Simple Summary
Salmonellosis affects the poultry industry globally and is a major cause of bacterial food poisoning in humans. A Salmonella infection in broiler chickens can result in lower growth rates, decreased feed efficiency and higher mortality rates. The inclusion of probiotics in chicken diets enhances their intestinal microbiota and enhances different patterns of cytokine production in response to Salmonella infection. The increasing demand from consumers for chicken products devoid of antibiotics has increased the need to identify alternatives to antibiotics for the management of Salmonella infection, colonization, and carcass contamination in poultry. Through the process of competitive exclusion, probiotics have been shown to inhibit the colonization of the gut by harmful bacteria such as Salmonella and Clostridium perfringens. Probiotic supplementation also regulates the gut flora to protect the host from infections as well as to create bacteriocins, which directly prevent the growth of pathogens. This study was therefore carried out to ascertain whether the supplementation of Pediococcus pentosaceus GT001 can influence the growth performance, meat quality, intestinal development and cecum microbiota of Salmonella typhimurium-challenged broiler chickens.
Abstract
This study evaluated the effects of Pediococcus pentosaceus GT001 on Salmonella typhimurium (S. typhimurium)-challenged broiler chickens. Two hundred Ross 708 broiler day-old chicks with comparable weight were distributed at random into four treatments with five replicates and ten chicks per replicate. The following were the treatment groups: (B) basal diet (control); (B + S) basal diet and birds were challenged with S. typhimurium at 1.0 × 107 cfu/g; (B + P) basal diet + Pediococcus pentosaceus GT001 at 4.0 × 108 cfu/g; (B + P + S) basal diet + P. pentosaceus GT001 at 4.0 × 108 cfu/g and birds were challenged with S. typhimurium at 1.0 × 107 cfu/g. There was a significant reduction (p < 0.05) in the body weight of the Salmonella-infected birds compared to the other treatment groups. However, the FCRs of the broilers were comparable among the different treatment groups (p > 0.05). The lipid profile and liver function indices measured were significantly enhanced in the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) compared to the group that was Salmonella-challenged (p < 0.05) but were similar to those in the control group. The serum antioxidant activities, such as the T-AOC, SOD, CAT, GHS-Px and MDA, were significantly improved in the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) (p < 0.05). The MDA was similar in the B + P and B + P + S groups, but both were significantly lower than the control and the Salmonella groups. The administration of P. pentosaceus GT001 enhanced the lipase and amylase levels in both the serum and intestine of the broilers (p < 0.05). The immunoglobin (IgA, IgG, IgM) and cytokine (IL-10 and IL-6) levels in the serum were significantly higher in the B, B + P and B + P + S treatment groups (p < 0.05). The immune-related organs (bursa and spleen) were significantly influenced in the birds fed with P. pentosaceus GT001. No significant variation was noted among all the dietary treatments in terms of the measured meat quality indices. The small intestinal digesta content of the Salmonella load was below a detectable range after 14 days of infection (p < 0.05). No significant differences were observed among the different treatment groups in terms of the breast pH, drip loss and meat color (p > 0.05). The inclusion of P. pentosaceus GT001 also modified the community structure in the cecum. This indicates that it has health benefits and could be incorporated in the broiler diet.
1. Introduction
A prevalent pathogenic bacterium that affects all animal and poultry species is Salmonella. It can have a negative impact on human health as well as the animal sector, particularly in the production of poultry. Salmonella typhimurium (S. typhimurium) is a common serotype that causes salmonellosis in broiler chickens. It is an intestinal bacterium that can colonize chickens [1,2]. Salmonella has 10 distinct serotypes, the most common of which is Salmonella enterica serovar enteritidis, which is found in about 60% of poultry samples [3]. Salmonellosis is a global issue for the poultry industry, especially in developing nations, and is a major cause of bacterial food poisoning in humans. It is also a key factor in lower productivity in the poultry industry [4]. A Salmonella infection in broiler chickens can result in lower growth rates, decreased feed efficiency and higher mortality rates. Earlier work suggests that adding multi-strain probiotics to chicken diets enhanced their intestinal microbiota and enhanced different patterns of cytokine production in response to Salmonella infection [5]. The increasing demand from consumers for chicken products devoid of antibiotics has increased the need to identify alternatives to antibiotics for the management of Salmonella infection, colonization, and carcass contamination in poultry.
