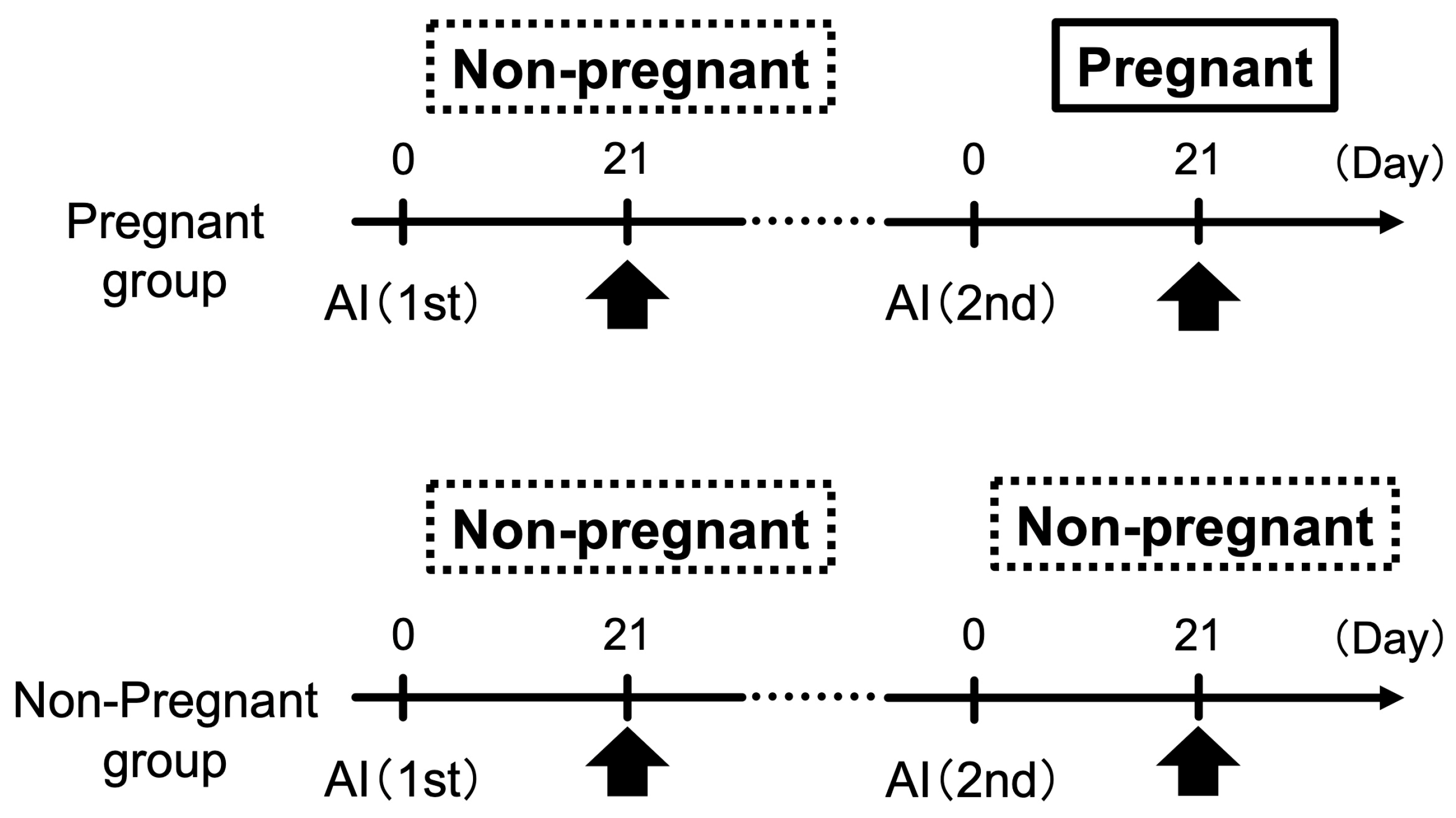
2.2. Experimental Design
2.2.1. Study 1
2.2.2. Study 2
2.2.3. Study 3
2.3. miRNA Extraction and RT-qPCR
2.4. Estimation of Threshold Values
2.5. Isolation of EVs in Plasma
2.6. Western Blotting
2.7. RT-PCR and Agarose Gel Electrophoresis of EV miRNA
2.8. Statistical Analysis
3. Results
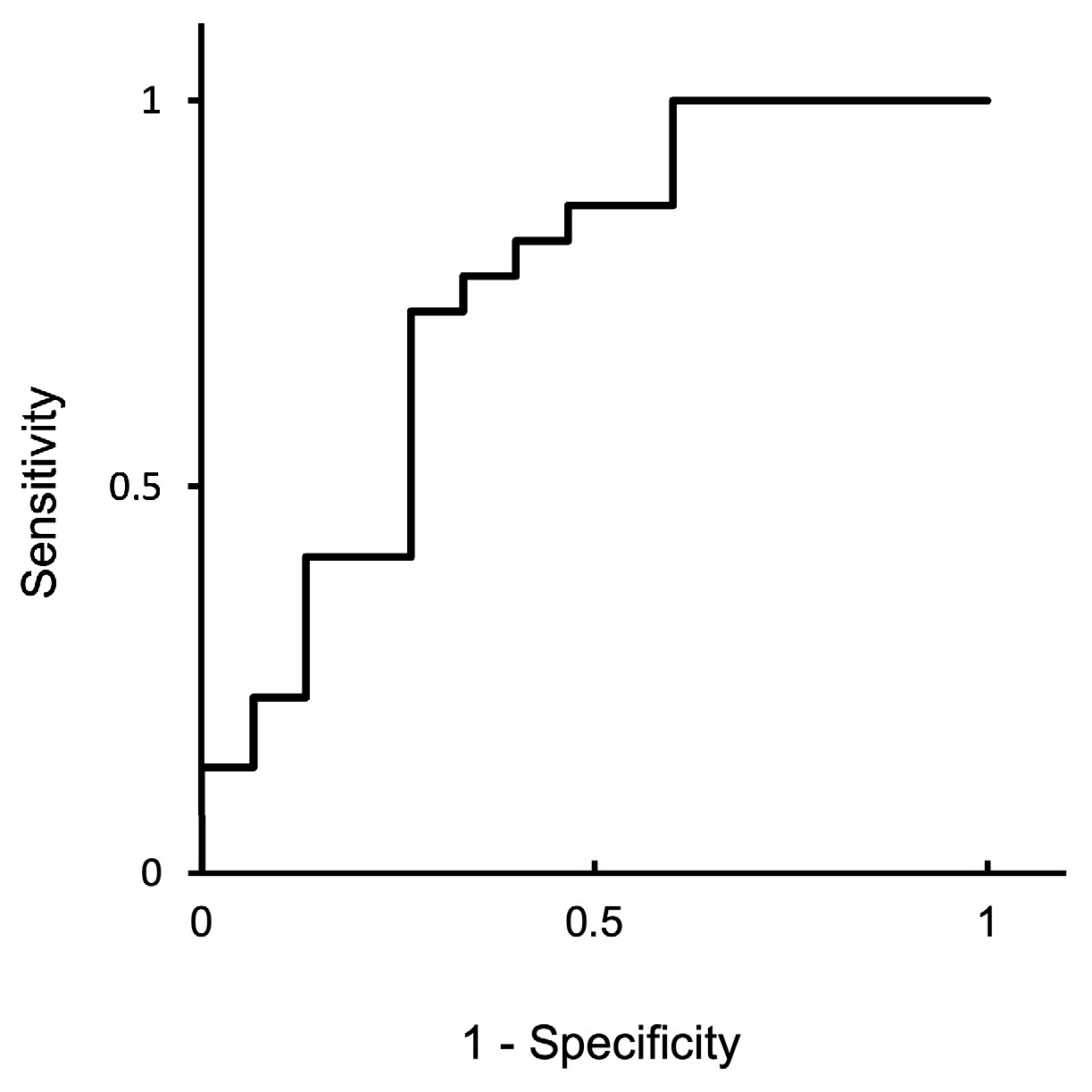
3.1. Study 1: Circulating miRNA in AI Cows and ROC Curve Analysis
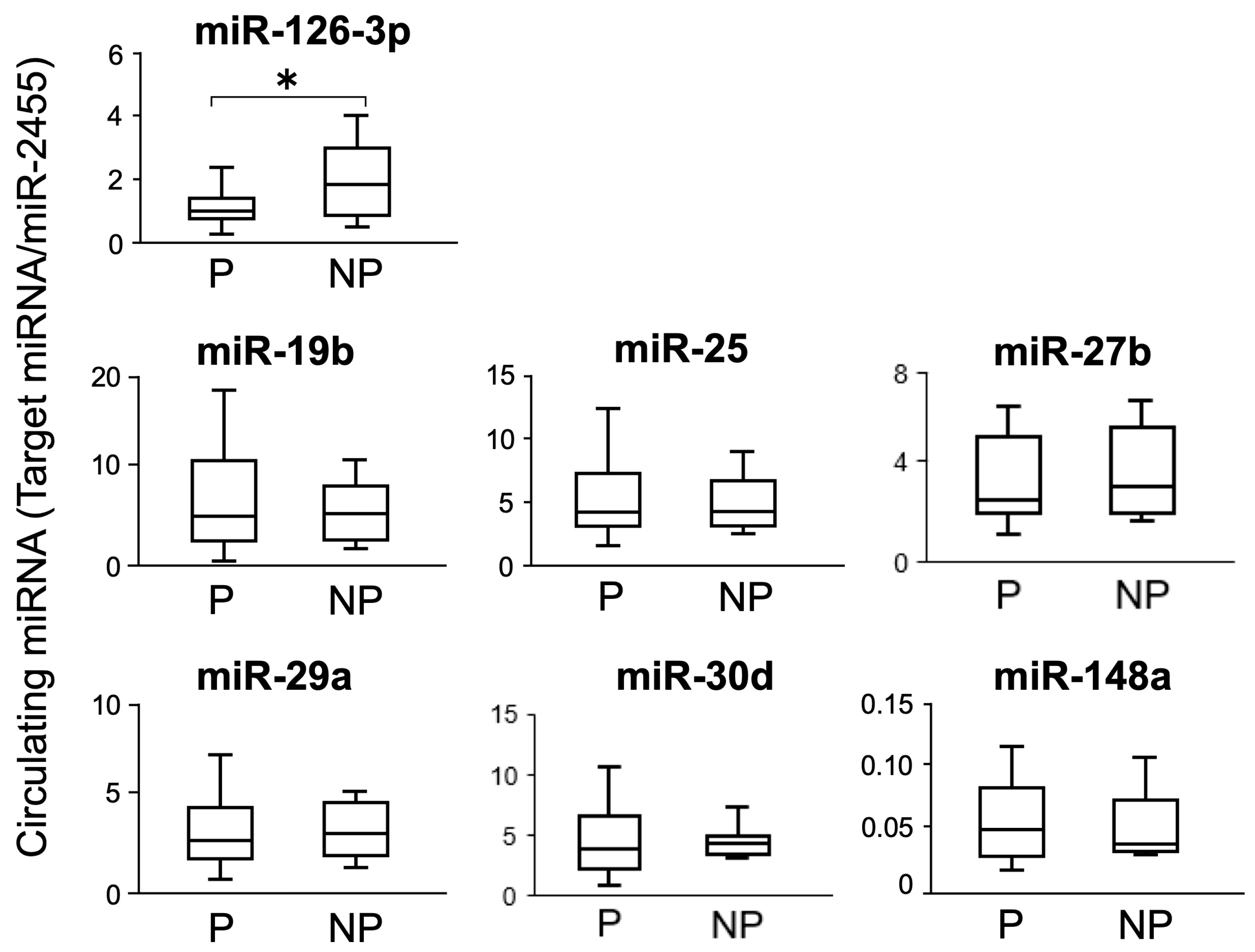
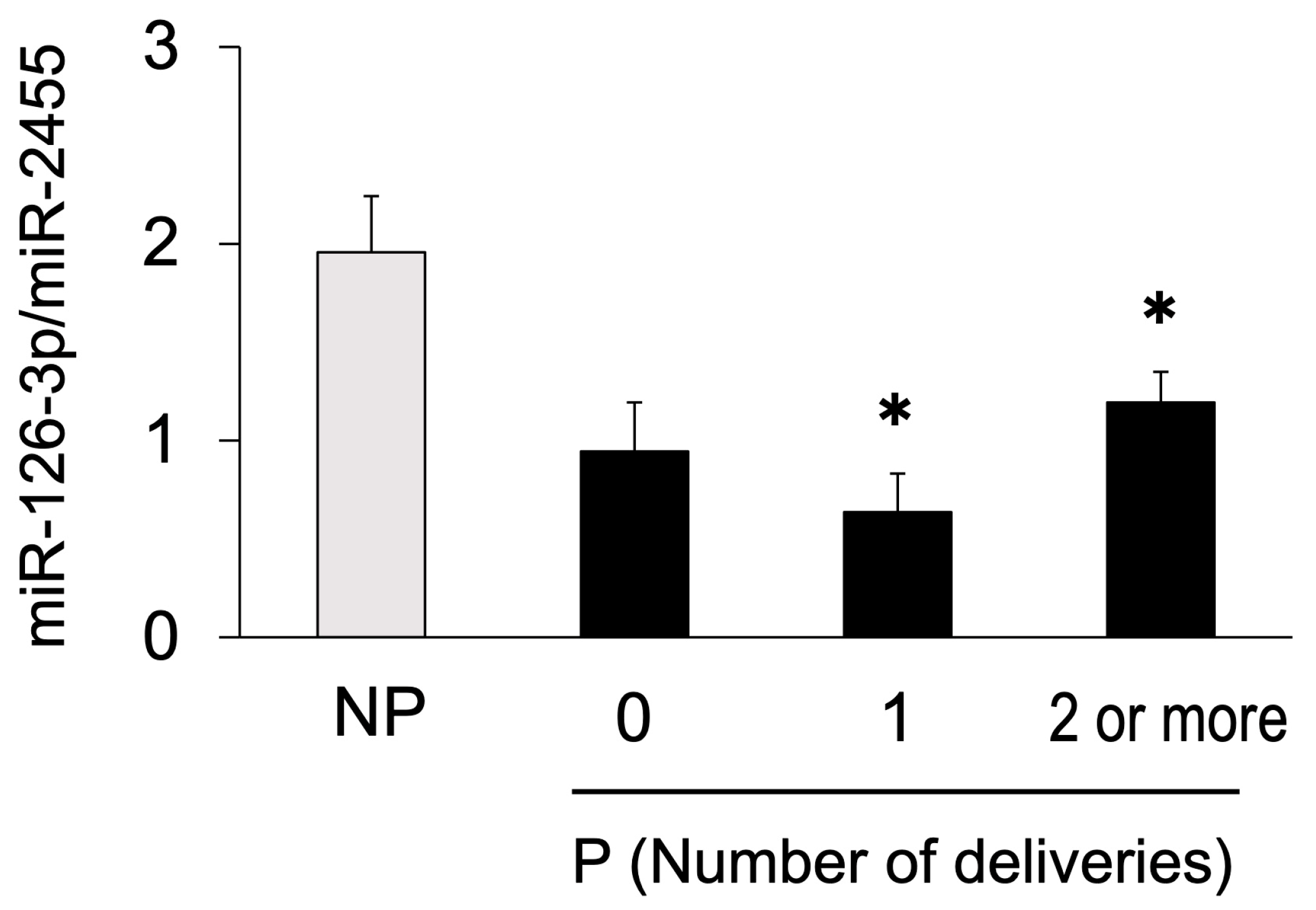
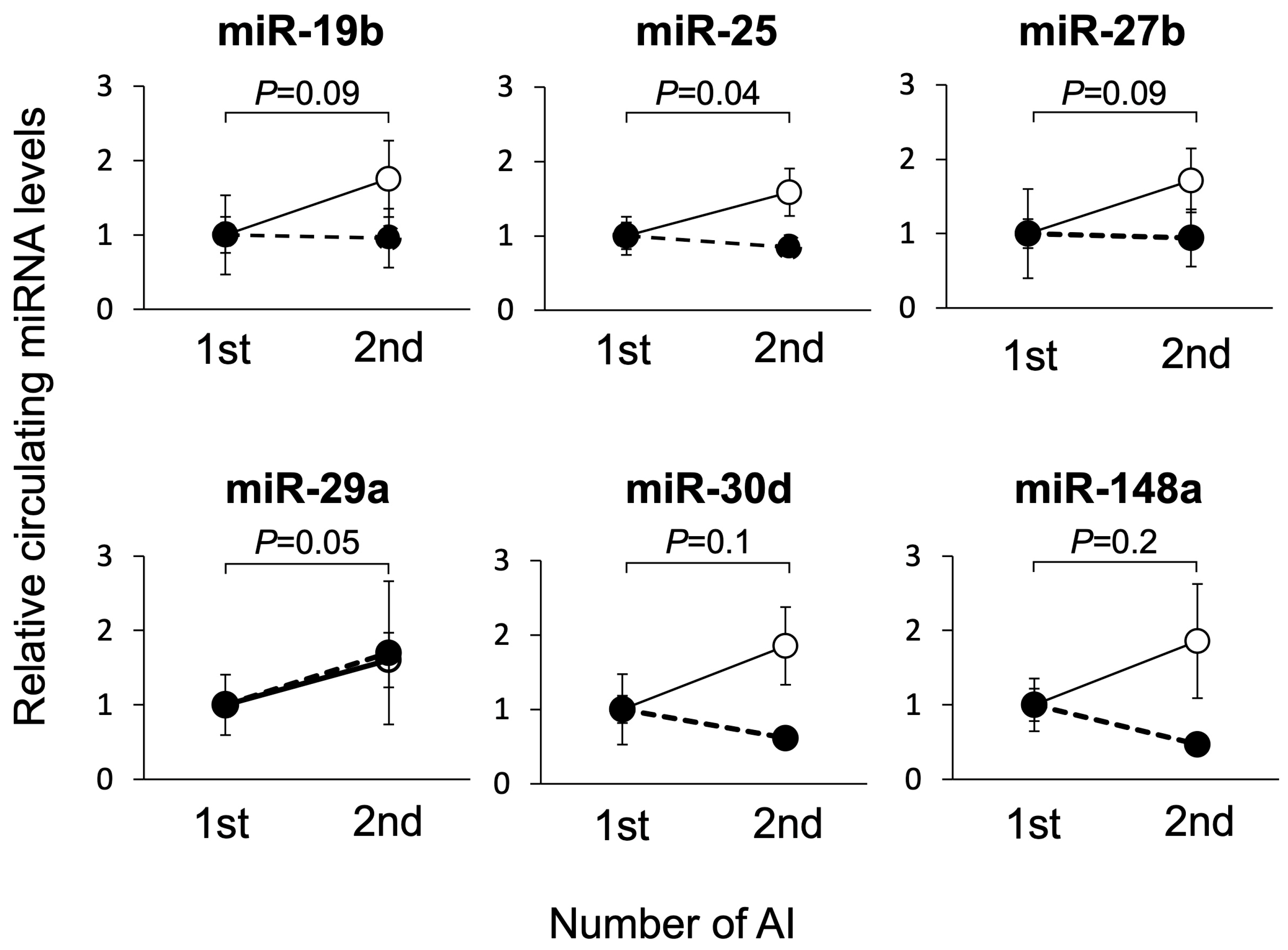
3.2. Study 2: Changes in Circulating miRNA Levels during Pregnant and Non-Pregnant States in the Same Individual on Day 21 of Gestation
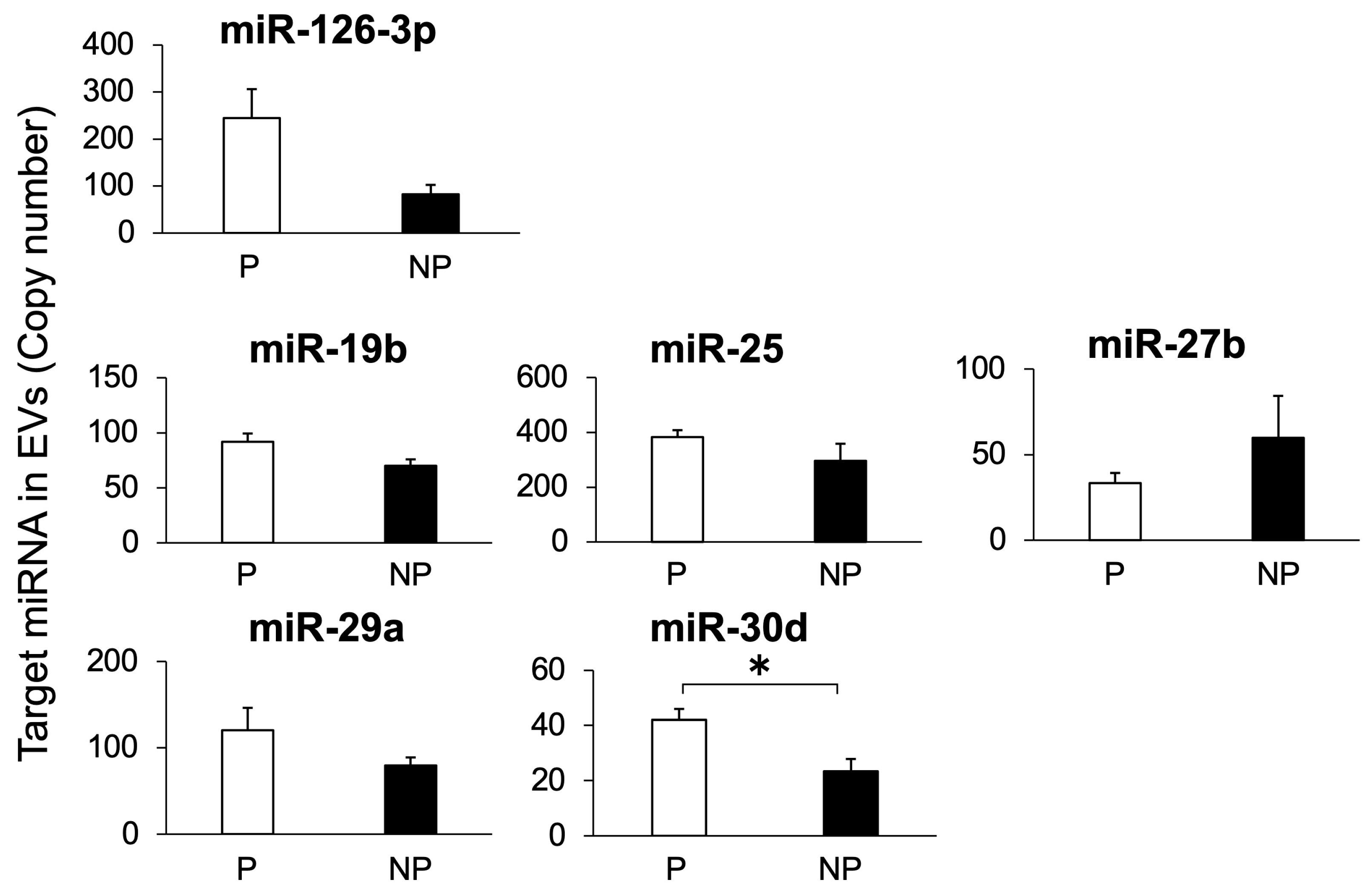
3.3. Study 3: Detection of Target miRNAs in EVs Isolated from Bovine Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucy, M.; Green, J.; Poock, S. Pregnancy determination in cattle: A review of available alternatives. Proc. Appl. Reprod. Strateg. Beef Cattle 2011, 21, 367–376. [Google Scholar]
- Fricke, P.M.; Ricci, A.; Giordano, J.O.; Carvalho, P.D. Methods for and implementation of pregnancy diagnosis in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 165–180. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Britt, J.H.A. A 100-year review: Practical female reproductive management. J. Dairy Sci. 2017, 100, 10292–10313. [Google Scholar] [CrossRef]
