Effect of Transcutaneous Electrical Nerve Stimulation on Gait Parameters in Dogs with Osteoarthritis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation Methods
2.1.1. Pressure-Sensitive Mat
2.1.2. Clinical Examination
2.1.3. Pain Questionnaires
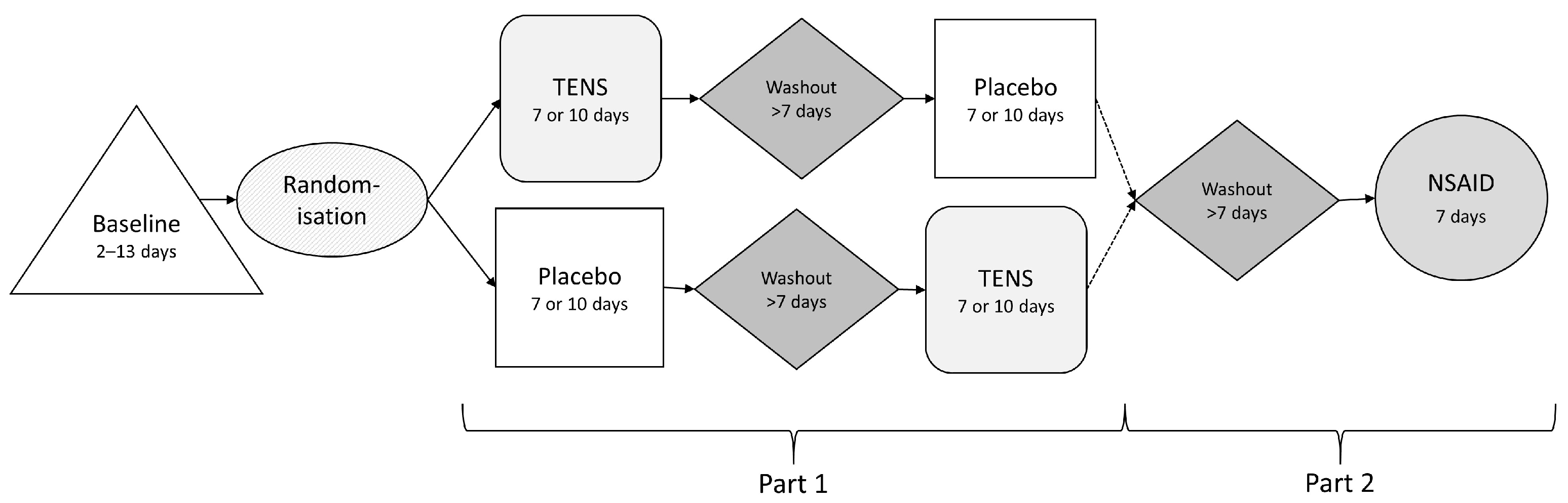
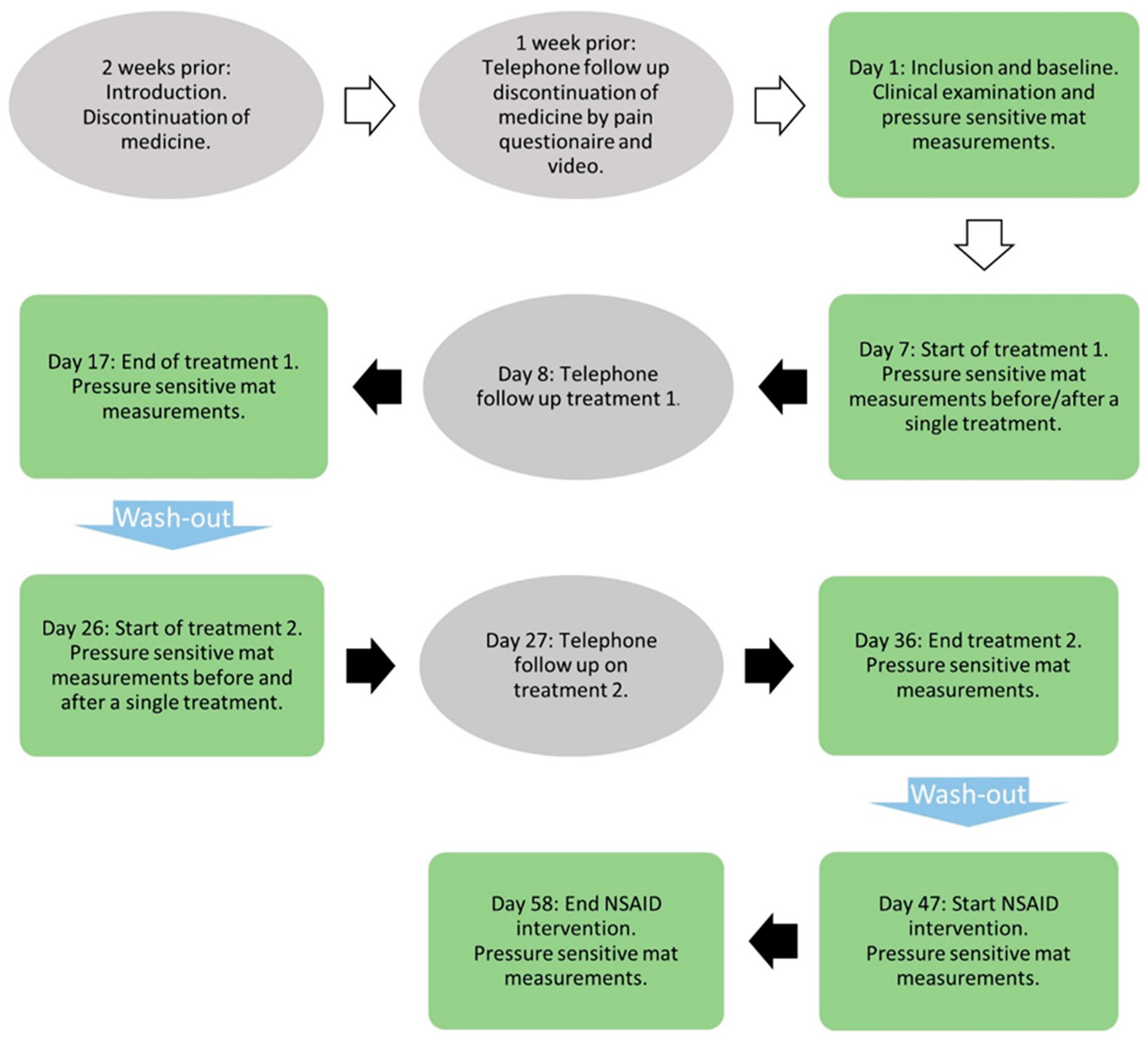
2.2. Study Protocol
2.2.1. Transcutaneous Electrical Nerve Stimulation and Placebo Treatment
2.2.2. NSAID Treatment
2.3. Data Management and Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Gait Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci. Rep. 2018, 8, 5641. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, B.N.; Egenvall, A.; Hedhammar, Å.; Olson, P. Mortality in over 350,000 Insured Swedish dogs from 1995–2000: I. Breed-, Gender-, Age- and Cause-specific Rates. Acta Vet. Scand. 2005, 46, 105. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Abood, S.K.; Fascetti, A.J.; Fleeman, L.M.; Michel, K.E.; Laflamme, D.P.; Bauer, C.; Kemp, B.L.E.; Van Doren, J.R.; Willoughby, K.N. Disease prevalence among dogs and cats in the United States and Australia and proportions of dogs and cats that receive therapeutic diets or dietary supplements. J. Am. Vet. Med. Assoc. 2006, 229, 531–534. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Longevity and mortality of owned dogs in England. Vet. J. 2013, 198, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A. Osteoarthritis: Joint Anatomy, Physiology, and Pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Braun, L.; Tichy, A.; Peham, C.; Bockstahler, B. Comparison of vertical force redistribution in the pads of dogs with elbow osteoarthritis and healthy dogs. Vet. J. 2019, 250, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.; Comerford, E. An update on mobility assessment of dogs with musculoskeletal disease. J. Small Anim. Pract. 2023, 64, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Amodie, D.M.; Cernicchiaro, N.; Lascelles, B.D.X.; Pavlock, A.M.; Roberts, C.; Bartram, D.J. Identification of canine osteoarthritis using an owner-reported questionnaire and treatment monitoring using functional mobility tests. J. Small Anim. Pract. 2022, 63, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Barbeau-Grégoire, M.; Otis, C.; Cournoyer, A.; Moreau, M.; Lussier, B.; Troncy, E. A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 10384. [Google Scholar] [CrossRef]
- Mille, M.A.; McClement, J.; Lauer, S. Physiotherapeutic Strategies and Their Current Evidence for Canine Osteoarthritis. Vet. Sci. 2023, 10, 2. [Google Scholar] [CrossRef]
- Pye, C.; Bruniges, N.; Peffers, M.; Comerford, E. Advances in the pharmaceutical treatment options for canine osteoarthritis. J. Small Anim. Pract. 2022, 63, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Mosley, C.; Edwards, T.; Romano, L.; Truchetti, G.; Dunbar, L.; Schiller, T.; Gibson, T.; Bruce, C.; Troncy, E. Proposed Canadian Consensus Guidelines on Osteoarthritis Treatment Based on OA-COAST Stages 1–4. Front. Vet. Sci. 2022, 9, 830098. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, R.A.; German, A.J. Investigation and management of canine osteoarthritis. Practice 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Musco, N.; Vassalotti, G.; Mastellone, V.; Cortese, L.; della Rocca, G.; Molinari, M.L.; Calabrò, S.; Tudisco, R.; Cutrignelli, M.I.; Lombardi, P. Effects of a nutritional supplement in dogs affected by osteoarthritis. Vet. Med. Sci. 2019, 5, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.; Robertson, S.; Simpson, W. 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2015, 51, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Elkholly, D.A.; Brodbelt, D.C.; Church, D.B.; Pelligand, L.; Mwacalimba, K.; Wright, A.K.; O’Neill, D.G. Side Effects to Systemic Glucocorticoid Therapy in Dogs Under Primary Veterinary Care in the UK. Front. Vet. Sci. 2020, 7, 515. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Dean, R.S.; Davis, G.N.D.; Murrell, J.C. An analysis of the relative frequencies of reported adverse events associated with NSAID administration in dogs and cats in the United Kingdom. Vet. J. 2015, 206, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Steagall, B.P.; Steagall, P.V.M.; Lascelles, B.D.X. Systematic Review of Nonsteroidal Anti-Inflammatory Drug-Induced Adverse Effects in Dogs. J. Vet. Intern. Med. 2013, 27, 1011–1019. [Google Scholar] [CrossRef]
- Luna, S.P.; Basílio, A.C.; Steagall, P.V.; Machado, L.P.; Moutinho, F.Q.; Takahira, R.K.; Brandão, C.V. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am. J. Vet. Res. 2007, 68, 258–264. [Google Scholar] [CrossRef]
- Läkemedelsverket. Smärtbehandling hos hund och katt. Inf. Från Läkemedelsverket 2005, 16, 17–27. [Google Scholar]