The use of feed additives like probiotics and other effective management techniques, such as immunization, biosecurity protocols, and regulatory compliance, is crucial for minimizing the effect of Salmonella on broiler chicken production and lowering the risk of foodborne disease for consumers. Through the process of competitive exclusion, probiotics have been shown to inhibit the colonization of the gut by harmful bacteria such as Salmonella and Clostridium perfringens [6]. According to other studies, probiotics enhanced broiler chicken performance by encouraging faster growth rates and feed conversion efficiency via various mechanisms [7]. P. pentosaceus had a variety of probiotic benefits, the most notable of which were immunological, antioxidant, growth enhancement [8] and cholesterol-lowering properties [9]. P. pentosaceus significantly enhanced complement 3 expression and immunoglobin M as well as reducing the damage to the intestinal villi and goblet cells, suggesting immune system stimulation. Moreover, it possessed broad-spectrum antimicrobial qualities [8]. Several functions were enhanced by P. pentosaceus, including growth, immunity, diseases resistance, and the activity of digestive enzymes. In addition to regulating the gut flora to protect the host from infections, P. pentosaceus can create bacteriocins, which directly prevent the growth of pathogens [10]. P. pentosaceus, a promising strain of lactic acid bacteria (LAB), is gradually attracting attention, leading to a rapid increase in experimental research [11]. This study assessed the effect of Pediococcus pentosaceus GT001 on the performance, immune function, antioxidant activities and intestinal development of Salmonella typhimurium-challenged broiler chickens. This study was also conducted to ascertain whether P. pentosaceus GT001 supplementation can decrease the S. typhimurium load in the poultry intestine as well as affect their cecum microbiota.
2. Materials and Methods
2.1. Bacteria Strain
In an earlier investigation, the P. pentosaceus GT001 utilized in this experiment was isolated, cultivated and examined in vitro (as part of the Ph.D. work). After inoculating a fresh culture in MRS broth medium for an entire night at 37 °C, the cultures were centrifuged for 15 min at 3000 rpm, washed twice in sterile phosphate-buffered saline (PBS) with a pH of 7.4, and then re-suspended in PBS to bring the concentration to 4.0 × 108 CFU/g. A total of 100 g of feed was well mixed with 10 mL of P. pentosaceus GT001.
2.2. Birds, Treatments, Design and Husbandry
A total of 200 Ross 708 broiler chicks at a day old and with an average weight of 39.19 g were acquired from Pluriton (Arendonk, Belgium). The chicks were split up into four experimental treatment groups at random in a completely randomized block design. The four experimental treatments comprised five replicates, with ten chicks per replicate, raised on a deep litter with an area of 3.0 m × 2.25 m. Feed and water were provided ad libitum, while the birds were maintained on a 24 h light schedule throughout the trial. A three-stage feeding regimen was implemented as follows: starter: 0–14 days of age; grower: 15–28 days of age and finisher: 29–42 days of age. The diets, comprising of maize and soybean meal in a mashed state, were formulated according to the requirements of the NRC [12] for the entire three-stage feeding regimen. Table 1 shows the nutritional values and content of the basal diets. The following were the treatment groups: basal diet (corn and soybean-based) (B); (B + S) basal diet + S. typhimurium challenged; (B + P) basal diet + P. pentosaceus − not S. typhimurium challenged; (B + S + P) basal diet + P. pentosaceus + S typhimurium challenged. After an hour of feed withdrawal, 100 g of basal meal was combined with about 10 mL of the probiotics, well mixed, and given to the birds (B + P and B + P + S). At the 3rd d of age, all the birds in the challenge groups (B + S and B + P + S) were orally gavaged with 1 mL of 1.0 × 107 S. typhimurium. In accordance with conventional management procedures, the room temperature was gradually lowered to 22 °C until the completion of the experiment after being kept at 34 °C for the first five days by the use of regulated heaters, fans, and opening doors and windows. On days 14 and 21, respectively, the chicks in all the treatment groups received vaccinations against Newcastle disease (YEBIO® Shandong, China) using the LaSota B1 strain of the virus (freeze-dried and live) and IBD against Gumboro disease (administered through the drinking water of the birds). The research area was regularly cleansed and de-infested to prevent infections from spreading.

Table 1.
Ingredients and chemical composition of the experimental diets.
2.3. Growth Performance
The weights of the chicks and feed were measured by replicate. The feed consumption and weight of the birds were measured weekly, and the feed conversion ratio (FCR) and body weight gain were calculated.
2.4. Serum Biochemistry
Using chicken-specific ELISA kits, the concentrations of total protein, albumin, globulin, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities, as well as malondialdehyde (MDA), interleukin-10 (IL-10), interleukin-6 (IL-6), and tumor necrosis factor-a (TNF-a), immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM), were measured according to the manufacturer’s protocol. The ELISA kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Two birds from each replicate in each treatment were sampled for 5 mL of blood through their jugular veins, placed into vacutainer tubes and left to clot at room temperature. The serum was extracted from the clotted blood samples after centrifugation for 15 min at room temperature at 3000 rpm.
