Enhancing the Anti-Tumor Efficacy of NK Cells on Canine Mammary Tumors through Resveratrol Activation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. CCK-8 Assay
2.3. Cytotoxicity Assay
2.4. Annexin V-FITC Apoptosis Detection
2.5. Clonogenic Assay
2.6. Wound-Healing Assay
2.7. Transwell Cell Migration Assay
2.8. Cytofluorimetric Analysis
2.9. Western Blotting Analysis
2.10. Animal Studies
2.11. Flow Cytometric Analysis
2.12. Histological Staining and Imaging
2.13. IHC Analysis
2.14. TUNEL Analysis
2.15. Statistical Analysis
3. Results
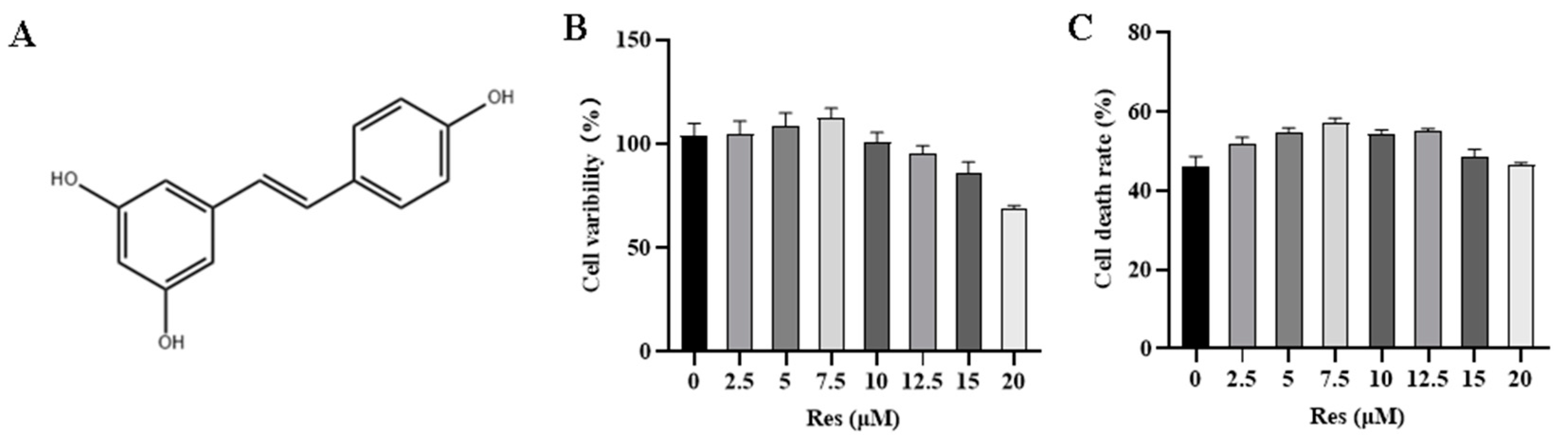
3.1. Effect of RES on NK Cell Activity and Target Cell Toxicity
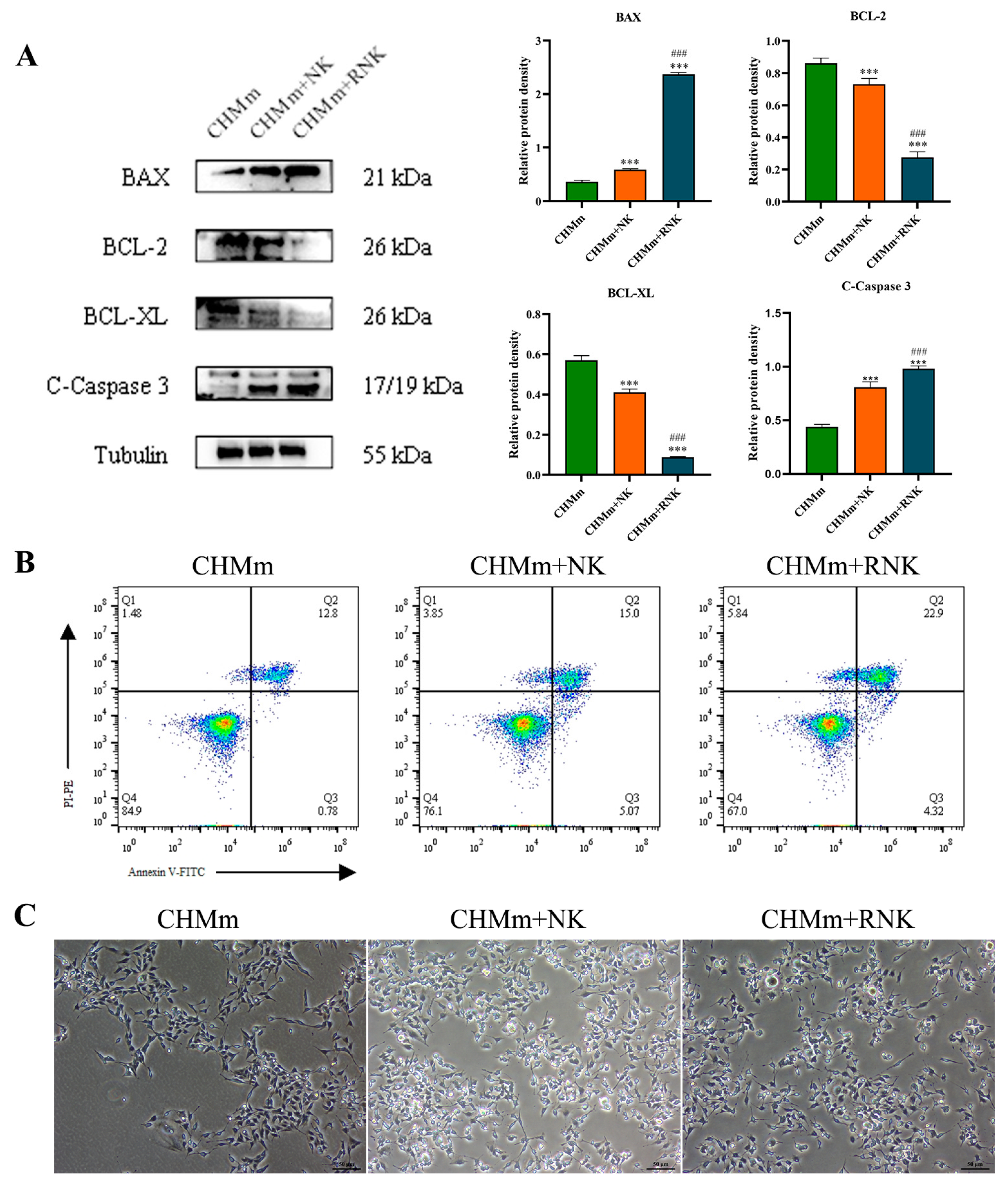
3.2. Res Pretreatment Enhances the Ability of NK Cells to Induce Apoptosis in CHMm
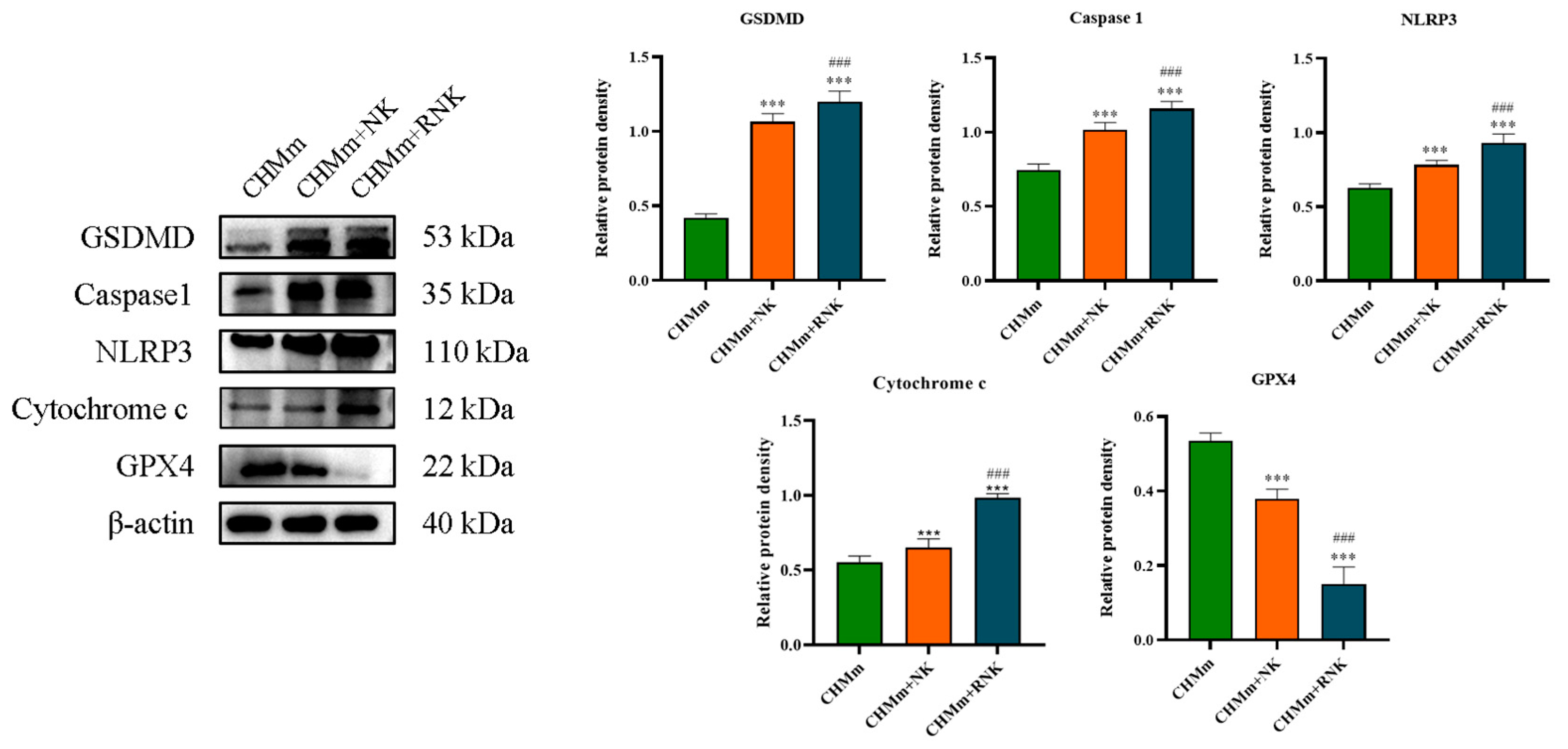
3.3. Res Pretreatment Enhances the Ability of NK Cells to Induce Pyroptosis and Ferroptosis in CHMm
3.4. Res Pretreatment Enhances the Inhibitory Effect of NK Cells on CHMm Cell Proliferation, Migration, and Invasion
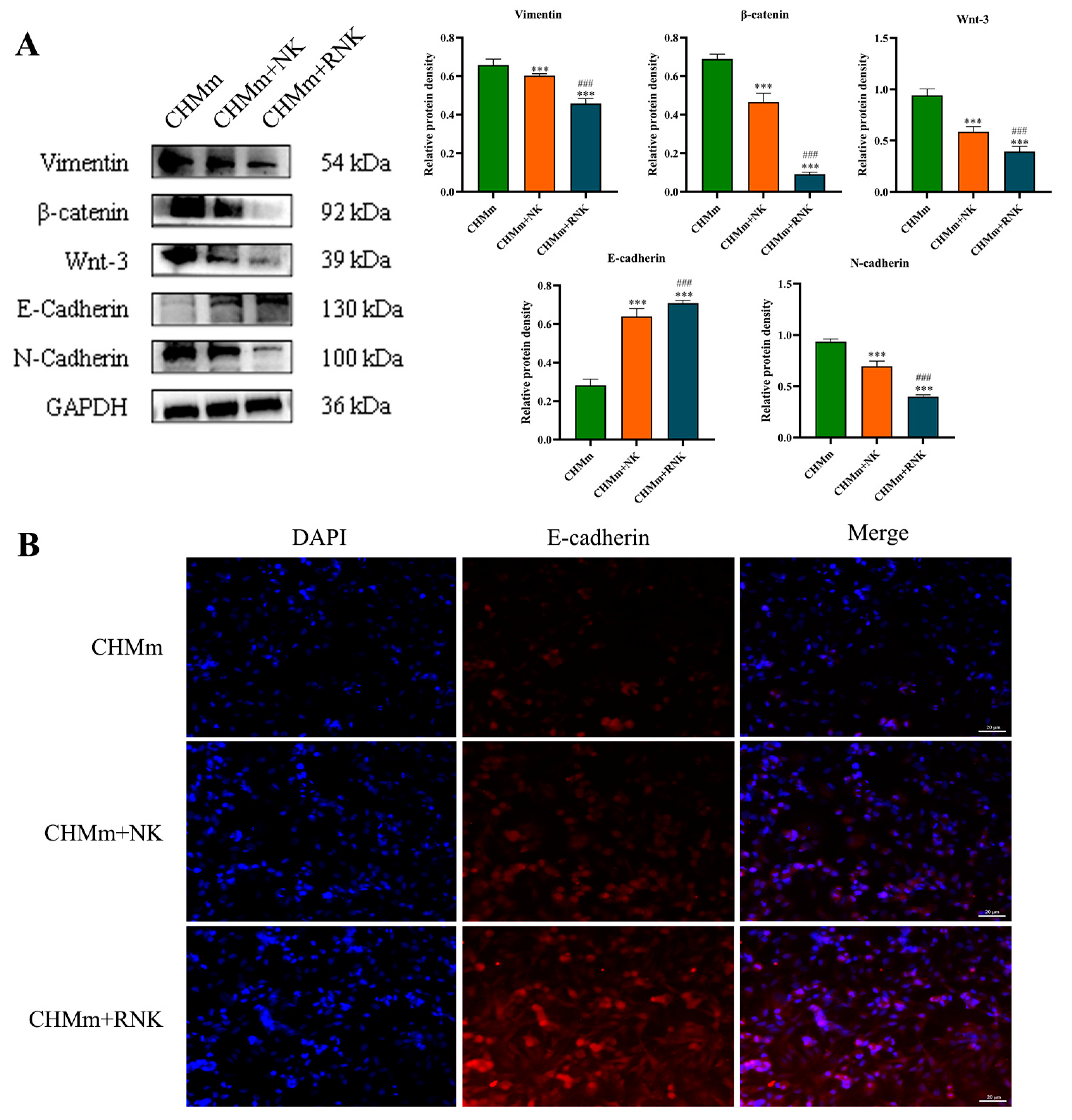
3.5. Res Pretreatment Enhances the Inhibitory Effect of NK Cells on EMT of CHMm Cells
3.6. Res Enhances the Inhibitory Effect of NK Cells on Tumors in Tumor-Bearing Mice