- Lascelles, B.D.; McFarland, J.M.; Swann, H. Guidelines for safe and effective use of NSAIDs in dogs. Vet. Ther. Res. Appl. Vet. Med. 2005, 6, 237–251. [Google Scholar]
- Mabry, K.; Hill, T.; Tolbert, M.K. Prevalence of gastrointestinal lesions in dogs chronically treated with nonsteroidal anti-inflammatory drugs. J. Vet. Intern. Med. 2021, 35, 853–859. [Google Scholar] [CrossRef]
- Davis, K.N.; Hellyer, P.W.; Carr, E.C.J.; Wallace, J.E.; Kogan, L.R. Qualitative study of owner perceptions of chronic pain in their dogs. J. Am. Vet. Med. Assoc. 2019, 254, 88–92. [Google Scholar] [CrossRef]
- Vance, C.G.T.; Dailey, D.L.; Rakel, B.A.; Sluka, K.A. Using TENS for pain control: The state of the evidence. Pain Manag. 2014, 4, 197–209. [Google Scholar] [CrossRef]
- Sluka, K.A.; Walsh, D. Transcutaneous electrical nerve stimulation: Basic science mechanisms and clinical effectiveness. J. Pain 2003, 4, 109–121. [Google Scholar] [CrossRef]
- Han, J.S.; Chen, X.H.; Sun, S.L.; Xu, X.J.; Yuan, Y.; Yan, S.C.; Hao, J.X.; Terenius, L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991, 47, 295–298. [Google Scholar] [CrossRef]
- Chen, Y.W.; Tzeng, J.I.; Lin, M.F.; Hung, C.H.; Wang, J.J. Transcutaneous electrical nerve stimulation attenuates postsurgical allodynia and suppresses spinal substance P and proinflammatory cytokine release in rats. Phys. Ther. 2015, 95, 76–85. [Google Scholar] [CrossRef]
- Vance, C.G.T.; Rakel, B.A.; Blodgett, N.P.; DeSantana, J.M.; Amendola, A.; Zimmerman, M.B.; Walsh, D.M.; Sluka, K.A. Effects of Transcutaneous Electrical Nerve Stimulation on Pain, Pain Sensitivity, and Function in People With Knee Osteoarthritis: A Randomized Controlled Trial. Phys. Ther. 2012, 92, 898–910. [Google Scholar] [CrossRef]
- Matsuo, H.; Uchida, K.; Nakajima, H.; Guerrero, A.R.; Watanabe, S.; Takeura, N.; Sugita, D.; Shimada, S.; Nakatsuka, T.; Baba, H. Early transcutaneous electrical nerve stimulation reduces hyperalgesia and decreases activation of spinal glial cells in mice with neuropathic pain. Pain 2014, 155, 1888–1901. [Google Scholar] [CrossRef]
- Johnson, M.I.; Paley, C.A.; Wittkopf, P.G.; Mulvey, M.R.; Jones, G. Characterising the Features of 381 Clinical Studies Evaluating Transcutaneous Electrical Nerve Stimulation (TENS) for Pain Relief: A Secondary Analysis of the Meta-TENS Study to Improve Future Research. Medicina 2022, 58, 803. [Google Scholar] [CrossRef]
- Gladwell, P.W.; Cramp, F.; Palmer, S. Foundational Research Could Improve Future Transcutaneous Electrical Nerve Stimulation Evaluations. Medicina 2022, 58, 149. [Google Scholar] [CrossRef]
- Johnson, M.I. Resolving Long-Standing Uncertainty about the Clinical Efficacy of Transcutaneous Electrical Nerve Stimulation (TENS) to Relieve Pain: A Comprehensive Review of Factors Influencing Outcome. Medicina 2021, 57, 378. [Google Scholar] [CrossRef]
- Johnson, M.I.; Paley, C.A.; Jones, G.; Mulvey, M.R.; Wittkopf, P.G. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 2022, 12, e051073. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Nüesch, E.; Sterchi, R.; Kalichman, L.; Hendriks, E.; Osiri, M.; Brosseau, L.; Reichenbach, S.; Jüni, P. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2009, 2009, CD002823. [Google Scholar] [CrossRef]
- Wu, L.C.; Weng, P.W.; Chen, C.H.; Huang, Y.Y.; Tsuang, Y.H.; Chiang, C.J. Literature Review and Meta-Analysis of Transcutaneous Electrical Nerve Stimulation in Treating Chronic Back Pain. Reg. Anesth. Pain Med. 2018, 43, 425–433. [Google Scholar] [CrossRef]
- Gibson, W.; Wand, B.M.; Meads, C.; Catley, M.J.; O’Connell, N.E. Transcutaneous electrical nerve stimulation (TENS) for chronic pain—An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019, 2019, CD011890. [Google Scholar] [CrossRef]
- Sato, K.L.; Sanada, L.S.; Rakel, B.A.; Sluka, K.A. Increasing Intensity of TENS Prevents Analgesic Tolerance in Rats. J. Pain 2012, 13, 884–890. [Google Scholar] [CrossRef]
- Hahm, S.C.; Song, E.; Jeon, H.; Yoon, Y.W.; Kim, J. Transcutaneous Electrical Nerve Stimulation Reduces Knee Osteoarthritic Pain by Inhibiting Spinal Glial Cells in Rats. Phys. Ther. 2019, 99, 1211–1223. [Google Scholar] [CrossRef]
- Krstić, N.; Lazarević-Macanović, M.; Prokić, B.; Mustur, D.; Rheumatology, I.; Stanisavljević, D. Testing the effect of different electrotherapeutic procedures in the treatment of canine ankylosing spondylitis. Acta Vet. 2010, 60, 585–595. [Google Scholar] [CrossRef]
- Mlacnik, E.; Bockstahler, B.A.; Müller, M.; Tetrick, M.A.; Nap, R.C.; Zentek, J. Effects of caloric restriction and a moderate or intense physiotherapy program for treatment of lameness in overweight dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2006, 229, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Levine, D.; Price, M.; Schneider, N.; Millis, D. The effect of TENS on osteoarthritic pain in the stifle of dogs. In Proceedings of the 2nd International Symposium on Rehabilitation and Physical Therapy in Veterinary Medicine, Knoxville, TN, USA, 10–14 August 2002; p. 199. [Google Scholar]
