Impact of Long-Term Supplementation with Probiotics on Gut Microbiota and Growth Performance in Post-Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. 16S rRNA Sequencing
2.4. Sequence Data Processing
2.5. Microbial Diversity Analysis
2.6. Bacterial Composition and Relative Abundance Analysis
3. Results
3.1. Growth Performance
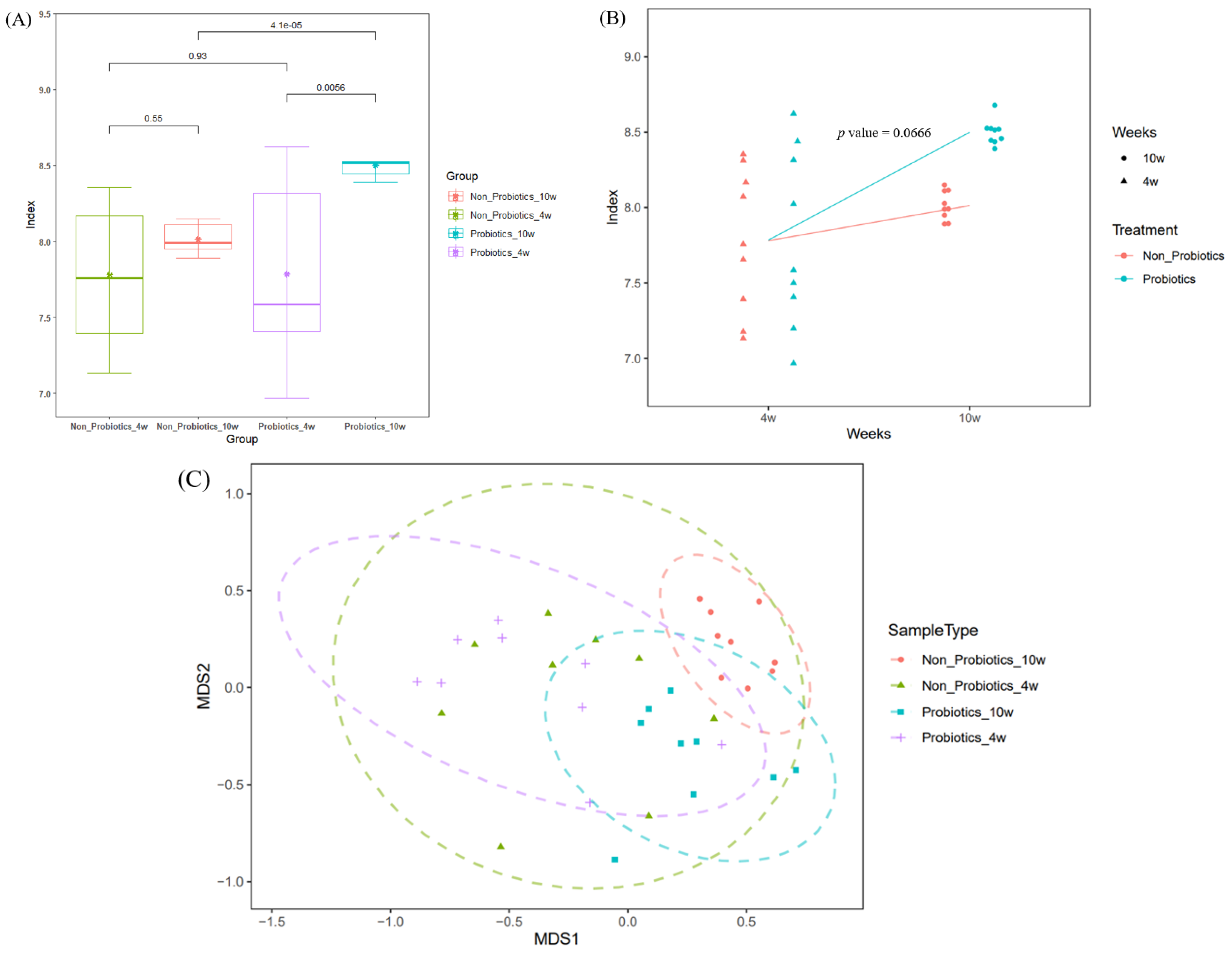
3.2. Microbial Diversity
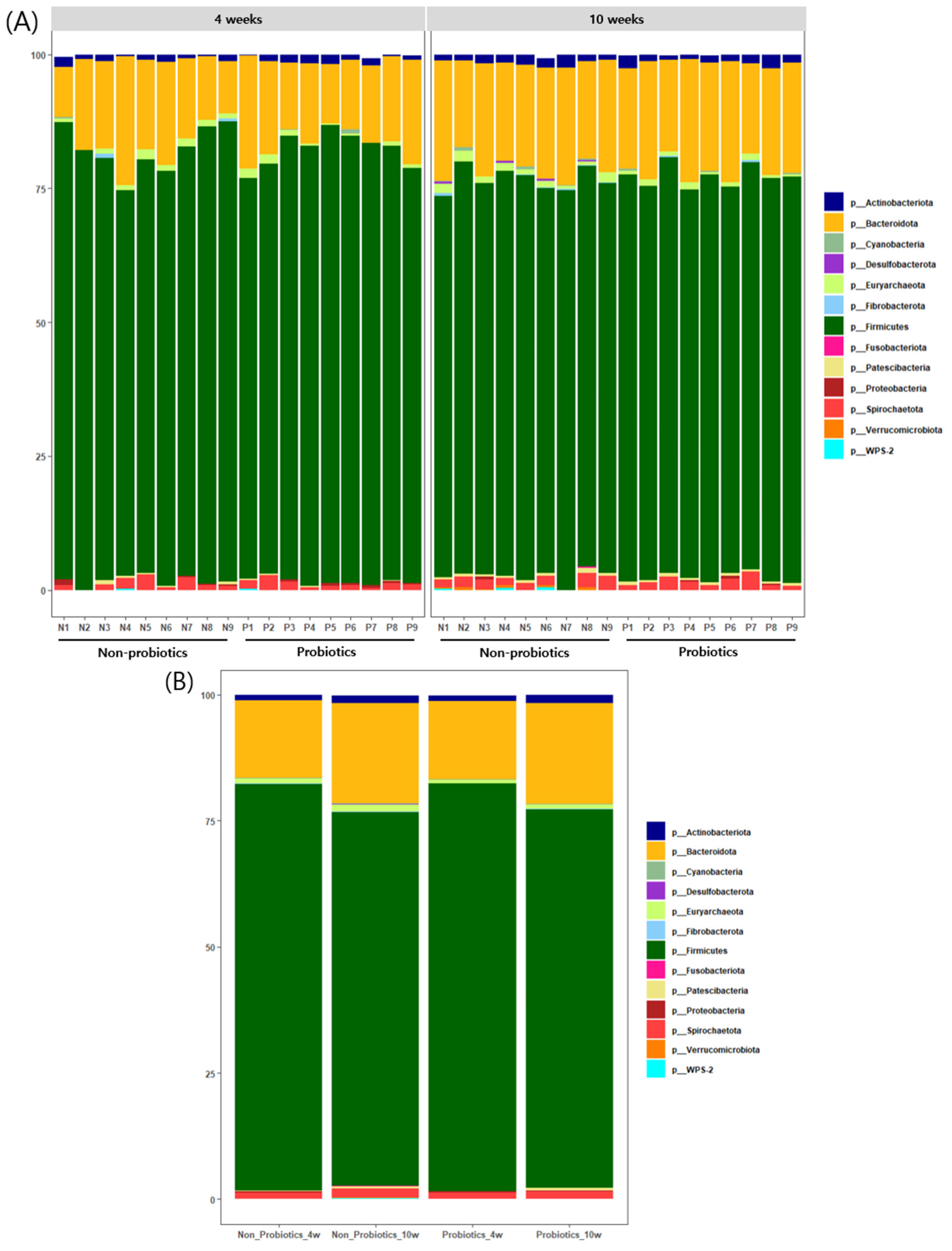
3.3. Bacterial Composition
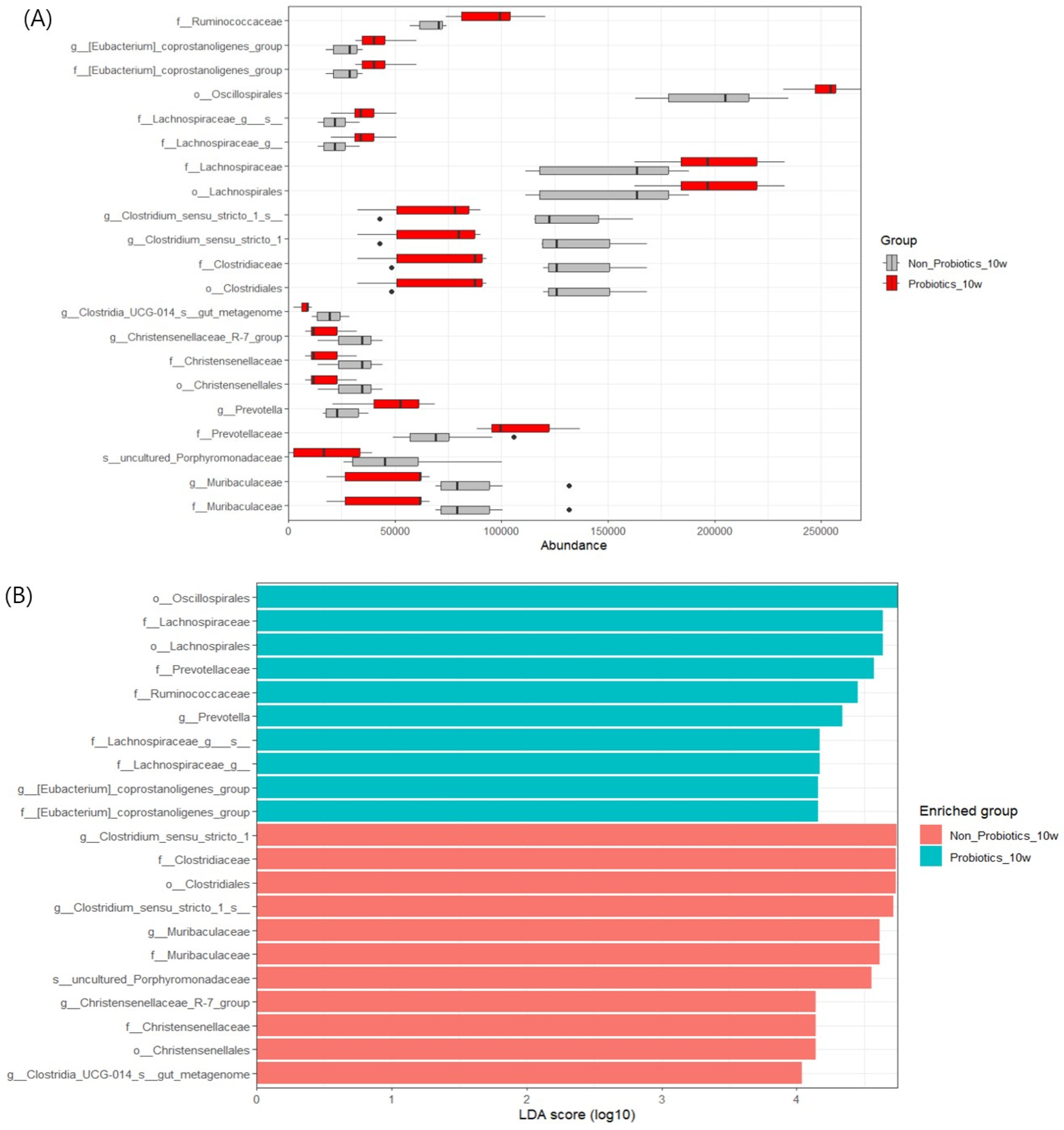
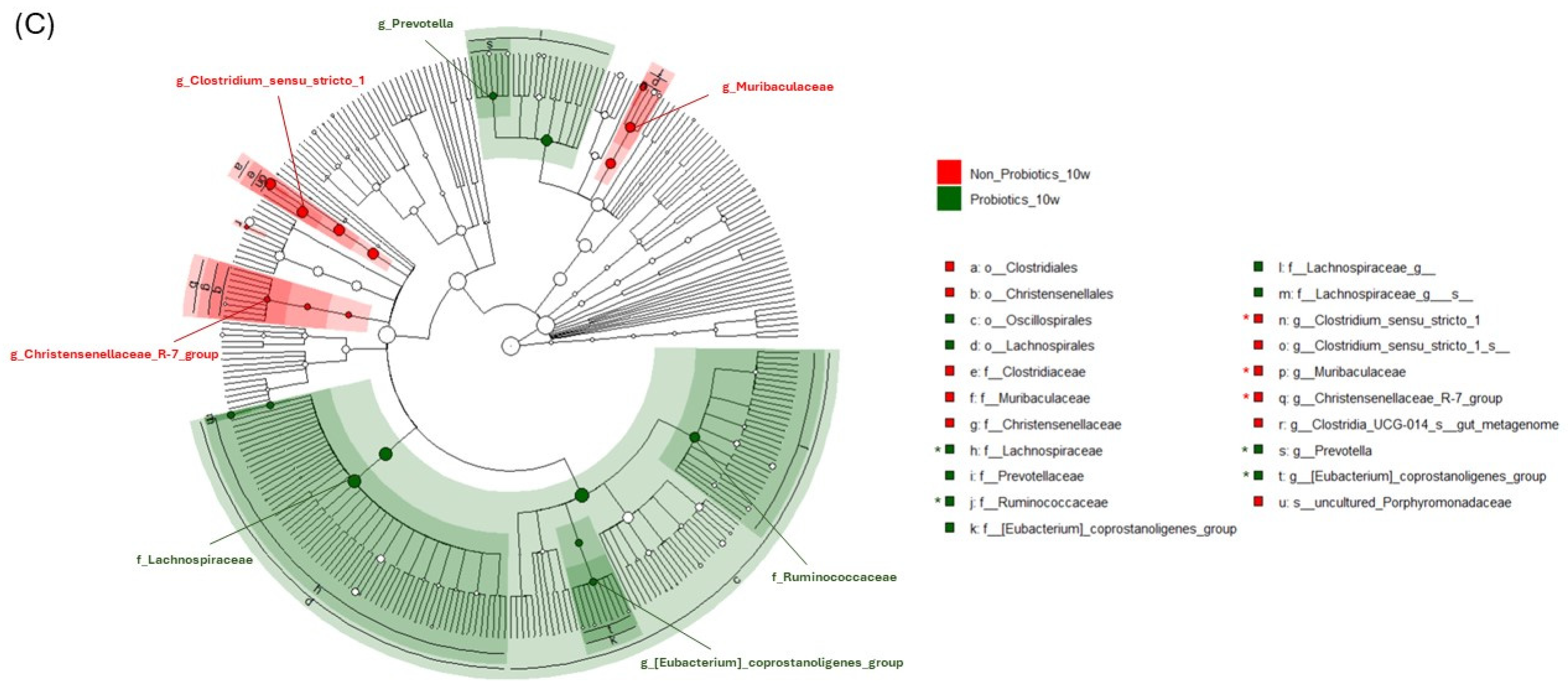
3.4. LEfSe Analysis to Find Significant Markers
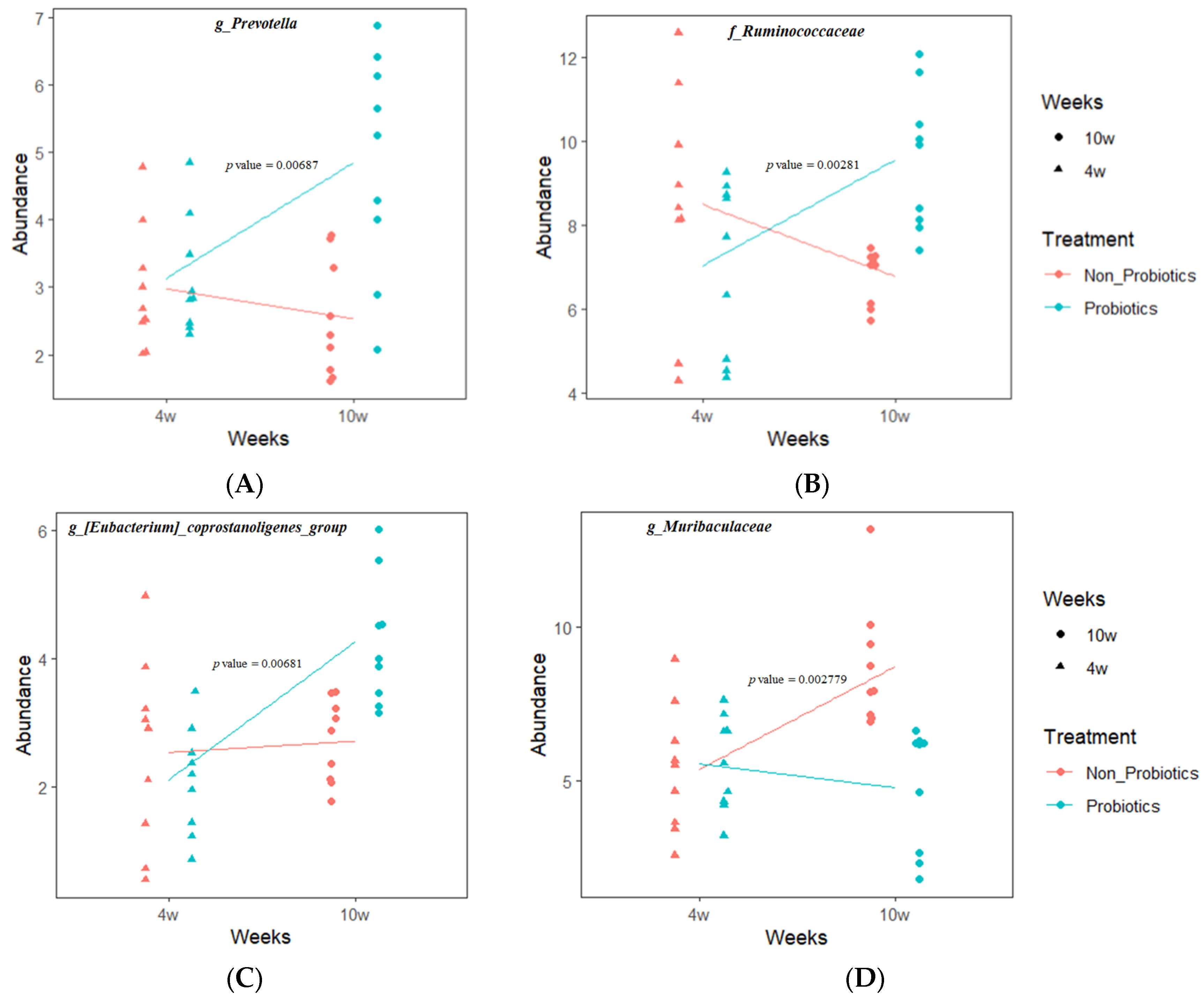
3.5. LMM Analysis on Relative Abundance Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musa, H.H.; Wu, S.; Zhu, C.; Seri, H.; Zhu, G. The potential benefits of probiotics in animal production and health. J. Anim. Vet. Adv. 2009, 8, 313–321. [Google Scholar]
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-derived probiotic Lactobacillus plantarum inhibits growth and adhesion of enterotoxigenic Escherichia coli and mediates host defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J. Probiotics, gut microbiota and health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K.J.N.r.i. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, R.; Zhu, J.; Huang, X.; Qu, J.J.M.B. Environmental filtering increases with elevation for the assembly of gut microbiota in wild pikas. Genes 2019, 12, 976–992. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ. Microbiol. 2016, 18, 1566–1577. [Google Scholar] [CrossRef]
- Xue, M.; Sun, H.; Wu, X.; Guan, L.L.; Liu, J.J.A.; Microbiology, E. Assessment of rumen microbiota from a large dairy cattle cohort reveals the pan and core bacteriomes contributing to varied phenotypes. Appl. Environ. Microbiol. 2018, 84, e00970-18. [Google Scholar] [CrossRef]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a possible link between the intestinal microbiota and feed efficiency in pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef]
- Ramayo-Caldas, Y.; Mach, N.; Lepage, P.; Levenez, F.; Denis, C.; Lemonnier, G.; Leplat, J.-J.; Billon, Y.; Berri, M.; Doré, J.; et al. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 2016, 10, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.P.; Pajarillo, E.A.B.; Oh, J.K.; Kim, H.; Kang, D. Revealing the combined effects of lactulose and probiotic enterococci on the swine faecal microbiota using 454 pyrosequencing. Microb. Biotechnol. 2016, 9, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, T.; Wang, X.; Kuang, S.; Xiao, Y.; Lu, W.; Bi, D. Probiotic mixture ameliorates heat stress of laying hens by enhancing intestinal barrier function and improving gut microbiota. Ital. J. Anim. Sci. 2017, 16, 292–300. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Xu, B.; Hao, L.; Su, W.; Jin, M.; Wang, Y. Bacillus subtilis and Enterococcus faecium co-fermented feed regulates lactating sow’s performance, immune status and gut microbiota. Microb. Biotechnol. 2021, 14, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Med. Ther. 2017, 17, 64. [Google Scholar] [CrossRef]
- Yeo, S.; Lee, S.; Park, H.; Shin, H.; Holzapfel, W.; Huh, C.S. Development of putative probiotics as feed additives: Validation in a porcine-specific gastrointestinal tract model. Appl. Microbiol. Biotechnol. 2016, 100, 10043–10054. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Fu, A.; Zhang, X.; Huang, Y.; Lee, K.H.; Yu, K.; Li, W.; Li, Y. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Benef. Microbes 2016, 7, 529–538. [Google Scholar] [CrossRef]
- Mallo, J.; Riopérez, J.; Honrubia, P. The addition of Enterococcus faecium to diet improves piglet’s intestinal microbiota and performance. Livest. Sci. 2010, 133, 176–178. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Duan, Y.; Zhao, Y.; Zhang, W.; Azad, M.A.K.; Wang, Z.; Blachier, F.; Kong, X. Dietary supplementation with Bacillus subtilis promotes growth and gut health of weaned piglets. Front. Vet. Sci. 2021, 7, 600772. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dun, Y.; Li, S.; Zhao, S.; Peng, N.; Liang, Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 1131. [Google Scholar] [CrossRef] [PubMed]