2.5. Intestinal Measurement and pH Assessment
Using a measuring tape, the duodenum, ileal, and jejunal lengths were measured in centimeters. The intestinal contents of the duodenum, ileum, and jejunum obtained from the slain birds (two bird per replicate) at day 42 were put into sterile plastic containers. A pH probe was then inserted directly into the digesta content to record the pH (SP-701/pH/mV/Temp.Meter, Suntex, Taipei, Taiwan).
2.6. Digestive Enzyme Measurement
With ELISA kits obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China), the serum digestive lipase and amylase enzymes were measured according to the manufacturer’s instructions. The intestinal digestive enzymes of the small intestinal digesta was homogenized using 0.9% physiological saline. The homogenate was then centrifuged at 5000× g for 15 min to collect the supernatant. Subsequently, the Nanjing Jiancheng detection kits were used to measure the lipase and amylase activity in the small intestinal digesta.
2.7. Intestinal Morphology Measurement
At day 42, portions from the middle of the duodenum, jejunum, and ileum were taken from the birds. These segments were then washed with a 0.9% salt solution and preserved for 48 h in a 10% formaldehyde–phosphate buffer. The slices were then stained with hematoxylin–eosin to allow for the measurements of the height and width of the intestinal villi as well as the depth of the intestinal crypts under a light microscope. Ten full, precisely aligned crypt–villus units were selected in triplicate for each intestinal cross-section and used to calculate the crypt depth to villus height ratio. The histological segments were examined using a Leica DM500 light (Wetzlar, Germany). The crypt depth was measured from the root of the lower limit of the crypt to the villi–crypt junction, while the villi height was measured vertically from the villi–crypt junction to the tip of the villi [13].
2.8. Organs and Meat Quality Assessment
At day 42, 2 birds similar to the mean weight were selected from each replicate for the measurement of the organs and meat quality. Following the process of euthanasia, the jugular vein was cut off, feathers and head shanks were removed and the remainder of the carcasses was dissected. For the purpose of measuring the meat quality, each bird’s left and right breasts were used. Using a microprocessor pH-meter (SevenCompact pH Meter, S220, Mettler Toledo, Columbus, OH, USA), the breast muscle’s pH was determined 15 min after slaughter. The starting and final weights of each sample were utilized to quantify the drip loss of the chicken breast meat samples and subsequently suspended and standardized for the surface area in cups at 4 °C for 48 h [14]. Using a Minolta colorimeter, the samples’ meat colors were measured at three distinct points across the breast flesh and reported as lightness (L*), redness (a*), and yellowness (b*) [14].
2.9. Salmonella and TVC Enumeration
Samples of the small intestinal digesta were aseptically collected, placed in sterile plastic containers and covered with liquid nitrogen for laboratory assessment. The digesta samples were stored at −40 °C until the microbial count examination. One gram of the digesta samples from the small intestine was serially diluted ten times in the lab using nine milliliters of peptone water. The samples that had been diluted (0.1 mL) were added to selective agar and the bacterial enumeration was determined in a biosafety cabinet. Salmonella was incubated using XLD agar, while TVC was incubated using Standard Plate Count agar. The microbial population was represented as log10 colony-forming units/g of the digesta.