3.7. Res Pretreatment Enhances NK Cell Therapy to Induce Tumor Tissue Apoptosis and Ferroptosis
3.8. Effect of Res Pretreatment of NK Cells on Proliferation and Metastasis Indicators in Breast Tumor Tissue
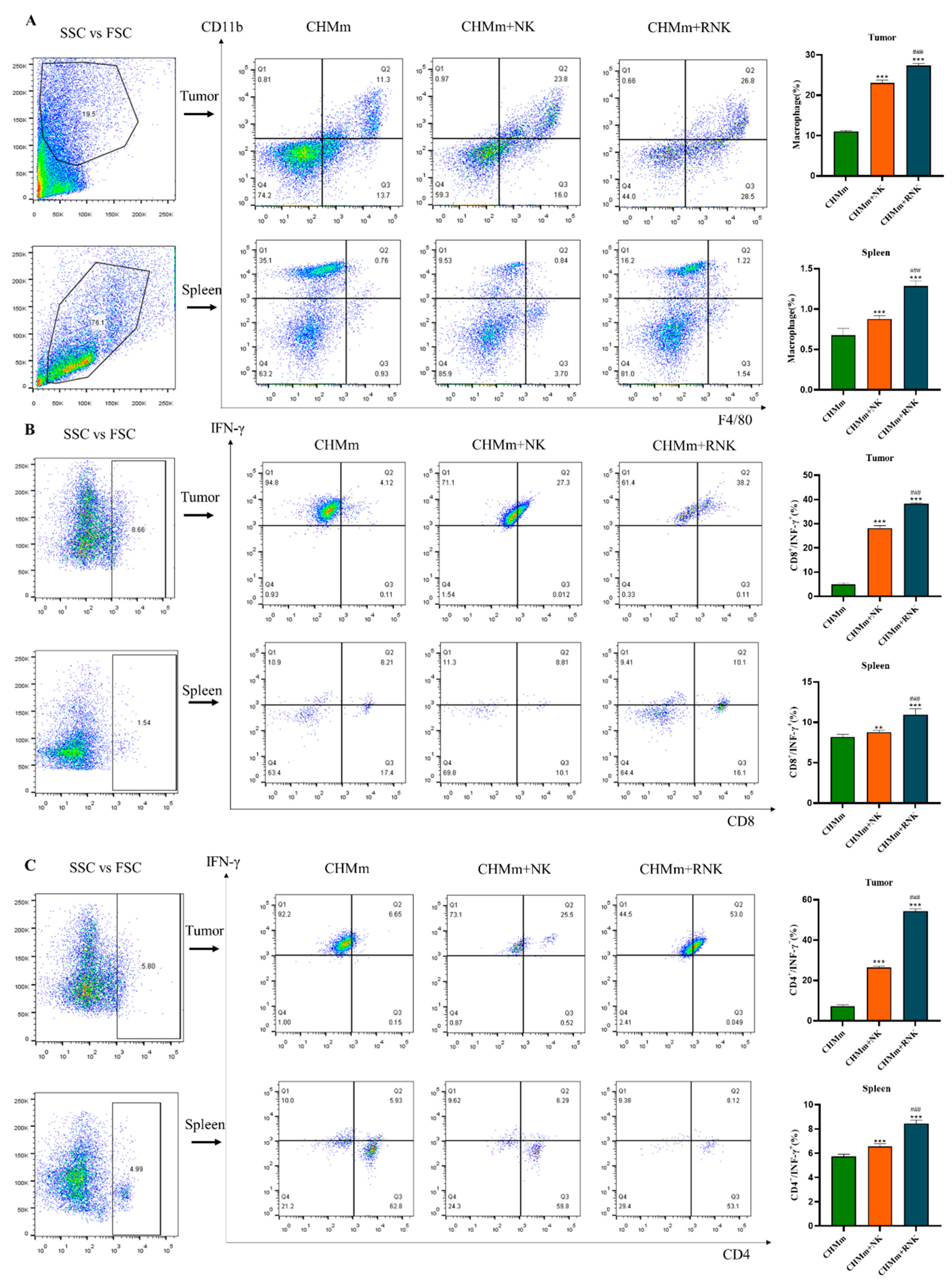
3.9. Res Enhances NK Cells and Enhances the Recruitment of Immune Cells in Mouse Spleen and Tumor Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosalova, N.; Huniadi, M.; Horňáková, Ľ.; Valenčáková, A.; Horňák, S.; Nagoos, K.; Vozar, J.; Cizkova, D. Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies. Int. J. Mol. Sci. 2024, 25, 2891. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Razeghian, E.; Kameh, M.C.; Shafiee, S.; Khalafi, F.; Jafari, F.; Asghari, M.; Kazemi, K.; Ilkhani, S.; Shariatzadeh, S.; Haj-Mirzaian, A. The role of the natural killer (NK) cell modulation in breast cancer incidence and progress. Mol. Biol. Rep. 2022, 49, 10935–10948. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Galán, L.; Arenas-Del Angel, M.C.; Zenteno, E.; Chávez, R.; Lascurain, R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell. Mol. Immunol. 2009, 6, 15–25. [Google Scholar] [CrossRef]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef]