- Trckova, M.; Faldyna, M.; Alexa, P.; Zajacova, Z.S.; Gopfert, E.; Kumprechtova, D.; Auclair, E.; D’Inca, R. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 2014, 92, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wei, S.; Wang, Z.; Zhu, C.; Hu, S.; Zheng, C.; Chen, Z.; Hu, Y.; Wang, L.; Ma, X. Effects of different forms of yeast Saccharomyces cerevisiae on growth performance, intestinal development, and systemic immunity in early-weaned piglets. J. Anim. Sci. Biotechnol. 2015, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Hmar, E.B.L.; Paul, S.; Sharma, H.K. An Insight into the Combination of Probiotics and their Implications for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Li, Y.; Zhao, J. The gut microbiome of healthy long-living people. Aging 2019, 11, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef] [PubMed]
- Massacci, F.R.; Berri, M.; Lemonnier, G.; Guettier, E.; Blanc, F.; Jardet, D.; Rossignol, M.N.; Mercat, M.-J.; Doré, J.; Lepage, P.; et al. Late weaning is associated with increased microbial diversity and Faecalibacterium prausnitzii abundance in the fecal microbiota of piglets. Anim. Microbiome 2020, 2, 2. [Google Scholar] [CrossRef]
- Uddin, M.K.; Hasan, S.; Mahmud, M.R.; Peltoniemi, O.; Oliviero, C. In-feed supplementation of resin acid-enriched composition modulates gut microbiota, improves growth performance, and reduces post-weaning diarrhea and gut inflammation in piglets. Animals 2021, 11, 2511. [Google Scholar] [CrossRef]
- Vieira, A.M.; Sessin, A.P.; Soratto, T.A.T.; Pires, P.G.d.S.; Cardinal, K.M.; Wagner, G.; Hauptli, L.; Lima, A.L.F.; Dahlke, F.; Netto, D.P.; et al. Effect of functional oils or probiotics on performance and microbiota profile of newly weaned piglets. Sci. Rep. 2021, 11, 19457. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.R.; Jian, C.; Uddin, M.K.; Huhtinen, M.; Salonen, A.; Peltoniemi, O.; Venhoranta, H.; Oliviero, C. Impact of Intestinal Microbiota on Growth Performance of Suckling and Weaned Piglets. Microbiol. Spectr. 2023, 11, e0374422. [Google Scholar] [CrossRef] [PubMed]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- De Weirdt, R.; Van de Wiele, T. Micromanagement in the gut: Microenvironmental factors govern colon mucosal biofilm structure and functionality. NPJ Biofilms Microbiomes 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Garg, N.; Hasan, S.; Shirodkar, S. Prevotella: An insight into its characteristics and associated virulence factors. Microb. Pathog. 2022, 169, 105673. [Google Scholar] [CrossRef] [PubMed]