2.10. Analysis of Cecum Microbial Ecology
On day 42, 5 cecum content samples were taken from each treatment, beaded with a Mini-BeadBeater for DNA extraction, and the DNA was extracted using the Power Fecal DNA Isolation Kit (MO BIO, Carlsbad, CA, USA). The DNA was quantified using a NanoDrop spectrophotometer (Nyxor Biotech, Paris, France) and stained using the Quant-iT Pico Green dsDNA Kit (Invitrogen Ltd., Paisley, UK). In order to perform the DNA MiSeq sequencing, the V4 region of the bacterial 16S rDNA was amplified by PCR using the universal primers 515F (50-CCTACGGGNGGCWGCAG-30) and 806R (50-GGACTACHVGGGTWTCTAAT-30), which included the sequence of a sample bar and the FLX Titanium adapters. The cycling parameters were as follows: 4 min of initial denaturation at 94 °C, 25 cycles of denaturation at 94 °C (30 s each cycle), 45 s of annealing at 50 °C, 30 s of elongation at 72 °C, and 5 min of final extension at 72 °C. For the MiSeq sequencing, three distinct PCR reactions were combined for each sample. The PCR products were isolated using 1.5% agarose gel electrophoresis and further purified with QIAqu. The PCR products were purified using a Gel Extraction Kit (Qiagen, Hilden, Germany). The Quant-iT Pico Green dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA) was used to quantify the amplicons. Each sample’s amplicons were combined at the same concentrations. The MiSeq Reagent Kit v2 (Illumina, San Diego, CA, USA) was used for the MiSeq sequencing, and libraries were produced using the TruSeq DNA PCR-Free Sample Prep Kit (Ilumina, San Diego, CA, USA). Paired-end readings were assigned to the samples with unique barcodes; the barcode and primer sequence were snipped. FLASH (https://ccb.jhu.edu/software/FLASH/MANUAL, accessed on 1 June 2024) was used for merging the paired-end readings. The length and quality of the resulting sequences were further examined and filtered. To obtain clean, high-quality tags in line with fqtrim (v0.94), the raw reads were put through quality filtering under predetermined filtering settings. The program V-search (v2.3.4) was utilized to filter the chimeric sequences. Using DADA2, (https://benjjneb.github.io/dada2/, accessed on 1 June 2024) the feature table and feature sequence were obtained after dereliction. Using the feature abundance, the relative abundance of each sample was normalized in line with the SILVA (version 132) classifier. The graphs were created using the R program, and the analysis of the alpha and beta diversities was computed using QIIME2 (https://view.qiime2.org/, accessed on 1 June 2024).
2.11. Statistical Analysis
Using a randomized complete block design (RCBD) with four replications, the general linear model (GLM) of Minitab® version 18.1 (Minitab version 18) was used to analyze the data as a one-way ANOVA. Tukey’s test was used to determine whether the treatment means differed from one another, and statistical significance was defined as p < 0.05. All the data are presented as the mean ± SEM. To reveal the similarities and contrasts between the four treatment groups on OTUs, a Venn diagram was used. To obtain the comparative study of the intergroup and group differences in terms of the individual fraction distance, the beta diversity analyses for the principal coordinate analysis (PCoA), the principal component analysis (PCA), and the nonmetric multidimensional scaling (NMDS) were employed. The permutational analysis of variance (PERMANOVA) was used to examine the beta diversity. The relative abundances at the phylum, family, and genus were examined using the Kruskal-Wallis test (p < 0.05).
3. Results
3.1. Growth Performance
The production parameter outcomes are presented in Table 2. The birds’ initial weights prior to the trial started were comparable for all the feeding regimens. There was no significant influence of P. pentosaceus GT001 supplementation on the daily feed intake (p = 0.677) and feed conversion ratio (p = 0.252) of the birds. The Salmonella-infected birds (B + S) showed significant (p < 0.05) decreased body weight changes (final weight, total weight gain and average daily gain) during the study. The body weight changes of the B, B + P and B + P + S groups were similar (p > 0.05) but significantly higher (p < 0.05) compared to the B + S group. The Salmonella-infected groups of B + S and B + P + S recorded mortality rates of 6.7% and 3.3%, respectively, compared to both the B- and B + P-treated birds.

Table 2.
Effect of Pediococcus pentosaceus GT001 on the production performance of broilers.
3.2. Serum Biochemistry
The results of the liver function and lipid profile are presented in Table 3. Significant differences (p < 0.05) were observed among all the indices measured. The B and B + P birds had similar albumin and creatinine content but were higher (p < 0.001) compared to their counterpart in the B + S and B + P + S groups. The globulin level of P. pentosaceus GT001 supplementation (B + P) was higher (p = 0.004) than B + S and B + S; nonetheless, it was similar to the B. Salmonella-infected group (B + S), which had the least significant total protein content, while the P. pentosaceus GT001 supplementation group (B + P) recorded the highest significant protein content (p = 0.001). The use of B + P in terms of the protein content was similar to B, while B + S showed similarity with the B + P + S-treated birds. With regards to the ALT and AST content, P. pentosaceus GT001 supplementation (B + P) recorded higher levels, while B + S recorded the lowest (p < 0.001) In terms of the T cholesterol, TG and HDL, the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) produced similar results, though lower (p < 0.001) compared to the groups of B and B + S. The LDL content for both the B + P and B + P + S groups were similar, though both were higher (p < 0.001) than the B and B + S groups.

Table 3.
Serum biochemistry activities of broilers fed with Pediococcus pentosaceus GT001 (n = 8).
