Fasting-Induced Molting Impacts the Intestinal Health by Altering the Gut Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Determination of Intestinal Morphology
2.4. Real-Time Quantitative PCR Analyses
2.5. DNA Extraction and 16S rDNA Sequencing Analysis
2.6. Cecal Contents Metabolite Analysis
2.7. Integration of Immune Data or Microbiota and Metabolites
2.8. Statistical Analysis
3. Results
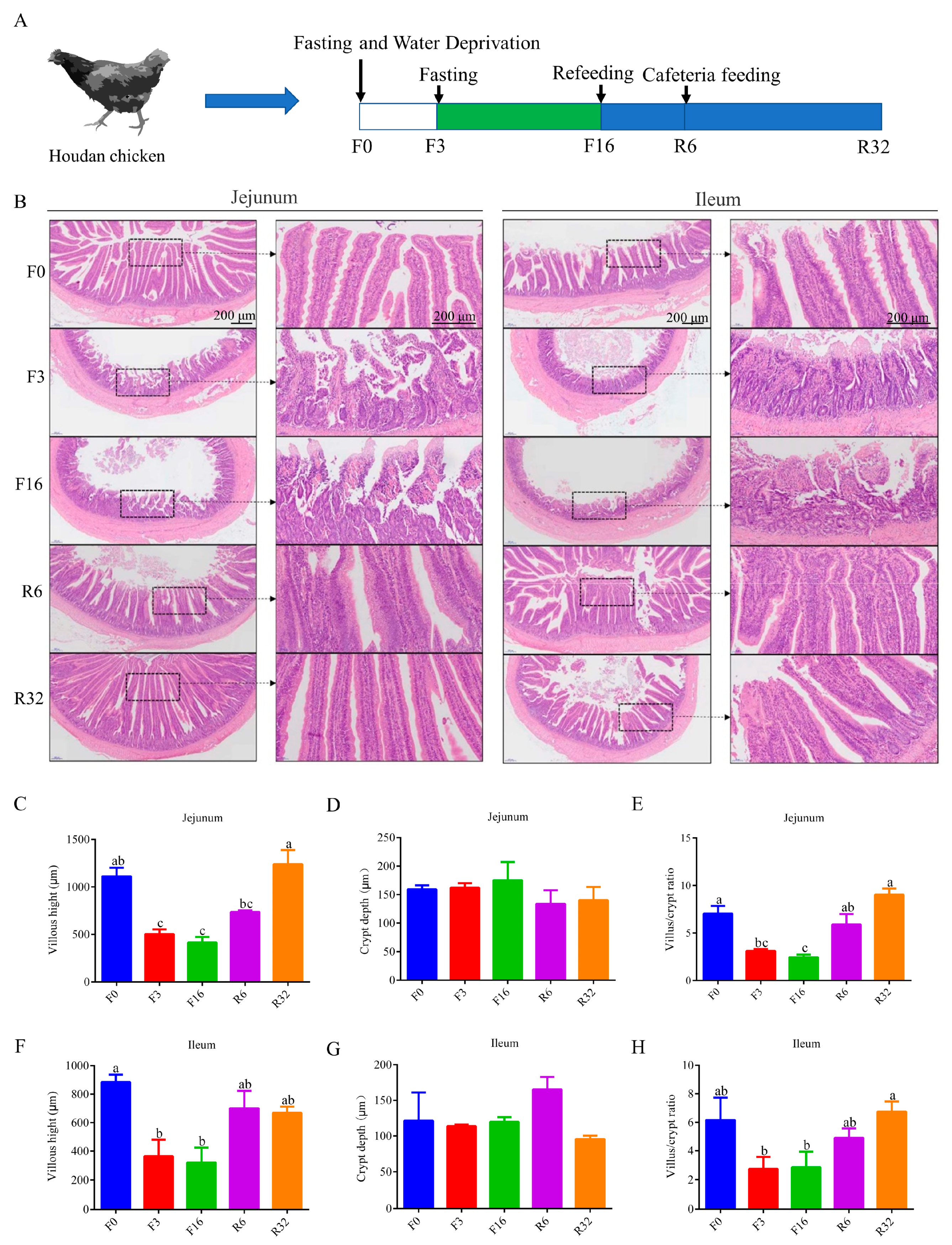
3.1. Intestinal Histological Changes during the FIM Process
3.2. Change of Intestinal Immune Regulation during the FIM Process
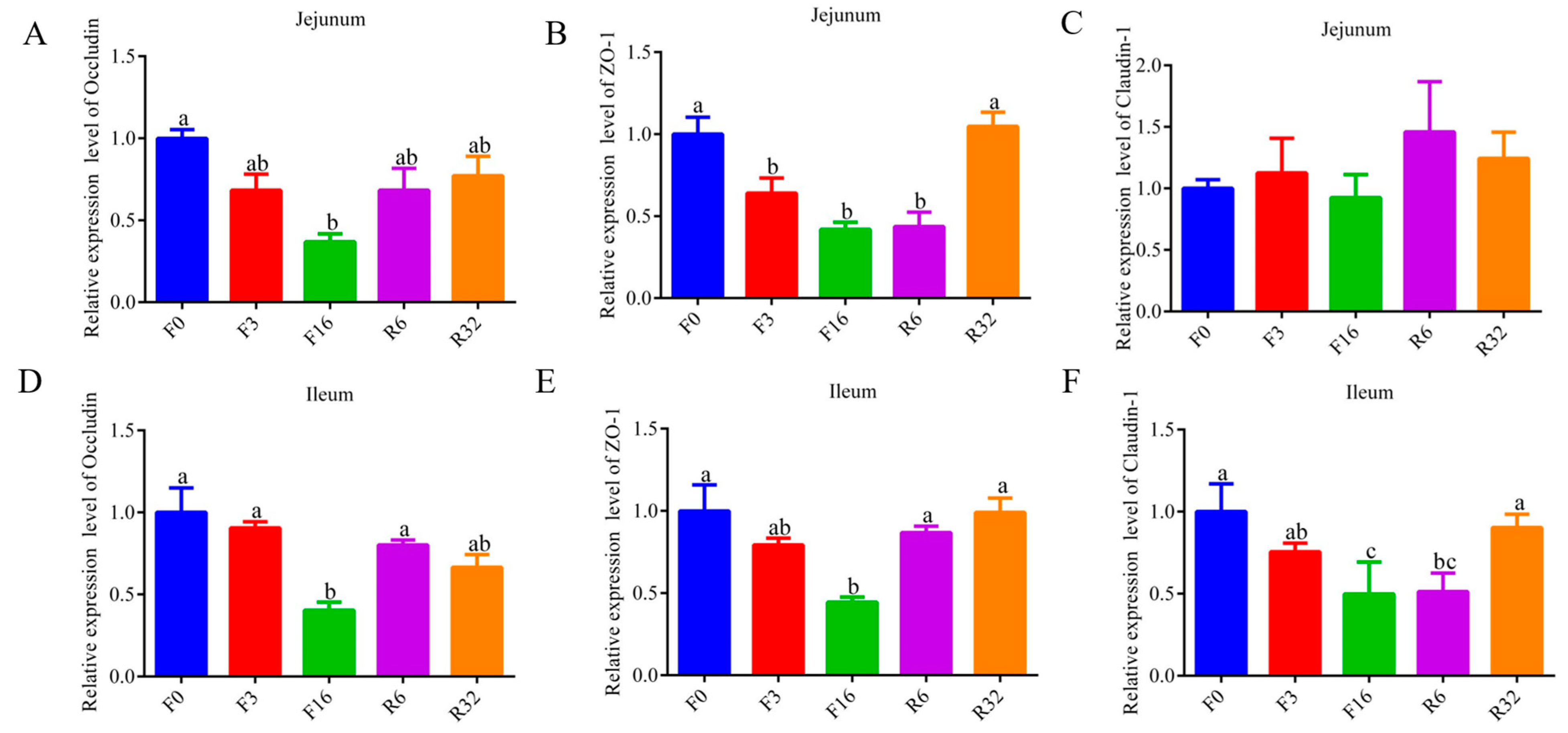
3.3. Change in Intestine Barrier during the FIM Process
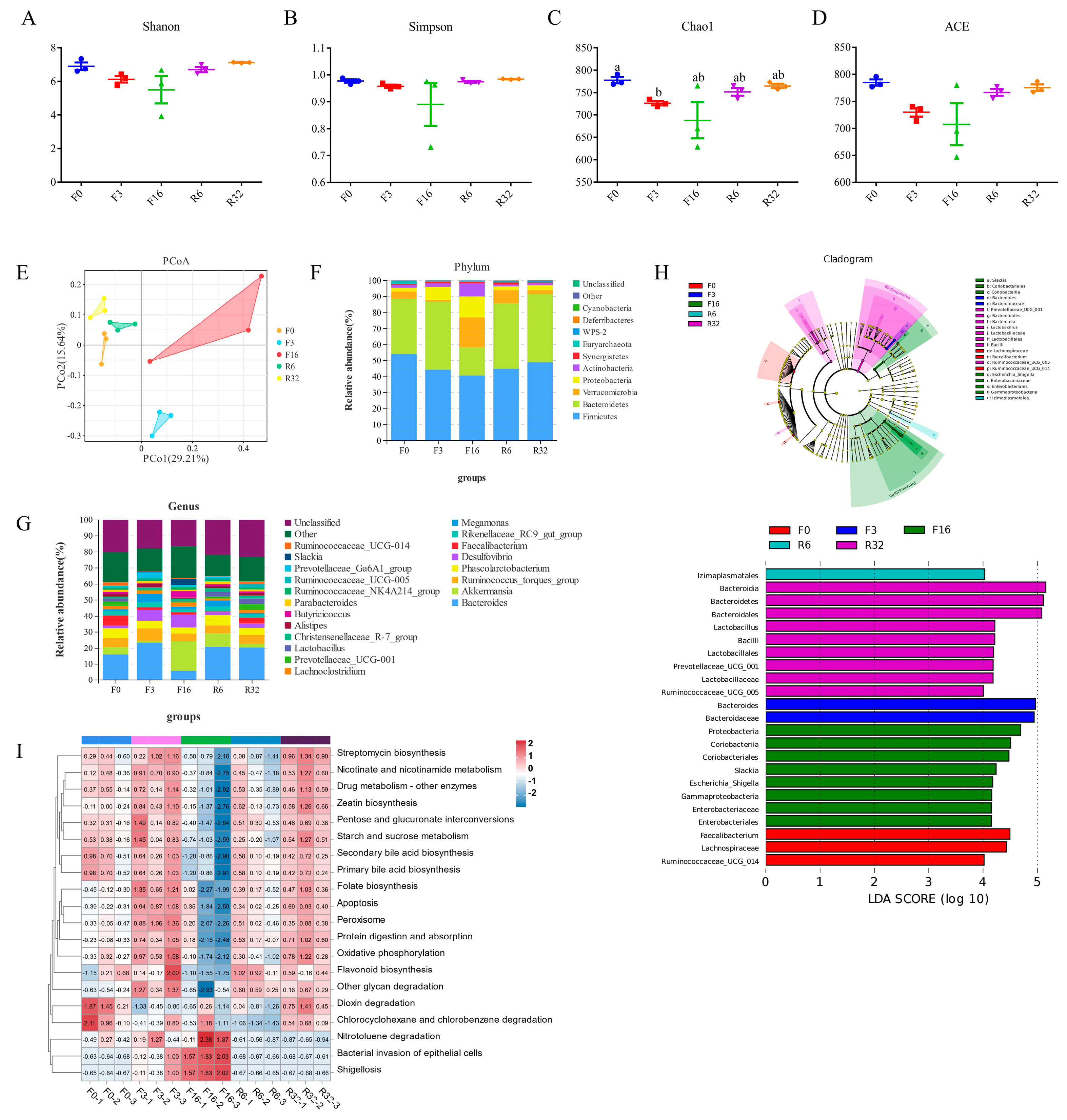
3.4. Alteration in the Gut Microbial Composition during the FIM Process
3.5. Change in Metabolites during the FIM Process
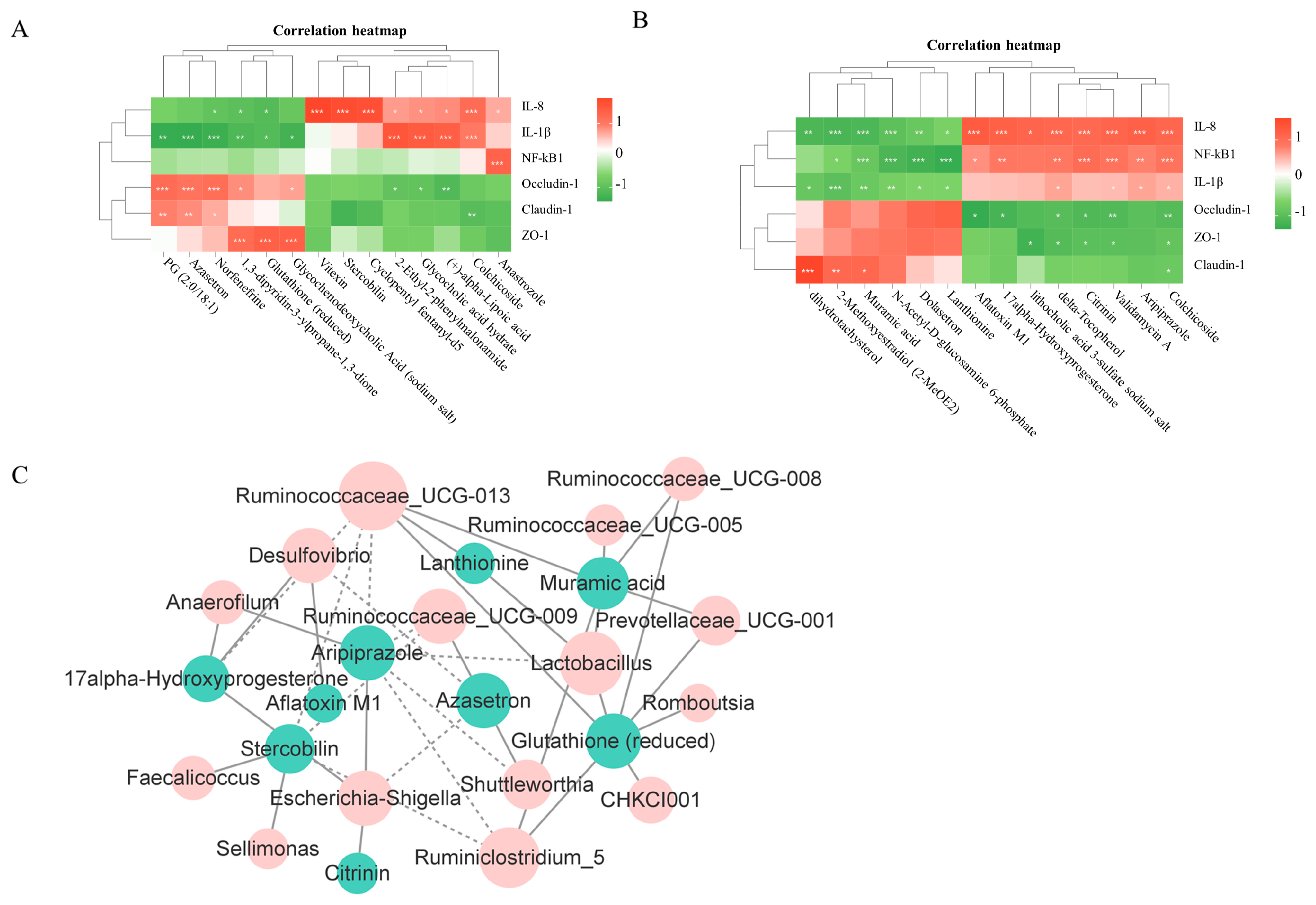
3.6. Correlation Analysis of Intestinal Iujury or Genera and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biggs, P.E.; Persia, M.E.; Koelkebeck, K.W.; Parsons, C.M. Further evaluation of nonfeed removal methods for molting programs. Poult. Sci. 2004, 83, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Wen, J.; Jia, Y.; Wang, L.; Lv, X.; Yang, W.; Qu, C.; Li, H.; Wang, H.; et al. Transcriptomic Analysis of Laying Hens Revealed the Role of Aging-Related Genes during Forced Molting. Genes 2021, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gong, Y.; Li, D.; Zhao, X.; Zhang, Y.; Zhang, J.; Geng, X.; Zhang, X.; Tian, Y.; Li, W.; et al. Effect of induced molting on ovarian function remodeling in laying hens. Poult. Sci. 2023, 102, 102820. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Schrader, J.; DiLorenzo, A.M.; Cardona, C.; Klingborg, D. Effects of induced molting on the well-being of egg-laying hens. J. Appl. Anim. Welf. Sci. 2006, 9, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.D. The physiology of induced molting. Poult. Sci. 2003, 82, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ning, Z.; Chen, Y.; Wen, J.; Jia, Y.; Wang, L.; Lv, X.; Yang, W.; Qu, C.; Li, H.; et al. Understanding Transcriptomic and Serological Differences between Forced Molting and Natural Molting in Laying Hens. Genes 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, R.W.; Bahr, J.M. Molt induced by gonadotropin-releasing hormone agonist as a model for studying endocrine mechanisms of molting in laying hens. Poult. Sci. 1989, 68, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, K.; Imai, K.; Suzuki, M.; Takikawa, H.; Hoshino, N.; Totsuka, K. Thyroxine-induced molting and gonadal function of laying hens. Poult. Sci. 1987, 66, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lin, Z.; Song, X.; Hu, C.; Qiu, M.; Yang, L.; Zhang, Z.; Pen, H.; Chen, J.; Xiong, X.; et al. Whole transcriptome analysis reveals the key genes and noncoding RNAs related to follicular atresia in broilers. Anim. Biotechnol. 2023, 34, 3144–3153. [Google Scholar] [CrossRef]
- Ran, J.S.; Yin, L.Q.; Li, J.J.; Tang, Y.Q.; Huang, J.; Ren, P.; Zhang, X.X.; Li, S.M.; Liu, Y.P. Integrated analysis of microRNA and mRNA interactions in ovary of counter-season breeding and egg-ceased geese (Anser cygnoides). Theriogenology 2022, 186, 146–154. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Grundler, F.; Le Maho, Y.; Wilhelmi de Toledo, F.; Zeller, G.; Habold, C.; Mesnage, R. Remodelling of the intestinal ecosystem during caloric restriction and fasting. Trends Microbiol. 