- Lian, G.; Mak, T.S.; Yu, X.; Lan, H.Y. Challenges and Recent Advances in NK Cell-Targeted Immunotherapies in Solid Tumors. Int. J. Mol. Sci. 2021, 23, 164. [Google Scholar] [CrossRef]
- Velichinskii, R.A.; Streltsova, M.A.; Kust, S.A.; Sapozhnikov, A.M.; Kovalenko, E.I. The Biological Role and Therapeutic Potential of NK Cells in Hematological and Solid Tumors. Int. J. Mol. Sci. 2021, 22, 11385. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Wang, J.; Zheng, X.; Wei, H.; Tian, Z.; Sun, R. Tumor Therapeutics Work as Stress Inducers to Enhance Tumor Sensitivity to Natural Killer (NK) Cell Cytolysis by Up-regulating NKp30 Ligand B7-H6. J. Biol. Chem. 2015, 290, 29964–29973. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, D.; Li, Y.; He, Y.; Guo, K. Resveratrol sensitized leukemia stem cell-like KG-1a cells to cytokine-induced killer cells-mediated cytolysis through NKG2D ligands and TRAIL receptors. Cancer Biol. Ther. 2012, 13, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, H.; Kim, J. In vivo Anti-Cancer Effects of Resveratrol Mediated by NK Cell Activation. J. Innate Immun. 2021, 13, 94–106. [Google Scholar] [CrossRef]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Chen, J.K. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J. Cell. Physiol. 