- Hyytiäinen, H.K.; Boström, A.; Asplund, K.; Bergh, A. A Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Electrotherapy. Animals 2023, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.M.; Batista, M.; Morales, M.; Santana, A.; Cuervo, B.; Rubio, M.; Cugat, R.; Sopena, J.; Carrillo, J.M. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet. Res. 2014, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, Z.; Asher, L.; Dean, R.S. Systematic Review of Outcome Measures Reported in Clinical Canine Osteoarthritis Research. Vet. Surg. 2016, 45, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.; Witte, P. Investigation of lameness in dogs: 1. Forelimb. Practice 2011, 33, 20–27. [Google Scholar] [CrossRef]
- Witte, P.; Scott, H. Investigation of lameness in dogs: 2. Hindlimb. Practice 2011, 33, 58–66. [Google Scholar] [CrossRef]
- Waxman, A.S.; Robinson, D.A.; Evans, R.B.; Hulse, D.A.; Innes, J.F.; Conzemius, M.G. Relationship Between Objective and Subjective Assessment of Limb Function in Normal Dogs with an Experimentally Induced Lameness. Vet. Surg. 2008, 37, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Horstman, C.; Conzemius, M. Accuracy and optimization of force platform gait analysis in Labradors with cranial cruciate disease evaluated at a walking gait. Vet. Surg. 2005, 34, 445–449. [Google Scholar] [CrossRef]
- Gibert, S.; Lequang, T.; Maitre, P.; Poujol, L.; Cachon, T.; Carozzo, C.; Fau, D.; Genevois, J.P.; Viguier, E. Sensitivity and specificity to determine lameness in dogs with a pressure walkway system. Comput. Methods Biomech. Biomed. Eng. 2010, 13, 61–62. [Google Scholar] [CrossRef]
- Quinn, M.M.; Keuler, N.S.; Lu, Y.; Faria, M.L.E.; Muir, P.; Markel, M.D. Evaluation of Agreement Between Numerical Rating Scales, Visual Analogue Scoring Scales, and Force Plate Gait Analysis in Dogs. Vet. Surg. 2007, 36, 360–367. [Google Scholar] [CrossRef]
- Rhodin, M.; Bergh, A.; Gustås, P.; Gómez Álvarez, C.B. Inertial sensor-based system for lameness detection in trotting dogs with induced lameness. Vet. J. 2017, 222, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Levine, D.; Marcellin-Little, D.J. Gait Changes Resulting from Orthopedic and Neurologic Problems in Companion Animals: A Review. Adv. Small Anim. Care 2023, 4, 1–20. [Google Scholar] [CrossRef]
- Besancon, M.F.; Conzemius, M.G.; Derrick, T.R.; Ritter, M.J. Comparison of vertical forces in normal greyhounds between force platform and pressure walkway measurement systems. Vet. Comp. Orthop.Traumatol. 2003, 16, 153–157. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Roe, S.C.; Smith, E.; Reynolds, L.; Markham, J.; Marcellin-Little, D.; Bergh, M.S.; Budsberg, S.C. Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. Am. J. Vet. Res. 2006, 67, 277–282. [Google Scholar] [CrossRef]
- Conzemius, M.G.; Torres, B.T.; Muir, P.; Evans, R.; Krotscheck, U.; Budsberg, S. Best practices for measuring and reporting ground reaction forces in dogs. Vet. Surg. 2022, 51, 385–396. [Google Scholar] [CrossRef]
- Avendano, J.N.; Langenbach, A.; Brunke, M.W.; Barnhard, J.A. Ground reaction forces, temporospatial parameters, range of motion, and limb characteristics were analyzed for small and medium size sound dogs with the use of pressure sensitive walkway. Am. J. Vet. Res. 2023, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brønniche Møller Nielsen, M.; Pedersen, T.; Mouritzen, A.; Vitger, A.D.; Nielsen, L.N.; Poulsen, H.H.; Miles, J.E. Kinetic gait analysis in healthy dogs and dogs with osteoarthritis: An evaluation of precision and overlap performance of a pressure-sensitive walkway and the use of symmetry indices. PLoS ONE 2020, 15, e0243819. [Google Scholar] [CrossRef]
- Häusler, K.A.; Braun, D.; Liu, N.C.; Penrose, F.; Sutcliffe, M.P.F.; Allen, M.J. Evaluation of the repeatability of kinetic and temporospatial gait variables measured with a pressure-sensitive treadmill for dogs. Am. J. Vet. Res. 2020, 81, 922–929. [Google Scholar] [CrossRef]
- Kano, W.T.; Rahal, S.C.; Agostinho, F.S.; Mesquita, L.R.; Santos, R.R.; Monteiro, F.O.B.; Castilho, M.S.; Melchert, A. Kinetic and temporospatial gait parameters in a heterogeneous group of dogs. BMC Vet. Res. 2016, 12, 9. [Google Scholar] [CrossRef]
- Adrian, D.; Brown, D. Kinetic symmetry indices and standing gait analysis: A review of current methods and data. Vet. J. 2022, 281, 105814. [Google Scholar] [CrossRef]
