A Review of the Nutritional Aspects and Composition of the Meat, Liver and Fat of Buffaloes in the Amazon
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Contextualization
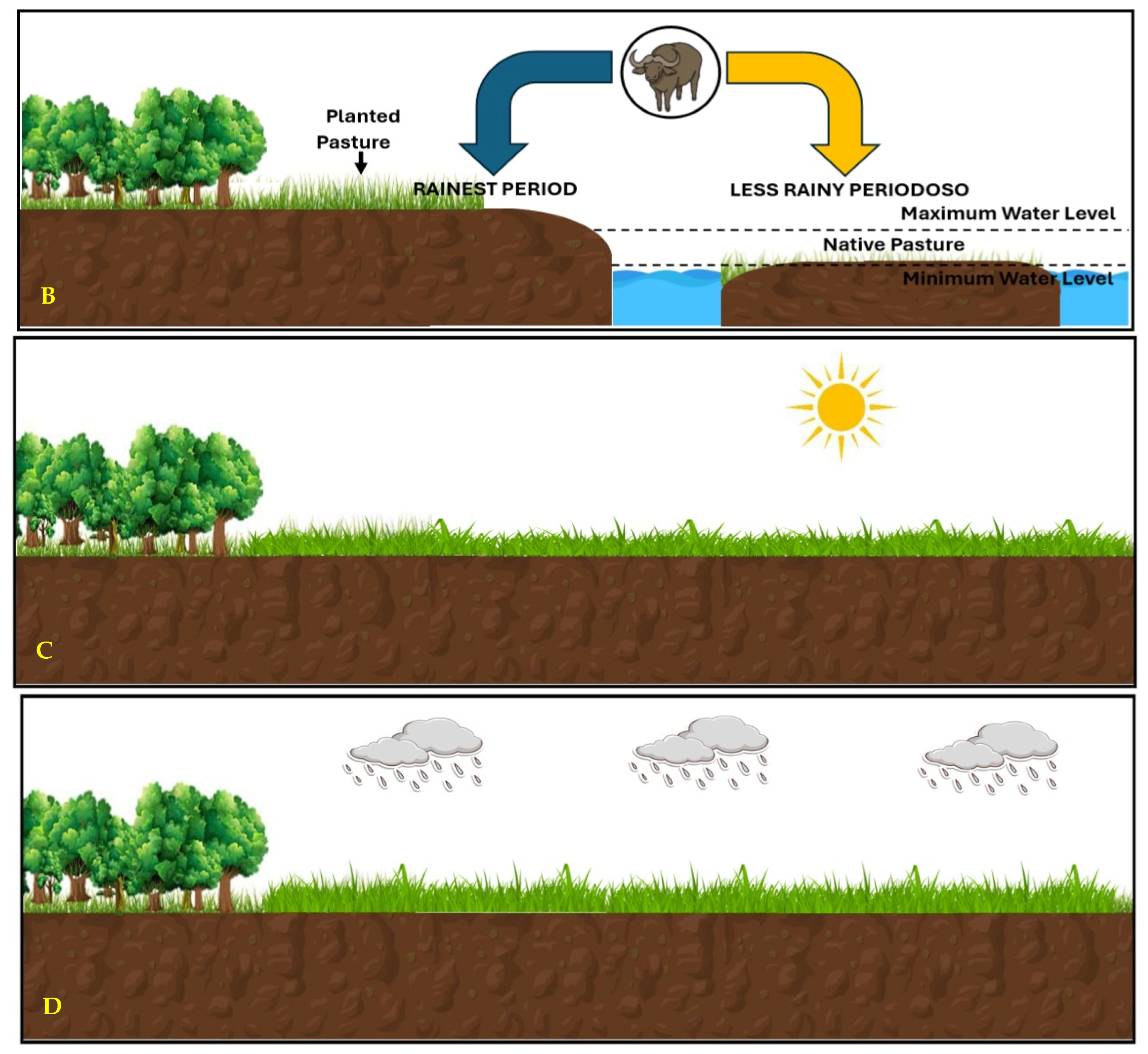
3.1. Buffalo Farming in the Amazon
3.2. Quality and Nutritional Value of Buffalo Meat and Liver
3.3. Characteristics of Nutrients and Their Relationship with Human Health
3.4. Vitamins
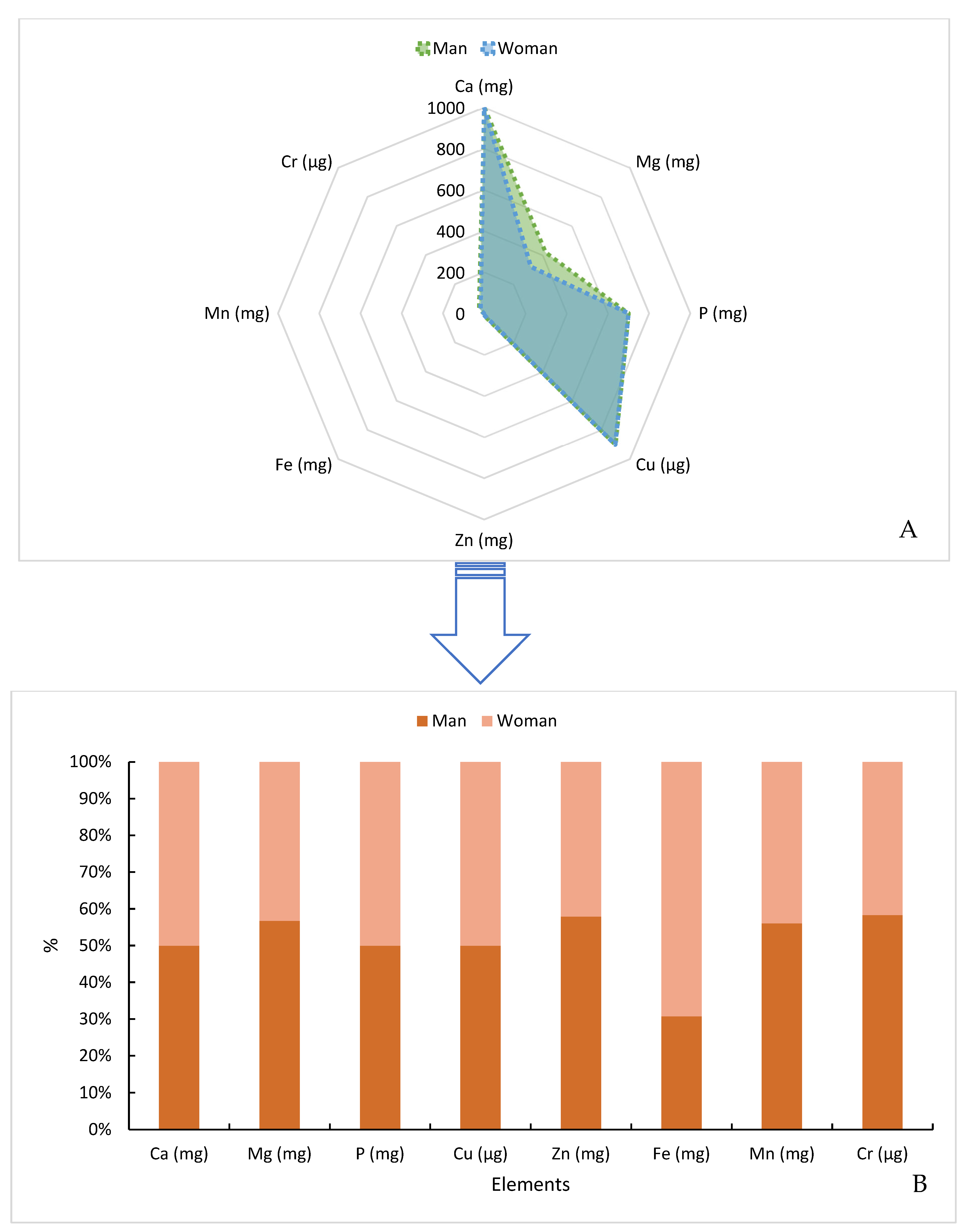
3.5. Minerals
3.6. Lipids
3.7. Fatty Acids Composition
3.8. Cholesterol
3.9. Nutritional Value of Lipids
3.10. Future Prospects
4. Conclusions
- These products have distinct characteristics compared to those from other regions.
- The rich Amazonian biodiversity directly influences the diet of aquatic buffaloes, giving unique properties to their products. Their meat, for example, reveals a differentiated nutritional value, enriched by specific elements of the local flora.
- Similarly, the liver exhibits remarkable concentrations of essential nutrients, while the fat exhibits specific fatty acid profiles that may benefit human health.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minervino, A.H.H.; Zava, M.; Vecchio, D.; Borghese, A. Bubalus bubalis: A short story. Front. Vet. Sci. 2020, 7, 570413. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Parma, P.; Iannuzzi, L. The Cytogenetics of the Water Buffalo: A Review. Animals 2021, 11, 3109. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Rosa, G.D.; Chay-Canul, A.; Álvarez-Macías, A.; Pereira, A.M.; Bragaglio, A.; Mora-Medina, P.; Rodríguez-González, D.; García-Herrera, R.; Hernández-Ávalos, I.; et al. The Challenge of Global Warming in Water Buffalo Farming: Physiological and Behavioral Aspects and Strategies to Face Heat Stress. Animals 2023, 13, 3103. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.R.; Garcia, A.R.; Almeida, A.M.D.; Bezerra, A.S.; LourenÇo-Júnior, J.D.B. Water buffalo production in the Brazilian Amazon Basin: A review. Trop. Anim. Health Prod. 2021, 53, 343. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.R.D.; Pantoja, M.H.D.A.; Silva, W.C.D.; Almeida, J.C.F.D.; Noronha, R.D.P.P.; Barbosa, A.V.C.; Lourenço-Júnior, J.D.B. Thermoregulatory reactions of female buffaloes raised in the sun and in the shade, in the climatic conditions of the rainy season of the Island of Marajó, Pará, Brazil. Front. Vet. Sci. 2022, 9, 998544. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.R.D.; Rodrigues, L.S.; Lourenço-Júnior, J.D.B.; Silva, A.G.M.E.; Rodrigues, T.C.G.D.C.; Silva, W.C.D.; Silva, T.C.D.; Castro, V.C.G.D.; Alfaia, C.M.; Prates, J.A.M.; et al. Evaluation of the Composition of the Cholesterol, Tocopherols, β-Carotene and Fatty Acids Profile of the Liver Tissue of Male Water Buffaloes (Bubalus bubalis) Managed in Different Ecosystems of the Eastern Amazon. Animals 2023, 13, 3785. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Institute of Geography And Statistics—IBGE. Directorate of Research, Coordination of Agriculture. Municipal Livestock Production. 2024. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/bubalinos/br (accessed on 10 January 2023).