3.3. Serum Antioxidant Activities
Table 4 shows the outcome of the serum antioxidant activities. There was a significant variation among all the treatment groups for all the indices measured under serum antioxidant activities. The birds treated with B + P recorded the highest significant (p = 0.001) T-AOC level compared to the Salmonella-infected groups. Furthermore, the B group showed similarity with the Salmonella-infected groups. The birds on the B + P produced the highest and most significant (p < 0.001) SOD and GHS-Px compared to the other treatments, while the use of B + S produced the least level. The MDA contents of the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) were comparable but (p < 0.001) lower than both group of B and B + S. Additionally, B + P was higher (p < 0.001) than B + P + S, B and B + S in terms of the CAT content, while the B and B + S groups showed comparable levels.

Table 4.
Pediococcus pentosaceus GT001 impact on the serum antioxidant activities of broilers (n = 8).
3.4. Serum Cytokines and Immunology
There were significant variations among the treatment groups in terms of the serum cytokines and immunology parameters, as shown in Table 5. The values obtained for TNF-α and IL 6 were higher in B + P, with B + S having the least significant levels (p < 0.01). The use of B + P produced the highest significant (p < 0.01) IL 10 content compared to B, B + S and B + P + S. However, the choice of the B, B + S and B + P + S treatments showed comparable levels during the study. No significant differences were noted in the serum IgG and IgA of treatments B, B + P and B + P + S, though a high significant variation was observed when compared to BD + S (p < 0.05). Regarding the serum IgM, B and B + P recorded the highest significant values, followed by B + P + S, with B + S recording the least value (p < 0.01).

Table 5.
Effects of probiotic Pediococcus pentosaceus GT001 on the serum cytokines and immunological indices of broilers (n = 8).
3.5. Digestive Enzymes
Table 6 presents the results of the serum and intestinal digestive enzymes of the broiler chickens. In terms of the serum amylase content, the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) were similar but (p < 0.01) higher compared to both the BD and BD + S groups. However, the birds in the B + S group recorded the least. Similarly, the serum lipase content was higher (p < 0.01) in the P. pentosaceus GT001-supplemented groups (B + P and B + P + S). A significant difference was noted in intestinal amylase. B + P had the highest significant value, followed by B, while the Salmonella-infected groups (B + S and B + P + S) recorded the least value (p < 0.05). With intestinal lipase, the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) had the highest significant values, but B + P + S was similar to B and B + S had the least significant value (p < 0.01).

Table 6.
Effect of Pediococcus pentosaceus GT001 on the digestive enzymes of broilers (n = 8).
3.6. Organs
The results of the internal organ are shown in Table 7. No significant differences (p > 0.05) were observed among the dietary treatments in the measured organ parameters expect the immune-related organs (spleen, bursa) and small intestine. Regarding the bursa, the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) and the B group showed similarity but were higher than the Salmonella-infected group (B + S) (p = 0.009). B + P and B were similar in terms of the spleen, though B + P was significantly higher compared to the Salmonella-infected groups (B + S and B + P + S). The B group was similar to B + P + S but higher than that of the B + S group. The small intestinal weights of B, B + P and B + P + S were high (p = 0.043), while B + S recorded the least, though similar to B.

Table 7.
Effects of Pediococcus pentosaceus GT001 on the weights of the organs of broilers (n = 8).
3.7. Meat Quality
The results in Table 8 show the impact of P. pentosaceus GT001 on meat quality. No significant variation was noted among all the dietary treatments in terms of the meat quality indices measured (p > 0.05).

Table 8.
Effect of Pediococcus pentosaceus GT001 on broiler meat quality.
3.8. Intestinal pH and Length
The outcomes concerning the intestinal length and pH are presented in Table 9. The intestinal length showed no significant differences among the treatments (p > 0.05). The lengthiest duodenum was observed in B + P, followed by B, then B + P + S, with B + S having the least. With the jejunal length, the B group recorded the highest value during the period of the trial, while B + S recorded the lowest length. Similarly, the longest ileum was noted in the B group, while the B + S group recorded the least. However, significant variations were noted in the intestinal pH. Both the B + P and B treatments recorded the highest duodenal pH, which were similar but higher than the B + S group (p < 0.01). The pH of the jejunum of B was higher compared to the other treatments (p < 0.01). The Salmonella-infected and P. pentosaceus GT001-supplemented groups (B + S, B + P and B + P + S) showed no significant difference during the study. A significance difference was noted in the ileum pH. The B groups showed the highest significant pH value compared to the other treatments (p < 0.01). The pH of B + S was also higher than the P. pentosaceus GT001-supplemented groups (B + P and B + P + S) (p < 0.01).

Table 9.
Effect of Pediococcus pentosaceus GT001 on the intestinal length and pH of broilers (n = 8).