- Kieves, N.R.; Hart, J.L.; Evans, R.B.; Duerr, F.M. Comparison of three walkway cover types for use during objective canine gait analysis with a pressure-sensitive walkway. Am. J. Vet. Res. 2019, 80, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Fanchon, L.; Grandjean, D. Accuracy of asymmetry indices of ground reaction forces for diagnosis of hind limb lameness in dogs. Am. J. Vet. Res. 2007, 68, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Lussier, B.; Ballaz, L.; Troncy, E. Kinetic measurements of gait for osteoarthritis research in dogs and cats. Can. Vet. J. 2014, 55, 1057–1065. [Google Scholar] [PubMed]
- Rumph, P.F.; Kincaid, S.A.; Baird, D.K.; Kammermann, J.R.; Visco, D.M.; Goetze, L.F. Vertical ground reaction force distribution during experimentally induced acute synovitis in dogs. Am. J. Vet. Res. 1993, 54, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Rumph, P.F.; Kincaid, S.A.; Visco, D.M.; Baird, D.K.; Kammermann, J.R.; West, M.S. Redistribution of vertical ground reaction force in dogs with experimentally induced chronic hindlimb lameness. Vet. Surg. 1995, 24, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.; Cowderoy, E.; Innes, J. Evaluation of Construct and Criterion Validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) Clinical Metrology Instrument and Comparison to Two Other Instruments. PLoS ONE 2013, 8, e58125. [Google Scholar] [CrossRef] [PubMed]
- Budsberg, S.C.; Torres, B.T.; Kleine, S.A.; Sandberg, G.S.; Berjeski, A.K. Lack of effectiveness of tramadol hydrochloride for the treatment of pain and joint dysfunction in dogs with chronic osteoarthritis. J. Am. Vet. Med. Assoc. 2018, 252, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Budsberg, S.C.; Johnston, S.A.; Schwarz, P.D.; DeCamp, C.E.; Claxton, R. Efficacy of etodolac for the treatment of osteoarthritis of the hip joints in dogs. J. Am. Vet. Med. Assoc. 1999, 214, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Malek, S.; Sample, S.J.; Schwartz, Z.; Nemke, B.; Jacobson, P.B.; Cozzi, E.M.; Schaefer, S.L.; Bleedorn, J.A.; Holzman, G.; Muir, P. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet. Res. 2012, 8, 185. [Google Scholar] [CrossRef]
- Miles, J.; Nielsen, M.; Mouritzen, A.; Pedersen, T.; Nielsen, L.N.; Vitger, A.; Poulsen, H.H. Gait analysis of lameness-free dogs: Experience with a Tekscan pressure-sensitive walkway system. In Proceedings of the BSAVA Congress, Gloucester, UK, 4–6 April 2019; British Small Animal Veterinary Association: Quedgeley, UK, 2019. [Google Scholar]
- Mejia, S.; Duerr, F.M.; Griffenhagen, G.; McGrath, S. Evaluation of the Effect of Cannabidiol on Naturally Occurring Osteoarthritis-Associated Pain: A Pilot Study in Dogs. J. Am. Anim. Hosp. Assoc. 2021, 57, 81–90. [Google Scholar] [CrossRef]
- Moreira, J.P.L.; Tichy, A.; Bockstahler, B. Comparison of the Vertical Force Distribution in the Paws of Dogs with Coxarthrosis and Sound Dogs Walking over a Pressure Plate. Animals 2020, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Bockstahler, B.A.; Vobornik, A.; Müller, M.; Peham, C. Compensatory load redistribution in naturally occurring osteoarthritis of the elbow joint and induced weight-bearing lameness of the forelimbs compared with clinically sound dogs. Vet. J. 2009, 180, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Pavarotti, G.S.; Hivernaud, V.; Brincin, M.; Roche, R.; Barreau, P.; Festy, F.; Gauthier, O. Evaluation of a Single Intra-Articular Injection of Autologous Adipose Tissue for the Treatment of Osteoarthritis: A Prospective Clinical Study in Dogs. Vet. Comp. Orthop. Traumatol. 2020, 33, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Duerr, F. Canine Lameness; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Goodyear, M.D.E.; Krleza-Jeric, K.; Lemmens, T. The Declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Hielm-Björkman, A.K.; Rita, H.; Tulamo, R.-M. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am. J. Vet. Res. 2009, 70, 727. [Google Scholar] [CrossRef] [PubMed]
- Nemery, E.; Gabriel, A.; Cassart, D.; Bayrou, C.; Piret, J.; Antoine, N.; Nilsson, M.; Steinwall, L.; Jacobson, I.; Martins, Â.; et al. Proceedings of the 9th international symposium on veterinary rehabilitation and physical therapy. Acta Vet. Scand. 2016, 58, 85. [Google Scholar] [CrossRef]
- Essner, A.; Zetterberg, L.; Hellström, K.; Gustås, P.; Högberg, H.; Sjöström, R. Psychometric evaluation of the canine brief pain inventory in a Swedish sample of dogs with pain related to osteoarthritis. Acta Vet. Scand. 2017, 59, 44. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Boston, R.C.; Coyne, J.C.; Farrar, J.T. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am. J. Vet. Res. 2007, 68, 631–637. [Google Scholar] [CrossRef]
- FASS vet.: Förteckning över Läkemedel för Veterinärmedicinskt Bruk. Available online: https://www.fass.se/LIF/startpage (accessed on 1 March 2024).