2023, 31, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Kamisoyama, H.; Isshiki, Y. Effects of fasting and refeeding on structures of the intestinal villi and epithelial cells in White Leghorn hens. Br. Poult. Sci. 1996, 37, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Simon, Á.; Gulyás, G.; Mészár, Z.; Bhide, M.; Oláh, J.; Bai, P.; Csősz, É.; Jávor, A.; Komlósi, I.; Remenyik, J.; et al. Proteomics alterations in chicken jejunum caused by 24 h fasting. PeerJ 2019, 7, e6588. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.; Zhao, W.; He, C.; Ding, J.; Dai, R.; Xu, K.; Xiao, L.; Luo, L.; Liu, S.; et al. Impaired Autophagy in Intestinal Epithelial Cells Alters Gut Microbiota and Host Immune Responses. Appl. Environ. Microbiol. 2018, 84, e00880-18. [Google Scholar] [CrossRef] [PubMed]
- Jarade, A.; Garcia, Z.; Marie, S.; Demera, A.; Prinz, I.; Bousso, P.; Di Santo, J.P.; Serafini, N. Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat. Immunol. 2022, 23, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.A.; Corrier, D.E.; Byrd, J.A.; Stanker, L.H.; Ricke, S.C. Feed deprivation affects crop environment and modulates Salmonella enteritidis colonization and invasion of leghorn hens. Appl. Environ. Microbiol. 1999, 65, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Corrier, D.E.; Nisbet, D.J.; Hargis, B.M.; Holt, P.S.; DeLoach, J.R. Provision of lactose to molting hens enhances resistance to Salmonella enteritidis colonization. J. Food Prot. 1997, 60, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Geng, X.; Zhang, Y.; Zhao, X.; Zhang, P.; Sun, G.; Li, W.; Li, D.; Han, R.; Li, G.; et al. Interaction Between Cecal Metabolites and Liver Lipid Metabolism Pathways During Induced Molting in Laying Hens. Front. Physiol. 