- Cruz-Cruz, L.A.D.L.; Roldán-Santiago, P.; Berdugo-Diaz, D.F.; Rodríguez-Florentino, R.; Berdugo-Gutiérrez, J.A. Characteristics of Buffalo Production and Research Systems in Southern Mexico. J. Buffalo Sci. 2022, 11, 19–31. [Google Scholar] [CrossRef]
- Ermetin, O. Evaluation of the application opportunities of precision livestock farming (PLF) for water buffalo (Bubalus bubalis) breeding: SWOT analysis. Arch. Anim. Breed. 2023, 66, 41–50. [Google Scholar] [CrossRef]
- Marrone, R.; Salzano, A.; Francia, A.D.; Vollano, L.; Matteo, R.D.; Balestrieri, A.; Anastasio, A.; Barone, C.M.A. Effects of feeding and maturation system on qualitative characteristics of buffalo meat (Bubalus bubalis). Animals 2020, 10, 899. [Google Scholar] [CrossRef]
- Andrade, B.F.; Rodrigues, L.M.; Paula, L.M.A.F.D.; Torres-Filho, R.D.A.; Fontes, P.R.; Ramos, E.M.; Ramos, A.D.L.S. A Comparative Study on Meat Quality Characteristics of Murrah Buffalo and Nellore Cattle Commercialized in Southeastern Brazil. Ruminants 2023, 3, 172–181. [Google Scholar] [CrossRef]
- Rodas-Gonzalez, A. 82 Critical Comparison of Water Buffalo (Bubalus bubalis) and Cattle on Growth Performance and Carcass Traits: A Review. J. Anim. Sci. 2023, 101, 144. [Google Scholar] [CrossRef]
- Stasio, L.D.; Brugiapaglia, A. Current knowledge on river buffalo meat: A critical analysis. Animals 2021, 11, 2111. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.F.M.; Amin, F.M.; Ahmad, H.; Nor, N.M.; Meng, G.Y.; Saad, M.Z.; Bakar, M.Z.A.; Abdullah, P.; Irawan, A.; Jayanegara, A.; et al. Effects of bypass fat on buffalo carcass characteristics, meat nutrient contents and profitability. Animals 2021, 11, 3042. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.F.; Magalhães, A.L.R.; Neves, M.L.M.W.; Carvalho, F.D.; Melo, A.A.S.; Vieira, G.H.P.; Lima, D.O.; Júnior, D.M.D.L.; Pessoa, R.A.S. Performance, carcass yield, and meat quality of buffalo (Bubalus bubalis) fed on diets with different levels of concentrate. S. Afr. J. Anim. Sci. 2023, 53, 133–138. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Silva, J.A.R.D.; Lourenço-Júnior, J.D.B.; Silva, A.G.M.E.; Almeida, A.M.D.; Mourato, M.P.; Castro, V.C.G.D.; Bezerra, A.S.; Silva, W.C.D.; Prates, J.A.M. Muscle mineral profile of water buffaloes (Bubalus bubalis) reared in different production systems of the Brazilian Eastern Amazon. Front. Vet. Sci. 2023, 10, 1057658. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, E.I.; Awokunmi, E.E.; Adesina, A.J. Lipid composition of variety meats (stomach, intestine, liver) of bull. NSUK J. Sci. Technol. 2012, 1, 1597–5527. [Google Scholar]
- Bertoni, A.; Álvarez-Macías, A.; Mota-Rojas, D.; Dávalos, J.L.; Minervino, A.H.H. Dual-purpose water buffalo production systems in tropical latin america: Bases for a sustainable model. Animals 2021, 11, 2910. [Google Scholar] [CrossRef] [PubMed]
- Borghese, A.; Chiariotti, A.; Barile, V.L. Buffalo in the World: Situation and Perspectives. Biotechnol. Appl. Buffalo Res. 2022, 1, 3–31. [Google Scholar]
- Valente, G.F.; Ferraz, G.A.E.S.; Santana, L.S.; Ferraz, P.F.P.; Mariano, D.D.C.; Santos, C.M.D.; Okumura, R.S.; Simonini, S.; Barbari, M.; Rossi, G. Mapping soil and pasture attributes for buffalo management through remote sensing and geostatistics in amazon biome. Animals 2022, 12, 2374. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, D.; Minervino, A.H.H.; Orihuela, A.; Bertoni, A.; Morales-Canela, D.A.; Álvarez-Macías, A.; José-Pérez, N.; Domínguez-Oliva, A.; Mota-Rojas, D. Handling and physiological aspects of the dual-purpose water buffalo production system in the mexican humid tropics. Animals 2022, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Álvarez-Macías, A.; Morales, D.A.; Dávalos, J.L.; Mota-Rojas, D. Description of four dual-purpose river Buffalo (Bubalis bubalis) production systems in tropical wetlands of Mexico Part 2: Sanitary management, milking, zootechnical and economic indicators. J. Buffalo Sci. 2022, 11, 8–18. [Google Scholar] [CrossRef]
- Bastianetto, E.; Oliveira, D.A.A.D.; McManus, C.; Bagolin, D.D.J.; Leite, R.C.; Melo, C.B.D. Genetic material from buffalo and cattle: Crucial importance in the formalization of bilateral trade between India and Brazil. Anim. Reprod. 2020, 17, e20200031. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, P.; Barbosa, J.D.; Brito, M.F.; Souza, V.C.D.; Costa, D.F.A. Phosphorus for Cattle and Buffaloes in Brazil: Clinical Signs and Diagnosis of Its Deficiency and Relevance, and Recommended Strategies to Alleviate Issues Observed under Grazing Conditions. Ruminants 2023, 3, 55–75. [Google Scholar] [CrossRef]
- Omran, F.I. Buffaloes and climatic change: Mitigation and adaptation. Egypt. J. Agric. Res. 2021, 99, 262–274. [Google Scholar] [CrossRef]