2010, 223, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Ryan, J.; Pan, D.; Wucherpfennig, K.W.; Letai, A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell 2022, 185, 1521–1538.e18. [Google Scholar] [CrossRef] [PubMed]
- Narni-Mancinelli, E.; Vivier, E. Advancing natural killer therapies against cancer. Cell 2022, 185, 1451–1454. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, Á.R.; Gonzalez, S.; López-Soto, A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. Int. J. Mol. Sci. 2020, 21, 3726. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Lieberman, J. Lighting a Fire: Can We Harness Pyroptosis to Ignite Antitumor Immunity? Cancer Immunol. Res. 2021, 9, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Pérez, A.; Oñate, C.; Andrés-Tovar, A.; Garzón-Tituaña, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, Z.; Chen, J.; Guo, S.; You, W.; Wang, S.; Ma, J.; Wang, H.; Wang, X.; Wang, H.; et al. Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer 2022, 10, e004381. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Ye, L.J.; Li, J.; Shi, J.L.; Huang, Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Raposo-Ferreira, T.M.M.; Brisson, B.K.; Durham, A.C.; Laufer-Amorim, R.; Kristiansen, V.; Puré, E.; Volk, S.W.; Sorenmo, K. Characteristics of the Epithelial-Mesenchymal Transition in Primary and Paired Metastatic Canine Mammary Carcinomas. Vet. Pathol. 