2022, 13, 862721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, X.; Ao, Y.; Nan, M.; Qiu, Z.; Jia, X.; Xiao, Y.; Liu, D.; Guo, X. ANXA1 affects murine hair follicle growth through EGF signaling pathway. Gene 2021, 771, 145343. [Google Scholar] [CrossRef]
- Sellick, C.A.; Hansen, R.; Stephens, G.M.; Goodacre, R.; Dickson, A.J. Metabolite extraction from suspension-cultured mammalian cells for global metabolite profiling. Nat. Protoc. 2011, 6, 1241–1249. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Li, Y.; Guo, Y.; Zhang, S.; Li, P.; Gao, P. Improving effects of Epimedium flavonoids on the selected reproductive features in layer hens after forced molting. Poult. Sci. 2020, 99, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Hai Ping, P.; Feng Bo, T.; Li, L.; Nan Hui, Y.; Hong, Z. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch. Biochem. Biophys. 2016, 604, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Kalafateli, M.; Tsounis, E.P.; Triantos, C. Exploring the role of IL-1β in inflammatory bowel disease pathogenesis. Front. Med. 2024, 11, 1307394. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Zhang, L.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of lentinan: Influence on IL-8 and TNFR1 expression in intestinal epithelial cells. PLoS ONE 2013, 8, e62441. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Gaskins, H.R. Another renaissance for bile acid gastrointestinal microbiology. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Pushpass, R.