- Newton, C.J.; O’Sullivan, L.M.; Underwood, K.R.; Grubbs, J.K.; Bakker, C.E.; Cammack, K.M.; Dinh, T.; Kruse, D.; O’Sullivan, L.M. Influence of Finishing Systems on Carcass Characteristics, Composition, and Fatty Acid Profile of Bison Bulls. Meat Muscle Biol. 2024, 8, 16999. [Google Scholar] [CrossRef]
- Nikolaou, K.; Koutsouli, P.; Laliotis, G.P.; Papachristou, D.; Bizelis, I. Análise comparativa de carcaças de bovinos bubalinos, locais e continentais com o sistema de classificação da União Europeia na Grécia. Ciência Da Carne 2023, 195, 109018. [Google Scholar]
- Silva, W.C.D.; Silva, J.A.R.D.; Júnior, A.G.; Alvarenga, A.B.B.; Barbosa, A.V.C.; Silva, É.B.R.; Santos, M.R.P.D.; Lourenço-Júnior, J.D.B.; Camargo-Júnior, R.N.C.; Silva, A.G.M.E. A new proposal for the use of the focal animal technique in buffaloes in the Eastern Amazon. Front. Vet. Sci. 2023, 10, 1266451. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.A.; Loewensteiner, D.A.; Murphy, B.P.; Pittard, S.; McMahon, C.R. Seasonal movements and site utilisation by Asian water buffalo (Bubalus bubalis) in tropical savannas and floodplains of northern Australia. Wildl. Res. 2020, 48, 230–239. [Google Scholar] [CrossRef]
- Mihailou, H.; Massaro, M. An overview of the impacts of feral cattle, water buffalo and pigs on the savannas, wetlands and biota of northern Australia. Austral Ecol. 2021, 46, 699–712. [Google Scholar] [CrossRef]
- Godinho, F.M.S.; Friedrich, M.T.; Modesto, E.C.; Mota, A.D.S.D. Perfil de ácidos graxos do leite de búfala produzido no sul do Brasil. Acta Scientiarum. Zootec. 2024, 46, e63400. [Google Scholar]
- Ponnampalam, E.N.; Priyashantha, H.; Vidanarachchi, J.K.; Kiani, A.; Holman, B.W. Effects of Nutritional Factors on Fat Content, Fatty Acid Composition, and Sensory Properties of Meat and Milk of Domesticated Ruminants: An Insight. Animals 2024, 14, 840. [Google Scholar] [CrossRef] [PubMed]
- Failla, S.; Contò, M.; Borghese, A.; Barile, V.L. Propriedades nutricionais da carne de búfalo terminada com feno de alfafa envolto em comparação com feno seco e dietas à base de silagem de milho. Rev. Científica Fac. Veterinária 2023, 33, 292–308. [Google Scholar]
- Mali, A.; Laskar, S.K.; Das, A.; Muthukumar, M.; Bhattacharya, D.K. Analysis of the texture profile of smoked low-fat buffalo meat sausages. Pharma. Innov. J. 2023, 12, 1684–1686. [Google Scholar]
- Baran, B.; Yilmaz, İ.; GEÇGEL, U. Determinação de alguns parâmetros de qualidade da carne bubalina. Tekirdağ Ziraat Fakültesi Derg. 2023, 20, 677–687. [Google Scholar] [CrossRef]
- Rébak, G.; Obregón, G.; Gómez, D.; Vázquez-Acosta, L.; Pino, M.; Obregón, J.; Molina, R.; Cantero, V.; Segóvia-Espinola, L. Chemical analysis of buffalo meat in a self-consumption system in northeastern of Argentina. Rev. Científica Fac. Veterinária 2023, 33, 292. [Google Scholar]
- Vaz, R.Z.; Sá, H.A.O.M.; Sartori, D.B.S.; Costa, P.T.; Fluck, A.C.; Kröning, A.B.; Ferreira, O.G.L.; Costa, O.A.D.; Restle, J. Trade and consumption of buffalo meat in Brazil. Meat Sci. 2024, 208, 109399. [Google Scholar] [CrossRef]
- Cruz-Monterrosa, R.; Mota-Rojas, D.; Ramírez-Bibriesca, E.; Mora-Medina, P.; Guerrero-Legarreta, I. Scientific findings on the quality of river buffalo meat and prospects for future studies. J. Buffalo Sci. 2020, 9, 170–180. [Google Scholar]
- Guerrero-Legarreta, I.; Napolitano, F.; Cruz-Monterrosa, R.; Mota-Rojas, D.; Mora-Medina, P.; Ramírez-Bribiesca, E.; Bertoni, A.; Berdugo-Gutiérrez, J.; Braghieri, A. River buffalo meat production and quality: Sustainability, productivity, nutritional and sensory properties. J. Buffalo Sci. 2020, 9, 159–169. [Google Scholar] [CrossRef]
- Alarcón-Rojo, A.; Mota-Rojas, D.; García-Galicia, I.; Ramírez-Bribiesca, E.; Olmos-Hernández, A.; Guerrero-Legarreta, I. Dark cutting in large ruminants: Effect of management and environmental factors. Agro Product. 2020, 13, 6. [Google Scholar] [CrossRef]
- Gobert, M.; Sayd, T.; Gatellier, P.; Sante-Lhoutellier, V. Application to proteomics to understand and modify meat quality. Meat Sci. 2014, 98, 539–543. [Google Scholar] [CrossRef]
- Badar, I.H.; Wang, Z.; Liu, H.; Chen, Q.; Xia, X.; Liu, Q.; Kong, B. Future prospects of high internal phase pickering emulsions stabilized by natural modified biopolymers as a potential fat substitute in meat products. Trends Food Sci. Technol. 2023, 140, 104176. [Google Scholar] [CrossRef]
- Bedani, R.; Cucick, A.C.C.; Albuquerque, M.A.C.D.; LeBlanc, J.G.; Saad, S.M.I. B-Group Vitamins as Potential Prebiotic Candidates: Their Effects on the Human Gut Microbiome. J. Nutr. 2024, 154, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Zanchim, M.C.; Kirsten, V.R.; Marchi, A.C.B.D. Markers of food consumption of diabetic patients assessed using a mobile application. Ciência Saúde Coletiva 2018, 23, 4199–4208. [Google Scholar] [CrossRef]