- Madore, E.; Huneault, L.; Moreau, M.; Dupuis, J. Comparison of trot kinetics between dogs with stifle or hip arthrosis. Vet. Comp. Orthop. Traumatol. 2007, 20, 102–107. [Google Scholar] [CrossRef]
- Lim, C.Y.; In, J. Considerations for crossover design in clinical study. Korean J. Anesthesiol. 2021, 74, 293–299. [Google Scholar] [CrossRef]
- Alon, G.; Allin, J.; Inbar, G.F. Optimization of pulse duration and pulse charge during transcutaneous electrical nerve stimulation. Aust. J. Physiother. 1983, 29, 195–201. [Google Scholar] [CrossRef]
- Sluka, K.A.; Bailey, K.; Bogush, J.; Olson, R.; Ricketts, A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain 1998, 77, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chung, J.M.; Willis, W.D. Inhibition of primate spinothalamic tract cells by TENS. J. Neurosurg. 1985, 62, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Gozani, S.N. Remote Analgesic Effects Of Conventional Transcutaneous Electrical Nerve Stimulation: A Scientific And Clinical Review With A Focus On Chronic Pain. J. Pain Res. 2019, 12, 3185–3201. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A. The basic science mechanisms of TENS and clinical implications. APS Bull. 2001, 11, 1–10. [Google Scholar]
- Leonard, G.; Goffaux, P.; Marchand, S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain 2010, 151, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Serra Bragança, F.M.; Hernlund, E.; Thomsen, M.H.; Waldern, N.M.; Rhodin, M.; Byström, A.; van Weeren, P.R.; Weishaupt, M.A. Adaptation strategies of horses with induced forelimb lameness walking on a treadmill. Equine Vet. J. 2021, 53, 600–611. [Google Scholar] [CrossRef]
- Vassalo, F.G.; Rahal, S.C.; Agostinho, F.S.; Mamprim, M.J.; Melchert, A.; Kano, W.T.; dos Reis Mesquita, L.; Doiche, D.P. Gait analysis in dogs with pelvic fractures treated conservatively using a pressure-sensing walkway. Acta Vet. Scand. 2015, 57, 68. [Google Scholar] [CrossRef] [PubMed]
- Fahie, M.A.; Cortez, J.C.; Ledesma, M.; Su, Y. Pressure Mat Analysis of Walk and Trot Gait Characteristics in 66 Normal Small, Medium, Large, and Giant Breed Dogs. Front. Vet. Sci. 2018, 5, 256. [Google Scholar] [CrossRef] [PubMed]
- Rincon Alvarez, J.; Anesi, S.; Czopowicz, M.; Corr, S.A. The Effect of Calibration Method on Repeatability and Reproducibility of Pressure Mat Data in a Canine Population. Vet. Comp. Orthop.Traumatol. 2020, 33, 428–433. [Google Scholar] [CrossRef]
- Light, V.A.; Steiss, J.E.; Montgomery, R.D.; Rumph, P.F.; Wright, J.C. Temporal-spatial gait analysis by use of a portable walkway system in healthy Labrador Retrievers at a walk. Am. J. Vet. Res. 2010, 71, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Seibert, R.; Marcellin-Little, D.J.; Roe, S.C.; DePuy, V.; Lascelles, B.D.X. Comparison of Body Weight Distribution, Peak Vertical Force, and Vertical Impulse as Measures of Hip Joint Pain and Efficacy of Total Hip Replacement. Vet. Surg. 2012, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Oosterlinck, M.; Bosmans, T.; Gasthuys, F.; Polis, I.; Van Ryssen, B.; Dewulf, J.; Pille, F. Accuracy of pressure plate kinetic asymmetry indices and their correlation with visual gait assessment scores in lame and nonlame dogs. Am. J. Vet. Res. 2011, 72, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.G. Cut-off Values for Gait Variables to Detect Forelimb Lameness in Individual Dogs. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2014. [Google Scholar]
- Conzemius, M.G.; Evans, R.B. Caregiver placebo effect for dogs with lameness from osteoarthritis. J. Am. Vet. Med. Assoc. 2012, 241, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Anders, A.; Nolte, I.; Schilling, N. Compensatory load redistribution in walking and trotting dogs with hind limb lameness. Vet. J. 2013, 197, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Dupuis, J.; Bonneau, N.H.; Desnoyers, M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet. Rec. 2003, 152, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.K.; Cross, A.R.; Renberg, W.C.; Dodd, C.E.; Sixby, K.A.; Fritsch, D.A.; Allen, T.A.; Jewell, D.E.; Richardson, D.C.; Leventhal, P.S.; et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2010, 236, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Characterization of weight-bearing compensation in dogs with bilateral hip osteoarthritis. Top. Companion Anim. Med. 2022, 49, 100655. [Google Scholar] [CrossRef]
- Venator, K.P.; Frye, C.W.; Gamble, L.J.; Wakshlag, J.J. Assessment of a Single Intra-Articular Stifle Injection of Pure Platelet Rich Plasma on Symmetry Indices in Dogs with Unilateral or Bilateral Stifle Osteoarthritis from Long-Term Medically Managed Cranial Cruciate Ligament Disease. Vet. Med. Res. Rep. 2020, 11, 31–38. [Google Scholar] [CrossRef]