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating bile acids as a link between the gut microbiota and cardiovascular health: Impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. . Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8(+) T cell immunity. Cell Metab. 2023, 35, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Brück, T.B. Molecular Mechanisms of Shigella Pathogenesis; Recent Advances. Int. J. Mol. Sci. 2023, 24, 2448. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, N.; Ge, Y.; Yang, Y.; Ren, F.; Wu, Z. Tryptophan and the innate intestinal immunity: Crosstalk between metabolites, host innate immune cells, and microbiota. Eur. J. Immunol. 2022, 52, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Courtois, F.; Seidman, E.G.; Delvin, E.; Asselin, C.; Bernotti, S.; Ledoux, M.; Levy, E. Membrane peroxidation by lipopolysaccharide and iron-ascorbate adversely affects Caco-2 cell function: Beneficial role of butyric acid. Am. J. Clin. Nutr. 2003, 77, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P. The Emerging Role of Vitamin B6 in Inflammation and Carcinogenesis. Adv. Food Nutr. Res. 2018, 83, 151–194. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorak, K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 2009, 15, 3329–3340. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; López-Maside, L.; Donapetry-García, C.; Fernández-Fernández, C.; Sixto-Leal, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Isaifan, D.; Crovella, S.; Soubra, L.; Al-Nesf, M.; Steinhoff, M. Fc Epsilon RI-Neuroimmune Interplay in Pruritus Triggered by Particulate Matter in Atopic Dermatitis Patients. Int. J. Mol. Sci. 2023, 24, 11851. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, Z.; Zhang, Y.; Zhang, Y.; Ren, G.; Zeng, Y.; Liu, H.; Guan, W.; Zhao, X.; Li, P.; et al. Proline uptake promotes activation of lymphoid tissue inducer cells to maintain gut homeostasis. Nat. Metab. 2023, 5, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Pereira, A.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Suzuki, T.; Nagata, A.; Hashidume, T.; Yoshikawa, Y.; Miyoshi, N. Intestinal microbial metabolite stercobilin involvement in the chronic inflammation of ob/ob mice. Sci. Rep. 2020, 10, 6479. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Zacchia, M.; Trepiccione, F.; Ingrosso, D. The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin. Toxins 2017, 9, 26. [Google Scholar] [CrossRef]
- Li, S.; Wen, X.; Yang, X.; Wang, L.; Gao, K.; Liang, X.; Xiao, H. Glutamine protects intestinal immunity through microbial metabolites rather than microbiota. Int. Immunopharmacol. 2023, 124, 110832. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Y.; Gong, Y.; Zhang, J.; Li, D.; Tian, Y.; Han, R.; Guo, Y.; Sun, G.; Li, W.; et al. Fasting-Induced Molting Impacts the Intestinal Health by Altering the Gut Microbiota. Animals 2024, 14, 1640. https://doi.org/10.3390/ani14111640
Zhang H, Zhang Y, Gong Y, Zhang J, Li D, Tian Y, Han R, Guo Y, Sun G, Li W, et al. Fasting-Induced Molting Impacts the Intestinal Health by Altering the Gut Microbiota. Animals. 2024; 14(11):1640. https://doi.org/10.3390/ani14111640
Chicago/Turabian StyleZhang, Hao, Yihui Zhang, Yujie Gong, Jun Zhang, Donghua Li, Yadong Tian, Ruili Han, Yujie Guo, Guirong Sun, Wenting Li, and et al. 2024. "Fasting-Induced Molting Impacts the Intestinal Health by Altering the Gut Microbiota" Animals 14, no. 11: 1640. https://doi.org/10.3390/ani14111640