- Karasova, D.; Crhanova, M.; Babak, V.; Jerabek, M.; Brzobohaty, L.; Matesova, Z.; Rychlik, I. Development of piglet gut microbiota at the time of weaning influences development of postweaning diarrhea–a field study. Res. Vet. Sci. 2021, 135, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Saladrigas-García, M.; D’Angelo, M.; Ko, H.-L.; Nolis, P.; Ramayo-Caldas, Y.; Folch, J.M.; Llonch, P.; Solà-Oriol, D.; Pérez, J.F.; Martín-Orúe, S.M. Understanding host-microbiota interactions in the commercial piglet around weaning. Sci. Rep. 2021, 11, 23488. [Google Scholar] [CrossRef] [PubMed]
- Valeriano, V.; Balolong, M.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Janssen, A.W.; Kersten, S. The role of the gut microbiota in metabolic health. FASEB J. 2015, 29, 3111–3123. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.; Lindberg, J. Fermentable non-starch polysaccharides increases the abundance of Bacteroides–Prevotella–Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef]
- Peng, S.; Wang, X.; Wang, Y.; Lv, T.; Zhao, H.; Wang, Y.; Zhu, S.; Qiu, H.; Zeng, J.; Dai, Q.; et al. Effects of dietary Bacillus and Non-starch polysaccharase on the intestinal microbiota and the associated changes on the growth performance, intestinal morphology, and serum antioxidant profiles in ducks. Front. Microbiol. 2021, 12, 786121. [Google Scholar] [CrossRef] [PubMed]