- Sharkey, M. The challenges of assessing osteoarthritis and postoperative pain in dogs. AAPS J. 2013, 15, 598–607. [Google Scholar] [CrossRef]
- Budsberg, S.C.; Jevens, D.J.; Brown, J.; Foutz, T.L.; DeCamp, C.E.; Reece, L. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am. J. Vet. Res. 1993, 54, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Abdelhadi, J.; Wefstaedt, P.; Galindo-Zamora, V.; Anders, A.; Nolte, I.; Schilling, N. Load redistribution in walking and trotting Beagles with induced forelimb lameness. Am. J. Vet. Res. 2013, 74, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bergh, A.; Gómez Álvarez, C.B.; Rhodin, M.; Gustås, P. Head and pelvic vertical displacement in dogs with induced swinging limb lameness: An experimental study. Acta Vet. Scand. 2018, 60, 81. [Google Scholar] [CrossRef] [PubMed]
- Phutthachalee, S.; Mählmann, K.; Seesupa, S.; Lischer, C. Upper body movement analysis of multiple limb asymmetry in 367 clinically lame horses. Equine Vet. J. 2021, 53, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.M.; DeCamp, C.E.; Soutas-Little, R.W.; Braden, T.D.; Richter, M.A. Effects of subject velocity on force plate-measured ground reaction forces in healthy greyhounds at the trot. Am. J. Vet. Res. 1993, 54, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Hans, E.C.; Zwarthoed, B.; Seliski, J.; Nemke, B.; Muir, P. Variance associated with subject velocity and trial repetition during force platform gait analysis in a heterogeneous population of clinically normal dogs. Vet. J. 2014, 202, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Mickelson, M.A.; Vo, T.; Piazza, A.M.; Volstad, N.J.; Nemke, B.W.; Muir, P. Influence of trial repetition on lameness during force platform gait analysis in a heterogeneous population of clinically lame dogs each trotting at its preferred velocity. Am. J. Vet. Res. 2017, 78, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Jevens, D.; Hauptman, J.; DeCamp, C.; Budsberg, S.; Soutas-Little, R. Contributions to variance in force-plate analysis of gait in dogs. Am. J. Vet. Res. 1993, 54, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Keebaugh, A.E.; Redman-Bentley, D.; Griffon, D.J. Influence of leash side and handlers on pressure mat analysis of gait characteristics in small-breed dogs. J. Am. Vet. Med. Assoc. 2015, 246, 1215–1221. [Google Scholar] [CrossRef]
- Salichs, M.; Badiella, L.; Sarasola, P.; Homedes, J. Enflicoxib for canine osteoarthritis: A randomized, blind, multicentre, non-inferiority clinical trial compared to mavacoxib. PLoS ONE 2022, 17, e0274800. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Main, D.C. Surgical trauma and chronically painful conditions—Within our comfort level but beyond theirs? J. Am. Vet. Med. Assoc. 2002, 221, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Salter, M.W. Neuronal Plasticity: Increasing the Gain in Pain. Science 2000, 288, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- 11th International Conference on Equine Exercise Physiology, Uppsala, Sweden 2022. Comp. Exerc. Physiol. 2022, 18, S1–S121. [CrossRef]
- Martimbianco, A.L.C.; Porfírio, G.J.M.; Pacheco, R.L.; Torloni, M.R.; Riera, R. Transcutaneous electrical nerve stimulation (TENS) for chronic neck pain. Cochrane Database Syst. Rev. 2019, 2019, CD011927. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, L.; Yonge, K.A.; Welch, V.; Marchand, S.; Judd, M.; Wells, G.A.; Tugwell, P. Transcutaneous electrical nerve stimulation (TENS) for the treatment of rheumatoid arthritis in the hand. Cochrane Database Syst. Rev. 2003, 2003, CD004377. [Google Scholar] [CrossRef]
- Gibson, W.; Wand, B.M.; O’Connell, N.E. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 2017, CD011976. [Google Scholar] [CrossRef]

| Dog | Age (Years) | Breed | Weight (kg) | Lameness at Inclusion | Diagnosis and Electrode Placement | Number of Days of Treatment | NSAID Treatment through the Whole Study |
|---|---|---|---|---|---|---|---|
| Dog 1 | 8 | Beagle | 13 | 1° LH | OA stifle LH. Cruciate ligament injury LH. DP. | 7 | No |
| Dog 2 | 8 | Labrador Retriever | 31 | 2° LF | OA metacarpal joint phalanx 4 and 5 LF, phalanx 5 RF and elbow LF. DP. | 10 | Yes |
| Dog 3 | 6.5 | Poodle, medium size | 7 | 1° LF | OA elbow LF. DP. | 10 | No |
| Dog 4 | 8 | Malinois | 27 | 1° LF | Moderate OA shoulder LF. Mild OA shoulder RF. Disc herniation L7S1. DP. | 10 | No |
| Dog 5 | 3 | Mixed breed | 15 | 1° LH | OA stifle LH. DP. | 10 | No |