- Ott, T.L. Symposium review: Immunological detection of the bovine conceptus during early pregnancy. J. Dairy Sci. 2019, 102, 3766–3777. [Google Scholar] [CrossRef] [PubMed]
- Balhara, A.K.; Gupta, M.; Singh, S.; Mohanty, A.K.; Singh, I. Early pregnancy diagnosis in bovines: Current status and future directions. Sci. World J. 2013, 2013, 958540. [Google Scholar] [CrossRef]
- Gifford, C.A.; Racicot, K.; Clark, D.S.; Austin, K.J.; Hansen, T.R.; Lucy, M.C.; Davies, C.J.; Ott, T.L. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 2007, 90, 274–280. [Google Scholar] [CrossRef]
- Green, J.C.; Okamura, C.S.; Poock, S.E.; Lucy, M.C. Measurement of interferon-Tau (IFN-τ) Stimulated Gene Expression in Blood leukocytes for Pregnancy Diagnosis within 18–20d after Insemination in Dairy Cattle. Anim. Reprod. Sci. 2010, 121, 24–33. [Google Scholar] [CrossRef]
- Kizaki, K.; Shichijo-Kizaki, A.; Furusawa, T.; Takahashi, T.; Hosoe, M.; Hashizume, K. Differential neutrophil gene expression in early bovine pregnancy. Reprod. Biol. Endocrinol. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Kojima, T.; Kato, S.; Kimura, K. Relationship between quantity of IFNT estimated by IFN-stimulated gene expression in peripheral blood mononuclear cells and bovine embryonic mortality after AI or ET. Reprod. Biol. Endocrinol. 2012, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Toji, N.; Shigeno, S.; Kizaki, K.; Koshi, K.; Matsuda, H.; Hashiyada, Y.; Imai, K.; Takahashi, T.; Ishiguro-Oonuma, T.; Hashizume, K. Evaluation of interferon-stimulated genes in peripheral blood granulocytes as sensitive responders to bovine early conceptus signals. Vet. J. 2017, 229, 37–44. [Google Scholar] [CrossRef]
- Yoshino, H.; Toji, N.; Sasaki, K.; Koshi, K.; Yamagishi, N.; Takahashi, T.; Ishiguro-Oonuma, T.; Matsuda, H.; Yamanouchi, T.; Hashiyada, Y.; et al. A predictive threshold value for the diagnosis of early pregnancy in cows using interferon-stimulated genes in granulocytes. Theriogenology 2018, 107, 188–193. [Google Scholar] [CrossRef]
- Yoshino, H.; Kizaki, K.; Iga, K.; Hirata, T.I.; Matsuda, H.; Yamanouchi, T.; Hashiyada, Y.; Toji, N.; Ishiguro-Oonuma, T.; Takahashi, T.; et al. Use of a prediction method for early pregnancy status utilizing receiver operating characteristic curve analysis of peripheral blood leukocyte interferon-stimulated genes in Japanese-Black cattle. Anim. Reprod. Sci. 2020, 214, 106283. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kizaki, K.; Hirata, T.I.; Iga, K.; Matsuda, H.; Yamanouchi, T.; Hashiyada, Y.; Imai, K.; Ishiguro-Oonuma, T.; Kanazawa, T.; et al. Interferon-stimulated gene expression in peripheral blood leucocytes as a convenient prediction marker for embryo status in embryo-transferred Japanese Black cows during the peri-implantation period. Vet. Sci. 2023, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, P.M.; Ribeiro, E.S.; Risco, C.; Ealy, A.D. Associations between pregnancy-associated glycoproteins and pregnancy outcomes, milk yield, parity, and clinical diseases in high-producing dairy cows. J. Dairy Sci. 2016, 99, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Fosgate, G.T.; Motimele, B.; Ganswindt, A.; Irons, P.C. A bayesian latent class model to estimate the accuracy of pregnancy diagnosis by transrectal ultrasonography and laboratory detection of pregnancy-associated glycoproteins in dairy cows. Prev. Vet. Med. 2017, 145, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Shiekhattar, R. MicroRNA biogenesis and cancer. Cancer Res. 2005, 65, 3509–3512. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.M.; Xu, Y.M.; Huang, L.F.; Wang, X.Z. Exosomes: Novel biomarkers for clinical diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal MiRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Okamoto, S.; Ninomiya, C.; Toji, N.; Kanazawa, T.; Ishiguro-Oonuma, T.; Takahashi, T.; Iga, K.; Kizaki, K. Analysis of circulating microRNA during early gestation in Japanese Black cattle. Domest. Anim. Endocrinol. 2022, 79, 106706. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Donadeu, F.X. Changes in circulating microRNA levels can be identified as early as Day 8 of pregnancy in cattle. PLoS ONE 2017, 12, e0174892. [Google Scholar] [CrossRef] [PubMed]
- Pohler, K.G.; Green, J.A.; Moley, L.A.; Gunewardena, S.; Hung, W.T.; Payton, R.R.; Hong, X.; Christenson, L.K.; Geary, T.W.; Smith, M.F. Circulating microRNA as candidates for early embryonic viability in cattle. Mol. Reprod. Dev. 2017, 84, 731–743. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Salilew-Wondim, D.; Hoelker, M.; Held-Hoelker, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D. Exploring maternal Serum microRNAs during early pregnancy in cattle. Theriogenology 2018, 121, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Donadeu, F.X. Circulating MiRNA signatures of early pregnancy in cattle. BMC Genom. 2016, 17, 184. [Google Scholar] [CrossRef]