- Juste, C.; Gérard, P. Cholesterol-to-coprostanol conversion by the gut microbiota: What we know, suspect, and ignore. Microorganisms 2021, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Sobolewska, S.; Kowalczuk-Vasilev, E.; Krasucki, W. Effect of dietary inulin source on piglet performance, immunoglobulin concentration, and plasma lipid profile. J. Vet. Res. 2014, 58, 453–458. [Google Scholar] [CrossRef][Green Version]
- Jung, H.; Kim, Y.; Han, I.K. Effects of fat sources on growth performance, nutrient digestibility, serum traits and intestinal morphology in weaning pigs. Asian-Australas. J. Anim. Sci. 2003, 16, 1035–1040. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.; Gálvez, E.J.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]


| Probiotics | Non-Probiotics | ||||||
|---|---|---|---|---|---|---|---|
| Animal ID | Body Weight (kg) | Weight Gain (kg) | Animal ID | Body Weight (kg) | Weight Gain (kg) | ||
| 4 w | 10 w | 4 w | 10 w | ||||
| 74-7 | 11.0 | 18.1 | 7.1 | 77-61 | 14.0 | 20.7 | 6.7 |
| 73-45 | 11.4 | 18.9 | 7.5 | 74-61 | 12.3 | 19.1 | 6.8 |
| 73-7 | 12.6 | 20.4 | 7.8 | 73-04 | 10.6 | 17.6 | 7.0 |
| 73-12 | 13.5 | 21.5 | 8.0 | 72-86 | 11.5 | 18.7 | 7.2 |
| 72-80 | 13.9 | 22.6 | 8.7 | 75-37 | 12.2 | 19.7 | 7.5 |