| Dog 6 | 8 | Mixed breed | 41 | 2° RH | OA stifle BH. DP. | 10 | No |
| Dog 7 | 6 | Mixed breed | 18 | 1° LH | OA hip BH. DP. | 10 | No |
| Dog 8 | 8 | Border Collie | 16 | 1° RH | OA lumbar spine. OA shoulder and phalanx BF. PP. | 10 | No |
| Dog 9 | 8 | Labrador Retriever | 37 | 3° RF | OA carpus and phalanx BF. OA hips BH. DP. | 10 | Yes |
| Dog 10 | 5 | Mixed breed | 17 | 3° LF | OA elbows and phalanx BF. Spondylosis spinal cord. DP. | 10 | No |
| Dog 11 | 7 | Flatcoated Retriever | 26 | 1° LF | OA carpus RF. Lameness LF. DP. | 10 | No |
| Dog 12 | 2 | Labrador Retriever | 30 | 1° LF | Fragmentation of processus coronoideus medialis elbow LF. OA elbow LF. DP. | 10 | No |
| Dog 13 | 7 | Staffordshire Bull Terrier | 13 | 1° LF | OA stifle LH. Operated cruciate ligament injury BH. Elbow dysplasia grade 2 BF. Hip dysplasia BH. DP. | 10 | No |
| Dog 14 | 9 | Mixed Breed | 30 | 1° LH | OA hips BH. OA lumbar spine. DP. | 10 | No |
| Dog 15 | 8 | Australian Cattle Dog | 20 | 1° LH | OA tarsus LH. DP. | 10 | No |
| Parameter | Time | Leg | TENS (Mean ± SD) | N | Placebo (Mean ± SD) | N | p-Value | NSAID (Mean ± SD) | N | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Stance time (sec) | Before | Lame | 0.19 ± 0.04 | 15 | 0.20 ± 0.04 | 15 | 0.18 ± 0.04 | 9 | ||
| Contralateral | 0.20 ± 0.04 | 15 | 0.20 ± 0.04 | 15 | 0.19 ± 0.03 | 9 | ||||
| Ipsilateral | 0.19 ± 0.04 | 15 | 0.20 ± 0.04 | 15 | 0.19 ± 0.05 | 9 | ||||
| Diagonal | 0.20 ± 0.04 | 15 | 0.20 ± 0.04 | 15 | 0.19 ± 0.05 | 9 | ||||
| After single | Lame | 0.20 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.80 | ||||
| Contralateral | 0.20 ± 0.04 | 15 | 0.20 ± 0.04 | 15 | 0.87 | |||||
| Ipsilateral | 0.20 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.14 | |||||
| Diagonal | 0.20 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.19 | |||||
| After multiple | Lame | 0.20 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.67 | 0.19 ± 0.04 | 10 | 0.66 | |
| Contralateral | 0.20 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.42 | 0.19 ± 0.03 | 10 | 0.36 | ||
| Ipsilateral | 0.19 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.98 | 0.19 ± 0.04 | 10 | 0.20 | ||
| Diagonal | 0.20 ± 0.04 | 15 | 0.19 ± 0.04 | 15 | 0.35 | 0.19 ± 0.04 | 10 | 0.06 | ||
| Swing time (sec) | Before | Lame | 0.25 ± 0.04 | 15 | 0.25 ± 0.04 | 15 | 0.25 ± 0.04 | 9 | ||
| Contralateral | 0.25 ± 0.04 | 15 | 0.25 ± 0.04 | 15 | 0.25 ± 0.04 | 9 | ||||
| Ipsilateral | 0.26 ± 0.03 | 15 | 0.26 ± 0.03 | 15 | 0.25 ± 0.03 | 9 | ||||
| Diagonal | 0.25 ± 0.03 | 15 | 0.26 ± 0.04 | 15 | 0.24 ± 0.04 | 9 | ||||
| After single | Lame | 0.25 ± 0.03 | 15 | 0.26 ± 0.03 | 15 | 0.26 | ||||
| Contralateral | 0.25 ± 0.04 | 15 | 0.25 ± 0.04 | 15 | 0.19 | |||||
| Ipsilateral | 0.26 ± 0.03 | 15 | 0.26 ± 0.03 | 15 | 0.86 | |||||
| Diagonal | 0.26 ± 0.03 | 15 | 0.26 ± 0.03 | 15 | 0.08 | |||||
| After multiple | Lame | 0.25 ± 0.03 | 15 | 0.25 ± 0.04 | 15 | 0.58 | 0.26 ± 0.04 | 10 | 0.80 | |
| Contralateral | 0.25 ± 0.04 | 15 | 0.25 ± 0.05 | 15 | 0.07 | 0.25 ± 0.04 | 10 | 0.33 | ||
| Ipsilateral | 0.26 ± 0.03 | 15 | 0.25 ± 0.04 | 15 | 0.25 | 0.25 ± 0.03 | 10 | 0.63 | ||
| Diagonal | 0.25 ± 0.03 | 15 | 0.26 ± 0.04 | 14 | 0.19 | 0.24 ± 0.03 | 10 | 0.39 | ||
| Stride time (sec) | Before | Lame | 0.44 ± 0.06 | 15 | 0.45 ± 0.07 | 15 | 0.43 ± 0.06 | 9 | ||
| Contralateral | 0.45 ± 0.06 | 15 | 0.45 ± 0.06 | 15 | 0.44 ± 0.07 | 9 | ||||
| Ipsilateral | 0.45 ± 0.06 | 15 | 0.45 ± 0.06 | 15 | 0.44 ± 0.08 | 9 | ||||
| Diagonal | 0.45 ± 0.06 | 15 | 0.45 ± 0.06 | 15 | 0.44 ± 0.07 | 9 | ||||
| After single | Lame | 0.45 ± 0.06 | 15 | 0.45 ± 0.05 | 15 | 0.34 | ||||
| Contralateral | 0.45 ± 0.06 | 15 | 0.45 ± 0.05 | 15 | 0.06 | |||||
| Ipsilateral | 0.46 ± 0.06 | 15 | 0.45 ± 0.06 | 15 | 0.47 | |||||
| Diagonal | 0.45 ± 0.06 | 15 | 0.45 ± 0.05 | 15 | 0.97 | |||||
| After multiple | Lame | 0.45 ± 0.06 | 15 | 0.44 ± 0.06 | 15 | 0.28 | 0.44 ± 0.07 | 10 | 0.78 | |
| Contralateral | 0.44 ± 0.06 | 15 | 0.45 ± 0.07 | 15 | 0.25 | 0.44 ± 0.06 | 10 | 0.59 | ||
| Ipsilateral | 0.45 ± 0.06 | 15 | 0.44 ± 0.06 | 15 | 0.29 | 0.44 ± 0.06 | 10 | 0.46 | ||
| Diagonal | 0.45 ± 0.06 | 20 | 0.45 ± 0.07 | 15 | 0.24 | 0.44 ± 0.07 | 10 | 0.42 | ||
| Stride length (cm) | Before | Lame | 88.96 ± 14.37 | 15 | 89.17 ± 13.80 | 15 | 87.36 ± 14.20 | 9 | ||
| Contralateral | 88.90 ± 14.35 | 15 | 89.07 ± 13.91 | 15 | 86.05 ± 14.90 | 9 | ||||
| Ipsilateral | 89.23 ± 14.09 | 15 | 88.94 ± 13.52 | 15 | 87.71 ± 13.86 | 9 | ||||
| Diagonal | 89.57 ± 14.46 | 15 | 89.38 ± 13.95 | 15 | 87.44 ± 14.54 | 9 | ||||
| After single | Lame | 88.22 ± 14.09 | 15 | 90.47 ± 14.77 | 15 | 0.52 | ||||
| Contralateral | 87.84 ± 14.06 | 15 | 91.04 ± 15.19 | 15 | 0.13 | |||||