2018, 55, 622–633. [Google Scholar] [CrossRef]
- Armando, F.; Ferrari, L.; Arcari, M.L.; Azzali, G.; Dallatana, D.; Ferrari, M.; Lombardi, G.; Zanfabro, M.; Di Lecce, R.; Lunghi, P.; et al. Endocanalicular transendothelial crossing (ETC): A novel intravasation mode used by HEK-EBNA293-VEGF-D cells during the metastatic process in a xenograft model. PLoS ONE 2020, 15, e0239932. [Google Scholar] [CrossRef]
- Chockley, P.J.; Chen, J.; Chen, G.; Beer, D.G.; Standiford, T.J.; Keshamouni, V.G. Epithelial-mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J. Clin. Investig. 2018, 128, 1384–1396. [Google Scholar] [CrossRef]
- Arnold, K.M.; Pohlig, R.T.; Sims-Mourtada, J. Co-activation of Hedgehog and Wnt signaling pathways is associated with poor outcomes in triple negative breast cancer. Oncol. Lett. 2017, 14, 5285–5292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tian, Q.; Gao, H.; Wu, K.; Wang, B.; Ge, G.; Jiang, S.; Wang, K.; Zhou, C.; He, J.; et al. PROX1 promotes breast cancer invasion and metastasis through WNT/β-catenin pathway via interacting with