- Tick, P.; Correia, P.; Garcia, B. Determinant of health in Brazil: The search for equity in health. Saúde E Soc. 2017, 26, 676–689. [Google Scholar]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kume, A.; Herbas, M. Potential of Vitamin E Deficiency, Induced by Inhibition of α-Tocopherol Efflux, in Murine Malaria Infection. Int. J. Mol. Sci. 2018, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a longterm care skilled nursing facility, King County, Washington, March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 377–381. [Google Scholar] [CrossRef]
- Lehninger, T.M.; Nelson, D.L.; Cox, M.M. Princípios de Bioquímica. Artmed 2014, 6, 1328. [Google Scholar]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- McGill, J.L.; Kelly, S.M.; Guerra-Maupome, M.; Winkley, E.; Henningson, J.; Narasimhan, B.; Sacco, R.E. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci. Rep. 2019, 9, 15157. [Google Scholar] [CrossRef]
- Choi, B.H.; Hwang, H.J.; Lee, J.E.; Oh, S.H.; Hwang, J.S.; Lee, B.Y.; Lee, P.C. Microbial production of retinyl palmitate and its application as a cosmeceutical. Antioxidants 2020, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.L.; Batista, M.C.C.; Silvino, V.O.; Moura, R.C.D.; Mendes, I.L.; Moura, M.S.B.D.; Batista, N.K.C.; Silva, K.F.; Barbosa, A.K.D.S. Nutritional importance of vitamins and minerals in COVID-19 infection. Res. Soc. Dev. 2020, 9, e804986103. [Google Scholar]
- El-Deen, N.N.; Neamat-Allah, A.; Rizk, L.; Fareed, R. Possible interrelation between phosphorous deficiency and vitamin d in buffaloes (Bubalus bubalis). Orig. Res. Artic. 2021, 58, 5–11. [Google Scholar]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2018, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, S.M.F. Biodisponibilidade de nutrientes. Manole 2008, 6, 903. [Google Scholar]
- Devi, L.S.; Hanah, S.S.; Vikram, R.; Haque, N.; Khan, M.H.; Girish, P.S.; Mitra, A. Composição química, ácidos graxos, aminoácidos, minerais e perfis vitamínicos do leite Mithun (Bos frontalis) criado em sistema semi-intensivo. Rev. Compos. Análise Aliment. 2023, 124, 105694. [Google Scholar]
- Spears, J.W. Trace Mineral Bioavailability in Ruminants. J. Nutr. 2003, 133, 1506S–1509S. [Google Scholar] [CrossRef]
- Souza, D.H.O.; Silva, M.I.D.O.D.; Fonseca, R.G.D.; Ferreira, J.C.D.S. A importância de uma alimentação saudável como forma de aumento da imunidade através das vitaminas e minerais. Res. Soc. Dev. 2021, 10, e103101220305. [Google Scholar]
- França, T.G.D.; Ishikawa, L.L.W.; Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Cunha, M.D.L.R.D.S.D.; Sartori, A. Impact of malnutrition on immunity and infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 374–390. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Kiernan, K.; Maciver, N.J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 2018, 9, 337099. [Google Scholar] [CrossRef]
- Padovani, R.M.; Amaya-Farfán, J.; Colugnati, F.A.B.; Domene, S.M.Á. Dietary reference intakes: Application of tables in nutritional studies. Rev. Nutr. 2006, 19, 741–760. [Google Scholar] [CrossRef]
- Tajik, H.; Rezaei, S.A.; Alamouti, M.R.P.; Moradi, M.; DalirNaghadeh, B. Mineral contents of muscle (Longissimus dorsi thoracis) and liver in river buffalo (Bubalus bubalis). J. Muscle Foods 2010, 21, 459–473. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Scanlon, T.; Kilminster, T.; Martins, C.; Greeff, J.; Milton, J.; Oldham, C.; Freire, J.P.B.; Mourato, M.P.; Almeida, A.M.D. Mineral profiling of muscle and hepatic tissues of Australian Merino, Damara and Dorper lambs: Effect of weight loss. J. Anim. Physiol. Anim. Nutr. 2020, 104, 823–830. [Google Scholar] [CrossRef]
- Langaro, A.; Tomkiel, L.C.E.; Mazon, L.R.; Carniel, T.K.; Dalcanton, F.; Zanetti, M. Determination of the quantity of sodium and potassium in organic and industrialized baby papers. Res. Soc. Dev. 2020, 9, e28591211114. [Google Scholar] [CrossRef]
- Domingues, B.C.; Ribeiro, T.R.; Neves, A.L.; Nascimento-Júniora, N.M. Suplementos Alimentares: Aspectos Químicos e Aplicações de Macro e Micronutrientes. Rev. Virtual Química 2023, 15, 1–41. [Google Scholar] [CrossRef]
- Silva, J.A.R.D.; Rodrigues, L.S.; Lourenço-Júnior, J.D.B.; Alfaia, C.M.; Costa, M.M.; Castro, V.C.G.D.; Bezerra, A.S.; Almeida, A.M.D.; Prates, J.A.M. Total lipids, fatty acid composition, total cholesterol and lipid-soluble antioxidant vitamins in the longissimus lumborum muscle of water buffalo (Bubalus bubalis) from different production systems of the Brazilian Eastern Amazon. Animals 2022, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Failla, S. Buffalo meat quality, processing, and marketing: Harnessing its benefits and nutraceutical potential. Rev. Cient. Fac. Veterinária 2023, 33, 105–113. [Google Scholar]
- Mahan, L.K.; Escott-Stump, S.; Raymond, J.L. Krause Alimentos, Nutrição e Dietoterapia; Elsevier: Amsterdam, The Netherlands, 2013; Volume 13, p. 1256. [Google Scholar]