| Ipsilateral | 88.70 ± 14.31 | 15 | 90.38 ± 14.53 | 15 | 0.90 | |||||
| Diagonal | 88.41 ± 14.05 | 15 | 90.76 ± 14.75 | 15 | 0.49 | |||||
| After multiple | Lame | 88.86 ± 13.45 | 15 | 90.14 ± 15.18 | 15 | 0.50 | 87.90 ± 14.36 | 10 | 0.96 | |
| Contralateral | 88.40 ± 13.41 | 15 | 92.60 ± 16.00 | 15 | 0.06 | 87.39 ± 14.24 | 10 | 0.66 | ||
| Ipsilateral | 89.03 ± 13.56 | 15 | 90.68 ± 15.52 | 15 | 0.67 | 87.98 ± 14.17 | 10 | 0.96 | ||
| Diagonal | 88.53 ± 13.32 | 15 | 93.04 ± 16.37 | 15 | 0.07 | 87.97 ± 14.26 | 10 | 0.81 | ||
| Peak vertical force (%BW) | Before | Lame | 61.15 ± 16.48 | 15 | 62.08 ± 16.56 | 15 | 59.33 ± 21.59 | 9 | ||
| Contralateral | 71.47 ± 20.97 | 15 | 72.57 ± 20.68 | 15 | 68.03 ± 23.22 | 9 | ||||
| Ipsilateral | 67.33 ± 22.68 | 15 | 69.05 ± 22.84 | 15 | 70.71 ± 18.81 | 9 | ||||
| Diagonal | 68.15 ± 22.52 | 15 | 70.85 ± 23.43 | 15 | 69.10 ± 18.99 | 9 | ||||
| After single | Lame | 60.47 ± 17.37 | 15 | 58.94 ± 12.03 | 15 | 0.57 | ||||
| Contralateral | 68.87 ± 20.03 | 15 | 68.98 ± 15.19 | 15 | 0.79 | |||||
| Ipsilateral | 67.60 ± 23.60 | 15 | 69.48 ± 28.22 | 15 | 0.81 | |||||
| Diagonal | 67.55 ± 23.25 | 15 | 69.40 ± 28.90 | 15 | 0.82 | |||||
| After multiple | Lame | 62.16 ± 17.73 | 15 | 61.31 ± 17.61 | 15 | 0.70 | 55.40 ± 16.09 | 10 | 0.07 | |
| Contralateral | 73.00 ± 20.99 | 15 | 69.49 ± 20.55 | 15 | 0.40 | 61.19 ± 16.90 | 10 | 0.08 | ||
| Ipsilateral | 72.03 ± 31.30 | 15 | 66.89 ± 22.89 | 15 | 0.26 | 70.55 ± 26.86 | 10 | 0.33 | ||
| Diagonal | 73.53 ± 30.14 | 15 | 67.84 ± 25.56 | 15 | 0.15 | 69.85 ± 27.11 | 10 | 0.31 | ||
| Vertical impulse (%BW*sec) | Before | Lame | 7.16 ± 2.70 | 15 | 7.60 ± 3.19 | 15 | 6.38 ± 3.07 | 9 | ||
| Contralateral | 8.49 ± 3.44 | 15 | 8.90 ± 3.86 | 15 | 7.53 ± 2.80 | 9 | ||||
| Ipsilateral | 7.59 ± 3.16 | 15 | 7.89 ± 3.26 | 15 | 7.80 ± 3.30 | 9 | ||||
| Diagonal | 7.76 ± 3.08 | 15 | 8.18 ± 3.14 | 15 | 7.92 ± 3.46 | 9 | ||||
| After single | Lame | 7.14 ± 2.71 | 15 | 7.00 ± 2.63 | 15 | 0.72 | ||||
| Contralateral | 8.26 ± 3.04 | 15 | 8.24 ± 3.24 | 15 | 0.88 | |||||
| Ipsilateral | 7.66 ± 3.20 | 15 | 7.67 ± 3.43 | 15 | 0.97 | |||||
| Diagonal | 7.79 ± 3.24 | 15 | 7.76 ± 3.38 | 15 | 0.99 | |||||
| After multiple | Lame | 7.36 ± 2.87 | 15 | 7.09 ± 2.74 | 15 | 0.75 | 6.18 ± 2.74 | 10 | 0.28 | |
| Contralateral | 8.89 ± 3.34 | 15 | 8.10 ± 3.27 | 15 | 0.28 | 6.85 ± 2.52 | 10 | 0.37 | ||
| Ipsilateral | 8.31 ± 4.87 | 15 | 7.36 ± 3.03 | 15 | 0.33 | 7.98 ± 3.96 | 10 | 0.02 ** | ||
| Diagonal | 8.54 ± 4.76 | 15 | 7.54 ± 3.55 | 15 | 0.26 | 8.05 ± 3.96 | 10 | 0.18 |
| Parameter | Time | TENS (Mean ± SD) | N | Placebo (Mean ± SD) | N | p-Value | NSAID (Mean ± SD) | N | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| SI limb Peak vertical force (%BW) | Before | 0.87 ± 0.11 | 15 | 0.87 ± 0.11 | 15 | 0.87 ± 0.09 | 9 | -- | |
| After single | 0.89 ± 0.11 | 15 | 0.86 ± 0.11 | 15 | 0.38 | -- | |||
| After multiple | 0.86 ± 0.12 | 15 | 0.90 ± 0.11 | 15 | 0.21 | 0.91 ± 0.07 | 10 | 0.07 | |
| SI sagittal Peak vertical force (%BW) | Before | 0.92 ± 0.09 | 15 | 0.92 ± 0.09 | 15 | 0.95 ± 0.06 | 9 | -- | |
| After single | 0.93 ± 0.09 | 15 | 0.93 ± 0.09 | 15 | 0.96 | -- | |||
| After multiple | 0.91 ± 0.10 | 15 | 0.94 ± 0.09 | 15 | 0.22 | 0.96 ± 0.06 | 10 | 0.52 | |
| SI transversefront Peak vertical force (%BW) | Before | 1.64 ± 0.21 | 8 | 1.64 ± 0.24 | 8 | 1.67 ± 0.21 | 4 | -- | |
| After single | 1.61 ± 0.23 | 8 | 1.63 ± 0.24 | 8 | 0.43 | -- | |||
| After multiple | 1.61 ± 0.19 | 8 | 1.61 ± 0.27 | 8 | 0.98 | 1.69 ± 0.31 | 4 | 0.05 | |
| SI transversehind Peak vertical force (%BW) | Before | 0.57 ± 0.06 | 7 | 0.56 ± 0.07 | 7 | 0.55 ± 0.06 | 5 | -- | |
| After single | 0.58 ± 0.06 | 7 | 0.56 ± 0.07 | 7 | 0.52 | -- | |||
| After multiple | 0.56 ± 0.06 | 7 | 0.57 ± 0.08 | 7 | 0.56 | 0.57 ± 0.06 | 6 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, A.; Hyytiäinen, H.K.; Rhodin, M.; Forterre, F.; Penell, J.; Bergh, A. Effect of Transcutaneous Electrical Nerve Stimulation on Gait Parameters in Dogs with Osteoarthritis. Animals 2024, 14, 1626. https://doi.org/10.3390/ani14111626
Pedersen A, Hyytiäinen HK, Rhodin M, Forterre F, Penell J, Bergh A. Effect of Transcutaneous Electrical Nerve Stimulation on Gait Parameters in Dogs with Osteoarthritis. Animals. 2024; 14(11):1626. https://doi.org/10.3390/ani14111626
Chicago/Turabian StylePedersen, Anja, Heli K. Hyytiäinen, Marie Rhodin, Franck Forterre, Johanna Penell, and Anna Bergh. 2024. "Effect of Transcutaneous Electrical Nerve Stimulation on Gait Parameters in Dogs with Osteoarthritis" Animals 14, no. 11: 1626. https://doi.org/10.3390/ani14111626