3.9. Small Intestinal Morphology
The results of the morphology of the broilers’ small intestines are shown in Table 10. The duodenal VH and CD varied significantly among the treatments, while a significant difference was not noted in the VH:CD. Treatment B was higher compared to the other treatments, followed by the P. pentosaceus GT001-supplemented groups (B + P and B + P + S), while the Salmonella-infected group (B + S) produced the least significant value (p < 0.01). However, the duodenal CD values were comparable among the treatments of B, B + P and B + P + S, though all were higher than the B + S group (p = 0.001). The jejunal VH of B recorded the highest significant difference, followed by B + P and B + P + S, while B + S recorded the least significant difference (p < 0.001). With the CD of the jejunum, the highest significant difference was noted in B + P, while the lowest significant difference was observed in the B + S group. The VH:CD levels were comparable among the dietary treatments. Significant differences were noted among the dietary groups in the ilea VH and CD values, while no significant difference was observed in the VH:CD value. Treatment B + P was higher (p < 0.001) in terms of the ilea VH and CD when compared to the other treatments. The VH and CD of B + P + S and B were similar, but both were higher than that of B + S.

Table 10.
Effect of Pediococcus pentosaceus GT001 on the small intestinal morphology of broilers.
3.10. Salmonella Load
The outcomes of the Salmonella count and total viable count during different periods are presented in Table 11. Significant variations were noted in both parameters at the different periods post infection. B + S, which was the Salmonella-infected group, recorded the highest significant