| 75-82 | 11.6 | 20.9 | 9.3 | 74-41 | 14.0 | 21.7 | 7.7 |
| 73-16 | 15.9 | 26.2 | 10.3 | 74-32 | 15.4 | 23.1 | 7.7 |
| 73-72 | 15.0 | 25.6 | 10.6 | 73-75 | 13.0 | 21.0 | 8.0 |
| 71-4 | 14.2 | 24.8 | 10.6 | 74-51 | 13.3 | 21.6 | 8.3 |
| Average | 13.2 | 22.1 | 8.9 | Average | 12.9 | 20.4 | 7.4 |
| SEM | 0.6 | 1.0 | 0.5 | SEM | 0.5 | 0.6 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kim, Y.-H.; Kim, S.-J.; Han, J.-H. Impact of Long-Term Supplementation with Probiotics on Gut Microbiota and Growth Performance in Post-Weaned Piglets. Animals 2024, 14, 1652. https://doi.org/10.3390/ani14111652
Park S-Y, Kim Y-H, Kim S-J, Han J-H. Impact of Long-Term Supplementation with Probiotics on Gut Microbiota and Growth Performance in Post-Weaned Piglets. Animals. 2024; 14(11):1652. https://doi.org/10.3390/ani14111652
Chicago/Turabian StylePark, Soo-Yeon, Yo-Han Kim, Sung-Jae Kim, and Jeong-Hee Han. 2024. "Impact of Long-Term Supplementation with Probiotics on Gut Microbiota and Growth Performance in Post-Weaned Piglets" Animals 14, no. 11: 1652. https://doi.org/10.3390/ani14111652
APA StylePark, S.-Y., Kim, Y.-H., Kim, S.-J., & Han, J.-H. (2024). Impact of Long-Term Supplementation with Probiotics on Gut Microbiota and Growth Performance in Post-Weaned Piglets. Animals, 14(11), 1652. https://doi.org/10.3390/ani14111652