- Markkandan, K.; Ahn, K.; Lee, D.J.; Kim, T.I.; Dang, C.; Hong, S.-E.; Yoon, H.-B.; Lim, H.-J.; Hong, C.P. Profiling and identification of pregnancy-associated circulating microRNAs in dairy cattle. Genes Genom. 2018, 40, 1111–1117. [Google Scholar] [CrossRef]
- Mishra, B.; Kizaki, K.; Koshi, K.; Ushizawa, K.; Takahashi, T.; Hosoe, M.; Sato, T.; Ito, A.; Hashizume, K. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and its expected roles in the bovine endometrium during gestation. Domest. Anim. Endocrinol. 2012, 42, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between MiRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Sánchez-Molano, E.; Psifidi, A.; Donadeu, F.X.; Banos, G. Association of plasma microRNA expression with age, genetic background and functional traits in dairy cattle. Sci. Rep. 2018, 8, 12955. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ping, J.L.; Ma, B.; Chen, Y.R.; Li, L.Q. Deregulation of MiR-126-3p in basal-like breast cancers stroma and its clinical significance. Pathol. Res. Pract. 2017, 213, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.F.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massagué, J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yang, C.; Yang, J.; Liu, P.; Jiang, K.; Shaukat, A.; Wu, H.; Deng, G. Placental exosome-mediated Bta-MiR-499-Lin28B/Let-7 axis regulates inflammatory bias during early pregnancy. Cell Death Dis. 2018, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Florijn, B.W.; Duijs, J.M.G.J.; Levels, J.H.; Dallinga-Thie, G.M.; Wang, Y.; Boing, A.N.; Yuana, Y.; Stam, W.; Limpens, R.W.A.L.; Au, Y.W.; et al. Diabetic nephropathy alters the distribution of circulating angiogenic microRNAs among extracellular vesicles, HDL, and Ago-2. Diabetes 2019, 68, 2287–2300. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in exosome isolation techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]

| miRNA | Accession No. | Sequence (5′-3′) |
|---|---|---|
| bta-miR-19b | MIMAT0004337 | ACACGTTTAGGTTCGTTTTGACTAAA |
| bta-miR-25 | MIMAT0003853 | GTAACGTGAACAGAGCCAGACTAAA |
| bta-miR-27b | MIMAT0003546 | AAGTGTCACCGATTCAAGACGAAA |
| bta-miR-29a | MIMAT0003518 | GATCGTGGTAGACTTTAGCCAATAAA |
| bta-miR-30d | MIMAT0003533 | ACATTTGTAGGGGCTGACCTTCGAAAA |