Salmonella level compared to the other groups (p < 0.01) at day 3, 7 and 14 post infection. No significant variation (p > 0.05) was observed among B and the P. pentosaceus GT001-supplemented groups (B + P and B + P + S). In terms of the total viable count, the Salmonella-challenged + probiotic (B + P + S) group recorded the highest significant value at day 3 post infection. The TVC at day 3 post infection of the B + P and B + S groups were comparable, but both were higher than the B treatment group (p < 0.01). The highest significant TVC value at day 7 post infection was noted in the B and B + S treatment groups, while the probiotic-supplemented groups (B + P and B + P + S) recorded the least values. At day 14 post infection, B observed the highest significant value of TVC, followed by B + S, while B + P and B + P + S recorded the least TVC values.

Table 11.
Effect of Pediococcus pentosaceus GT001 on the Salmonella load after Salmonella infection in broilers.
3.11. Analysis of Cecum Microbial Ecology
3.11.1. Microbial Effective Sequence
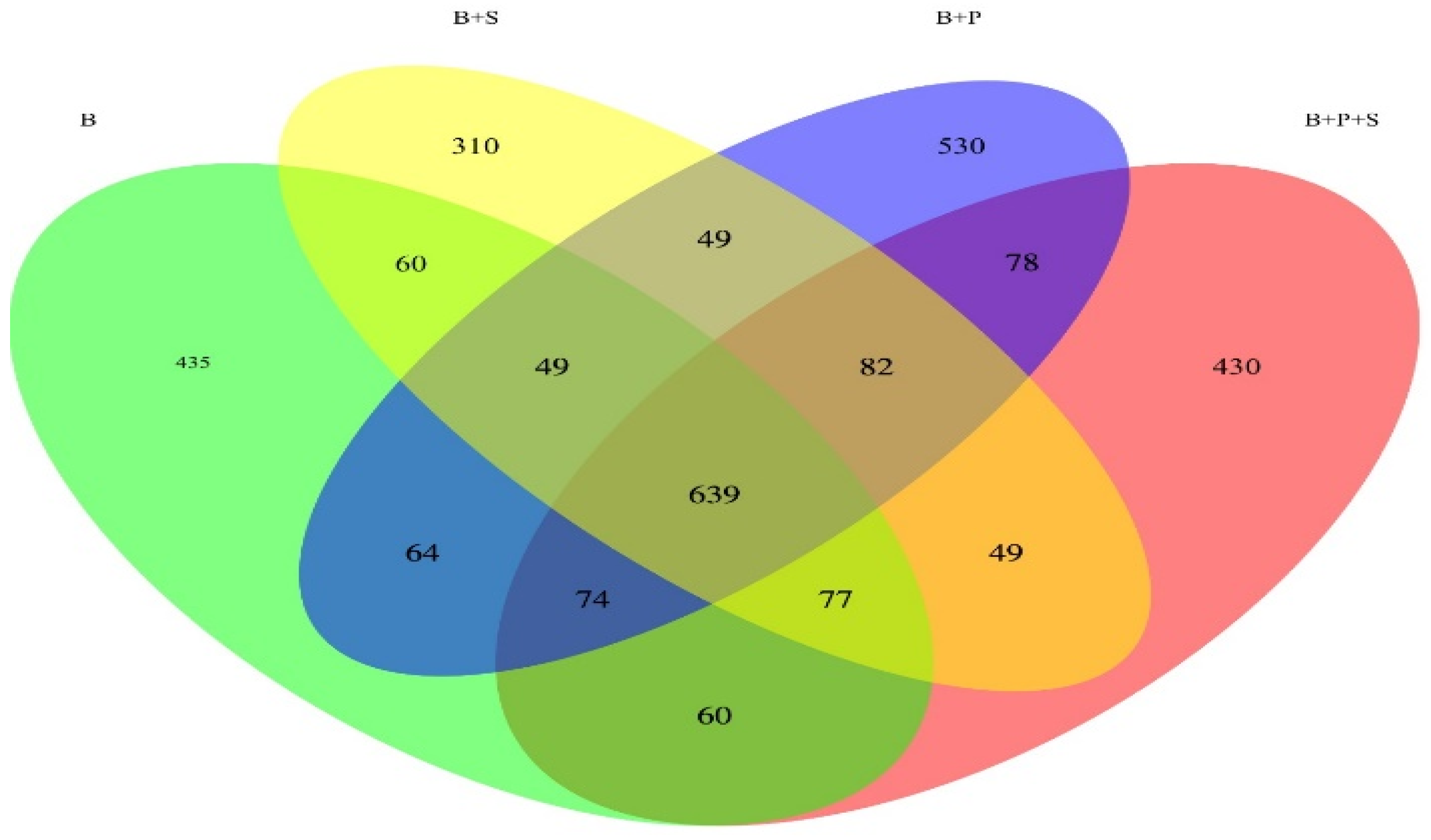
Figure 1 displays a Venn diagram based on the OTUs in the total sequences of each group. The Venn diagram explains the overlap (639 OTU, core) with the four displayed groups combined. Across all four treatments, a total of 435, 310, 530 and 430 unique OTU were exposed. To be precise, 60 OTU were found in both the B and B + S treatments; 49 OUT were found in both the B + S and B + P treatments; 49 OUT were found in the B + P and B + P + S treatments, and 60 OTU were found in the B + P + S and B treatments
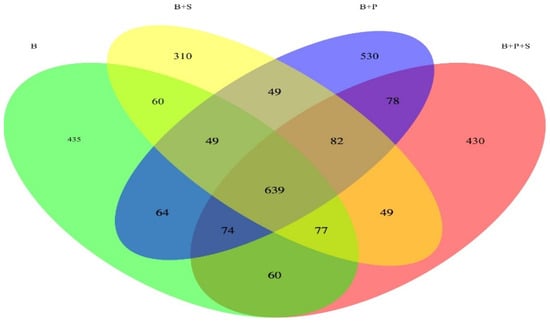
Figure 1.