- Jiang, B.; Qin, C.; Shi, D. Multi-omics reveals the mechanism of rumen microbiome and its metabolome together with host metabolome participating in the regulation of milk production traits in dairy buffaloes. Front. Microbiol. 2024, 15, 1301292. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.F.M.; Ahmad, H.; Nor, N.M.; Goh, Y.M.; Zamri-Saad, M.; Bakar, M.Z.A.; Salleh, A.; Abdullah, P.; Jayanegara, A.; Hassim, H.A. The impact of feed supplementations on Asian buffaloes: A review. Animals 2021, 11, 2033. [Google Scholar] [CrossRef]
- Silva, L.K.X.; Lourenço-Júnior, J.D.B.; Silva, A.O.A.D.; Sousa, J.S.D.; Reis, A.N.D.; Miranda, M.D.S.; Santos, S.D.S.D.; Ohashi, O.M.; Martorano, L.G.; Filho, G.N.D.R.; et al. Increased quality of in natura and cryopreserved semen of water buffaloes supplemented with saturated and unsaturated fatty acids from the palm oil industry. Anim. Reprod. 2020, 17, e20200522. [Google Scholar] [CrossRef]
- Dąbrowski, G.I.; Konopka, I. Update on food sources and biological activity of odd-chain, branched and cyclic fatty acids––A review. Trends Food Sci. Technol. 2022, 119, 514–529. [Google Scholar] [CrossRef]
- Kawanishi, K.; Coker, J.K.; Grunddal, K.V.; Dhar, C.; Hsiao, J.; Zengler, K.; Varki, N.; Varki, A.; Gordts, P.L. Dietary Neu5Ac intervention protects against atherosclerosis associated with human-like Neu5Gc loss—Brief report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2730–2739. [Google Scholar] [CrossRef]
- Lozano, M.S.R.; Ngapo, T.M.; Huerta-Leidenz, N. Tropical beef: Is there an axiomatic basis to define the concept? Foods 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Maheswarappa, N.B.; Muthupalani, M.; Mohan, K.; Banerjee, R.; Sen, A.R.; Barbuddhe, S.B. Buffalo Meat Processing and Value Addition. Asiat. Water Buffalo A Sustain. Healthy Red Meat Source 2022, 1, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, J.H.G.G.; Gonçalves, J.S. Dietary supplements, immunity and COVID-19: What is the evidence? Vittalle—J. Health Sci. 2020, 32, 10–21. [Google Scholar]
- Uttam, V.; Vohra, V.; Chhotaray, S.; Santhosh, A.; Diwakar, V.; Patel, V.; Gahlyan, R.K. Exome-wide comparative analyses revealed differentiating genomic regions for performance traits in Indian native buffaloes. Anim. Biotechnol. 2023, 35, 2277376. [Google Scholar] [CrossRef] [PubMed]
- Vergara-López, J. Ruminant grazing feeding and methane production. Rev. Fac. Agron. Univ. Zulia 2023, 40, e2340Spl05. [Google Scholar] [CrossRef]
- Neglia, G.; Matera, R.; Cotticelli, A.; Salzano, A.; Cimmino, R.; Campanile, G. Precision livestock farming of buffalo species: A sustainable approach for the future. Rev. Científica Fac. Veterinária 2023, 33, 124. [Google Scholar]
- Walaa, F.; Muzammal, A.; Majid, A. Identification, Biochemical Characterization, and Safety Attributes of Locally Isolated Lactobacillus fermentum from Bubalus bubalis (Buffalo) Milk as a Probiotic. Microorganisms 2022, 10, 954. [Google Scholar] [CrossRef]
- Beltrão, N.P.F.; Junior, M.V.D.C.F. Lipids in the reproduction of sires. Trop. Anim. Health Prod. 2023, 55, 324. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Alles, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hariyani, A.S.; Hazra, T.; Thesiya, A.J.; Ahuja, K.K. Comparative study of fatty acids, triacylglycerols and nutritional indices of ghee of goats, cows and buffaloes. Indian J. Small Rumin. (The) 2024, 30, 159–167. [Google Scholar] [CrossRef]
- Filho, L.C.P.M.; Seó, H.L.; Daros, R.R.; Enriquez-Hidalgo, D.; Wendling, A.V.; Machado, L.C.P. Voisin rational grazing as a sustainable alternative for livestock production. Animals 2021, 11, 3494. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Alex, R.; Gowane, G.; Vohra, V.; Joshi, P.; Kumar, R.; Verma, A. Weighted single step GWAS reveals genomic regions associated with economic traits in Murrah buffaloes. Anim. Biotechnol. 2024, 1, 2319622. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Poltronieri, F. Tratado de Nutrição e dietoterapia. Guanab. Koogan 2019, 1, 1112. [Google Scholar]
- Khalil, W.A.; Hassan, M.A.; El-Harairy, M.A.; Abdelnour, S.A. Supplementation of Thymoquinone Nanoparticles to Semen Extender Boosts Cryotolerance and Fertilizing Ability of Buffalo Bull Spermatozoa. Animals 2023, 13, 2973. [Google Scholar] [CrossRef]
- Kerman, B.E.; Self, W.; Yassine, H.N. Can the gut microbiome inform the effects of omega-3 fatty acid supplementation trials on cognition? Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 116–124. [Google Scholar] [CrossRef]
- Castellanos-Perilla, N.; Borda, M.G.; Aarsland, D.; Barreto, G.E. An analysis of omega-3 clinical trials and a call for personalized supplementation for dementia prevention. Expert Rev. Neurother. 2024, 24, 1–12. [Google Scholar] [CrossRef]