| bta-miR-126-3p | MIMAT0004328 | GTAATAATGAAAACCATGCGCAAA |
| bta-miR-148a | MIMAT0003522 | AGTCACGTGATGTCTTGAAACAAAA |
| bta-miR-2455 | MIMAT0012037 | AGACACGAGCCCCTCCGTCCCTAAA |
| Status | miR-126-3p Values *1 | Ultrasonic Detection *2 |
|---|---|---|
| True positives, TP | + | + |
| False positives, FP | + | − |
| True negatives, TN | − | − |
| False negatives, FN | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninomiya, C.; Yoshino, H.; Ishiguro-Oonuma, T.; Iga, K.; Kanazawa, T.; Takahashi, T.; Kizaki, K. Potential of Circulating miRNA Biomarkers and Exosomes for Early Pregnancy Diagnoses in Cattle. Animals 2024, 14, 1592. https://doi.org/10.3390/ani14111592
Ninomiya C, Yoshino H, Ishiguro-Oonuma T, Iga K, Kanazawa T, Takahashi T, Kizaki K. Potential of Circulating miRNA Biomarkers and Exosomes for Early Pregnancy Diagnoses in Cattle. Animals. 2024; 14(11):1592. https://doi.org/10.3390/ani14111592
Chicago/Turabian StyleNinomiya, Chiaki, Hitomi Yoshino, Toshina Ishiguro-Oonuma, Kosuke Iga, Tomomi Kanazawa, Toru Takahashi, and Keiichiro Kizaki. 2024. "Potential of Circulating miRNA Biomarkers and Exosomes for Early Pregnancy Diagnoses in Cattle" Animals 14, no. 11: 1592. https://doi.org/10.3390/ani14111592