hnRNPK. Int. J. Biol. Sci. 2022, 18, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Tian, Y.; Zhou, G.L.; Yue, H.R.; Zhou, X.J.; Ma, H.Y.; Ge, J.; Wang, X.; Cao, X.C.; Yu, Y. CMTM7 inhibits breast cancer progression by regulating Wnt/β-catenin signaling. Breast Cancer Res. 2023, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Armando, F.; Godizzi, F.; Razzuoli, E.; Leonardi, F.; Angelone, M.; Corradi, A.; Meloni, D.; Ferrari, L.; Passeri, B. Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives. Animals 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 protein as a tumour proliferation marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Green, A.R.; Ashankyty, I.; Elmouna, A.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A. Impact of intratumoural heterogeneity on the assessment of Ki67 expression in breast cancer. Breast Cancer Res. Treat. 2016, 158, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gutiérrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef]
- Lu, X. OX40 and OX40L Interaction in Cancer. Curr. Med. Chem. 2021, 28, 5659–5673. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Guo, C. Crosstalk between macrophages and natural killer cells in the tumor microenvironment. Int. Immunopharmacol. 2021, 101, 108374. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Borcherding, N.; Ogunsakin, A.; Lemke-Miltner, C.D.; Gibson-Corley, K.N.; Rajan, A.; Choi, A.B.; Wongpattaraworakul, W.; Chan, C.H.F.; Salem, A.K.; et al. The anti-tumor effects of cetuximab in combination with VTX-2337 are T cell dependent. Sci. Rep. 2021, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Cancel, J.C.; Crozat, K.; Dalod, M.; Mattiuz, R. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front. Immunol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Jin, S.; Tong, D.; Liu, X.; Liu, Y.; Zheng, J. Enhancing the Anti-Tumor Efficacy of NK Cells on Canine Mammary Tumors through Resveratrol Activation. Animals 2024, 14, 1636. https://doi.org/10.3390/ani14111636
Zhu T, Jin S, Tong D, Liu X, Liu Y, Zheng J. Enhancing the Anti-Tumor Efficacy of NK Cells on Canine Mammary Tumors through Resveratrol Activation. Animals. 2024; 14(11):1636. https://doi.org/10.3390/ani14111636
Chicago/Turabian StyleZhu, Tingting, Shengzi Jin, Danning Tong, Xingyao Liu, Yun Liu, and Jiasan Zheng. 2024. "Enhancing the Anti-Tumor Efficacy of NK Cells on Canine Mammary Tumors through Resveratrol Activation" Animals 14, no. 11: 1636. https://doi.org/10.3390/ani14111636