- Fathy, A.; Hassan, R.A.; Soliman, E.S. Productive and reproductive traits and clinical epidemiological measures in discriminant analysis between baladi buffaloes and crossbred. Adv. Anim. Vet. Sci. 2023, 11, 45–56. [Google Scholar] [CrossRef]
- Fűrész, A.; Penksza, K.; Sipos, L.; Turcsányi-Járdi, I.; Szentes, S.; Fintha, G.; Penksza, P.; Viszló, L.; Szalai, F.; Wagenhoffer, Z. Examination of the Effects of Domestic Water Buffalo (Bubalus bubalis) Grazing on Wetland and Dry Grassland Habitats. Plants 2023, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Almadaly, E.A.; Abdel-Salam, A.B.S.; Sahwan, F.M.; Kahilo, K.A.; Abouzed, T.K.; El-Domany, W.B. Fertility-associated biochemical components in seminal plasma and serum of buffalo (Bubalus bubalis) bulls. Front. Vet. Sci. 2023, 9, 1043379. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Z.D.; Aref, A.T.; Miladinovic, D.; Mah, C.Y.; Raj, G.V.; Hoy, A.J.; Butler, L.M. Peri-prostatic adipose tissue: The metabolic microenvironment of prostate cancer. BJU Int. 2018, 121, 9–21. [Google Scholar] [CrossRef]
- Mills, J.; Driscoll, M. The hidden health impacts of industrial livestock systems: Transforming livestock systems for better human, animal and planetary health. Cabi Digit. Libr. 2022, 316, 77. [Google Scholar]
- Efferding, S.; McCune, D. The Vertical Diet: A Simple, Sensible, and Sustainable Lifestyle Plan to Improve Body Composition F Or Optimal Health and Performance. Vic. Belt Publ. 2021, 1, 240. [Google Scholar]
- Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian dairy products: Ethnic foods with health benefits. Microorganisms 2021, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Assis, J.R.; Assisi, A.C.M.; Gallardo, W.B. Nutrigenômica aplicada ao perfil de ácidos graxos e marmoreio na carne de ruminantes. BIOFARM—J. Biol. Pharm. Agric. Manag. 2020, 16, 196–214. [Google Scholar]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; Vecchia, C.L.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Aterosclerose 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.R.D.A.; Carvalho, F.F.R.D.; Neves, M.L.M.W.; Neto, J.D.P.; Vieira, G.H.P.; Pessoa, R.A.S. Carcass and meat traits of bubaline finished on sugarcane-based diets supplemented with spineless cactus as a replacement for wheat bran. Anim. Biosci. 2022, 35, 47. [Google Scholar] [CrossRef]
- Ramadan, F.M.; Alim, T.S.A. Chemical Properties and Oxidative Stability of Modified of Table Margarine from Buffalo Fat Fractions with Vegetable Oil Oleogels. J. Food Dairy Sci. 2024, 14, 323–329. [Google Scholar] [CrossRef]
- Camcı, S.; Erdem, H. Some carcass quality characteristics of Anatolian buffaloes slaughtered at a private slaughterhouse in Samsun Province. Cabi Digit. Libr. 2022, 10, 1646–1653. [Google Scholar]
- Pantoja, L.S.G.; Amante, E.R.; Rodrigues, A.M.D.C.; Silva, L.H.M.D. World scenario for the valorization of byproducts of buffalo milk production chain. J. Clean. Prod. 2022, 364, 132605. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Dietary strategies to enrich milk with healthy fatty acids–A review. Ann. Anim. Sci. 2022, 22, 523–536. [Google Scholar] [CrossRef]
- Kaur, L.; Elamurugan, A.; Chian, F.M.; Zhu, X.; Boland, M. Protein and Lipid Digestibility of Pasture-Raised and Grain-Finished Beef: An In Vitro Comparison. Foods 2023, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.F.M.; Ahmad, H.; Nor, N.M.; Meng, G.Y.; Saad, M.Z.; Bakar, M.Z.A.; Rahman, N.A.; Irawan, A.; Jayanegara, A.; Hassim, H.A. In-vitro ruminal ecosystem in buffaloes on concentrates and fat supplementation. Adv. Anim. Vet. Sci. 2023, 11, 1313. [Google Scholar]
- Singh, R.K.; Dey, A.; Thakur, S.; Singh, M.; Lailer, P.C. Modulation of Murrah Buffalo (Bubalus bubalis) Rumen Functions for In Vitro Fatty Acid Bio-Hydrogenation, Methane Production and Fermentation Pattern of Total Mixed Ration Supplemented with Allium sativum (Garlic) Essential Oils. Fermentation 2023, 9, 615. [Google Scholar] [CrossRef]
- Gomes, A.L.M.; Magalhães, J.A.; Neves, J.P.; Silva, L.R.L.D.; Gomes, R.V.D.S.; França, R.G.D.O.; Nogueira, T.R. Effects of arginine, glutamine and omega-3 supplementation on the inflammatory response and nutritional status of cancer patients. Res. Soc. Dev. 2020, 9, e193953285. [Google Scholar] [CrossRef]
- Poli, A.; Agostoni, C.; Visioli, F. Dietary fatty acids and inflammation: Focus on the n-6 series. Int. J. Mol. Sci. 2023, 24, 4567. [Google Scholar] [CrossRef]


| Nutrients | Buffalo | Cattle |
|---|---|---|
| Proteins | 21.13 g | 19.23 g |
| Isoleucine | 5.0 g | 4.8 g |
| Leucine | 8.1 g | 8.1 g |
| Lysine | 8.4 g | 8.9 g |
| Methionine + Cystine | 3.9 g | 4.0 g |
| Phenylalanine + Tyrosine | 6.7 g | 8.0 g |
| Threonine | 3.8 g | 4.6 g |
| Tryptophan | 1.0 g | 1.1 g |
| Valine | 4.5 g | 5.0 g |
| Myristic acid | 15.3 mg | 9.9 mg |
| Palmitic acid | 189.3 mg | 143.9 mg |
| Stearic acid | 173.7 mg | 117.7 mg |
| Oleic acid | 253.0 mg | 267.3 mg |
| Total SFA | 383.0 mg | 275.7 mg |
| Total PUFA | 255.7 mg | 267.9 mg |
| Total MUFA | 182.0 mg | 175.0 mg |
| Cholesterol | 41.3 mg | 60–90 mg |
| Thiamine | 0.045 mg | 0.15 mg |
| Riboflavin | 1.7 mg | 0.26 mg |
| Niacin | 20 mg | 6.30 mg |
| Pyridoxine | 0.253 mg | 0.30 mg |
| Cobalamin | 2.131 mg | 1.00 mg |
| Iron | 2.56 mg | 1.2 mg |
| Zinc | 4.0 mg | 3.2 mg |
| Magnesium | 24.2 mg | 25.7 mg |
| Calcium | 5.1 mg | 7.4 mg |
| Phosphorus | 181.1 mg | 109.4 mg |
| Potassium | 290.3 mg | 312.4 mg |
| Sodium | 74.6 mg | 61.9 mg |
| Nutrients | Buffalo | Cattle |
|---|---|---|
| Proteins | 21.4 g | 20.7 g |
| Total lipids | 40.72 mg | 5.4 g |
| Total cholesterol | 2.57 mg | 393 mg |
| Lauric acid | 0.15 mg | 3.25 mg |
| Myristic acid | 0.34 mg | 0.005 mg |
| Palmitic acid | 17.66 mg | 24.4 mg |
| Stearic acid | 25.24 mg | 18.8 mg |
| Oleic acid | 18.15 mg | 6.77 mg |
| MUFA | 22.77 mg | 34.4 mg |
| PUFA | 24.22 mg | 4.92 mg |
| SFA | 47.67 mg | 60.7 mg |
| Sodium | 60.04 mg | 76 mg |
| Potassium | 274 mg | 265 mg |
| Calcium | 5.60 mg | 4 mg |
| Magnesium | 6.20 mg | 12 mg |
| Iron | 20.86 mg | 5.6 mg |
| Zinc | 94.6 mg | 3.5 mg |
| Phosphorus | 11.71 mg | 334 mg |
| Thiamine | 0.04 mg | 0.14 mg |
| Riboflavin | 0.50 mg | 0.90 mg |
| Cobalamin | 1.66 mcg | 83.1 mcg |
| Mineral | Functions |
|---|---|
| Cácio (Ca) | Components bone mineralization, metabolic regulation, blood clotting, muscle contraction, nerve impulse transmission. |
| Phosphorus (P) | Components DNA and RNA, part of high-energy compounds (ATP), regulation of allosteric enzymes, component of phospholipids. |
| Potassium (K) | Regulation of osmotic pressure, nerve impulse transmission, regulation of acid-base balance, muscle contraction, and control of water balance. |
| Sulfur (S) | Sulfur amino acid component, biotin and thiamine component, mucopolysaccharides component, detoxification reactions. |
| Sodium (Na) | Regulation of osmotic pressure, nerve conduction, active transport of nutrients, regulation of acid-base balance, muscle contraction, and control of water balance. |
| Chlorine (Cl) | Regulation of osmotic pressure, regulation of acid-base balance, control of water balance, and formation of hydrochloric acid in gastric juice. |
| Magnesium (Mg) | Cofactor of more than 300 enzymes, components of bones, and neuromuscular activity. |
| Mineral | Function |
|---|---|
| Iron (Fe) | Oxygen transport and storage (hemoglobin, myoglobin), electron transport, enzyme component (catalase, tryptophan 5-monooxygenase, phenylalanine 4-monooxygenase, aconitase). |
| Zinc (Zn) | Component of more than 70 enzymes (alcohol dehydrogenase, DNA polymerase, RNA polymerase, carbonic anhydrase, carboxypeptidase, pyruvate dehydrogenase), gene expression, membrane stability. |
| Copper (Cu) | Component of many enzymes (lysyl oxidase, tyrosinase, cytochrome oxidase, superoxide dismutase). |
| Iodine (I) | Component of thyroid hormones |
| Manganese (Mg) | Enzyme component (pyruvate carboxylase, arginase, mitochondrial superoxide dismutase), enzyme activator (glycosyl transferases). |
| Cobalt (Co) | Component from vitamin B12. |
| Molibidênio (Mo) | Enzyme component (xanthine oxidase, sulfite oxidase, aldeide oxidase). |
| Selenium (Se) | Enzyme component (glutathione peroxidase, iodothyronine deiodase type I). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.S.; Silva, J.A.R.d.; Silva, W.C.d.; Silva, É.B.R.d.; Belo, T.S.; Sousa, C.E.L.; Rodrigues, T.C.G.d.C.; Silva, A.G.M.e.; Prates, J.A.M.; Lourenço-Júnior, J.d.B. A Review of the Nutritional Aspects and Composition of the Meat, Liver and Fat of Buffaloes in the Amazon. Animals 2024, 14, 1618. https://doi.org/10.3390/ani14111618
Rodrigues LS, Silva JARd, Silva WCd, Silva ÉBRd, Belo TS, Sousa CEL, Rodrigues TCGdC, Silva AGMe, Prates JAM, Lourenço-Júnior JdB. A Review of the Nutritional Aspects and Composition of the Meat, Liver and Fat of Buffaloes in the Amazon. Animals. 2024; 14(11):1618. https://doi.org/10.3390/ani14111618
Chicago/Turabian StyleRodrigues, Laurena Silva, Jamile Andrea Rodrigues da Silva, Welligton Conceição da Silva, Éder Bruno Rebelo da Silva, Tatiane Silva Belo, Carlos Eduardo Lima Sousa, Thomaz Cyro Guimarães de Carvalho Rodrigues, André Guimarães Maciel e Silva, José António Mestre Prates, and José de Brito Lourenço-Júnior. 2024. "A Review of the Nutritional Aspects and Composition of the Meat, Liver and Fat of Buffaloes in the Amazon" Animals 14, no. 11: 1618. https://doi.org/10.3390/ani14111618
APA StyleRodrigues, L. S., Silva, J. A. R. d., Silva, W. C. d., Silva, É. B. R. d., Belo, T. S., Sousa, C. E. L., Rodrigues, T. C. G. d. C., Silva, A. G. M. e., Prates, J. A. M., & Lourenço-Júnior, J. d. B. (2024). A Review of the Nutritional Aspects and Composition of the Meat, Liver and Fat of Buffaloes in the Amazon. Animals, 14(11), 1618. https://doi.org/10.3390/ani14111618