Venn diagram based on the OTUs in the total sequences of each treatment. B—basal diet (corn and soybean-based); B + S—basal diet + Salmonella typhimurium challenged; B + P—basal diet + P. pentosaceus − not Salmonella typhimurium challenged; B + P + S—basal diet + P. pentosaceus + Salmonella typhimurium challenged.
3.11.2. Microbial Diversity
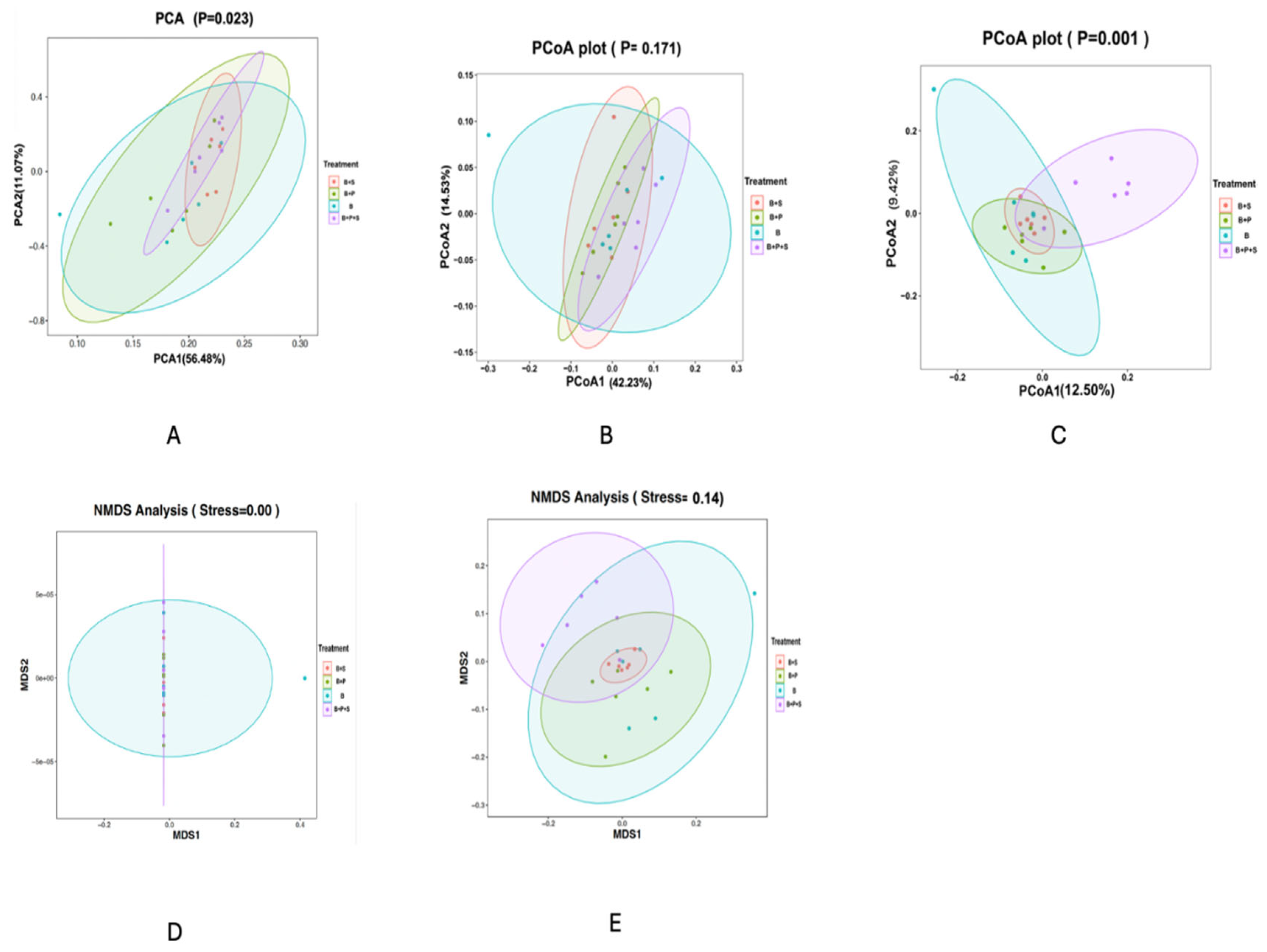
Table 12 displays the indicators of the alpha diversity of the following indices: Goods coverage, Shannon, Simpson, Chao1 and Observed OUTs. No significant differences were observed in all the indices measured among the dietary treatments (p > 0.05). Figure 2 displays the beta diversity indicators of the PCoA, PCA, and NMDS that were obtained to calculate the intragroup and intergroup distances. It was observed that the differences among the treatments were not significant in the PCA (Figure 2A), weighted PCoA (Figure 2B), and unweighted NMDS (Figure 2E) plots. However, a significant difference was estimated in the unweighted PCoA (Figure 2C) and weighted NMDS (Figure 2D) plots (p < 0.05). The statistically significant p values were estimated in the intragroup and intergroup distances.

Table 12.
Dietary inclusion of Pediococcus pentosaceus GT001 on the composition of the cecal microbiota of broilers by alpha diversity measures.
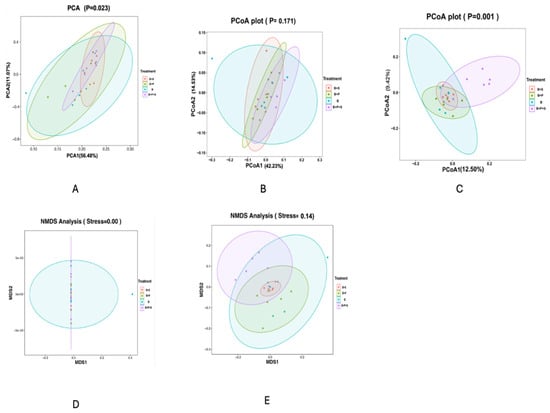
Figure 2.
Effect of dietary inclusion of P. pentosaceus GT001 on the cecal microbiota composition of broilers by indices of beta diversity. (A) PCA; (B) weighted PCoA; (C) unweighted PCoA; (D) weighted NMDS; (E) unweighted NMDS. B—basal diet (corn and soybean-based); B + S—basal diet + Salmonella typhimurium challenged; B + P—basal diet + P. pentosaceus − not Salmonella typhimurium challenged; B + P + S—basal diet + P. pentosaceus + Salmonella typhimurium challenged.
3.11.3. Microbial Composition
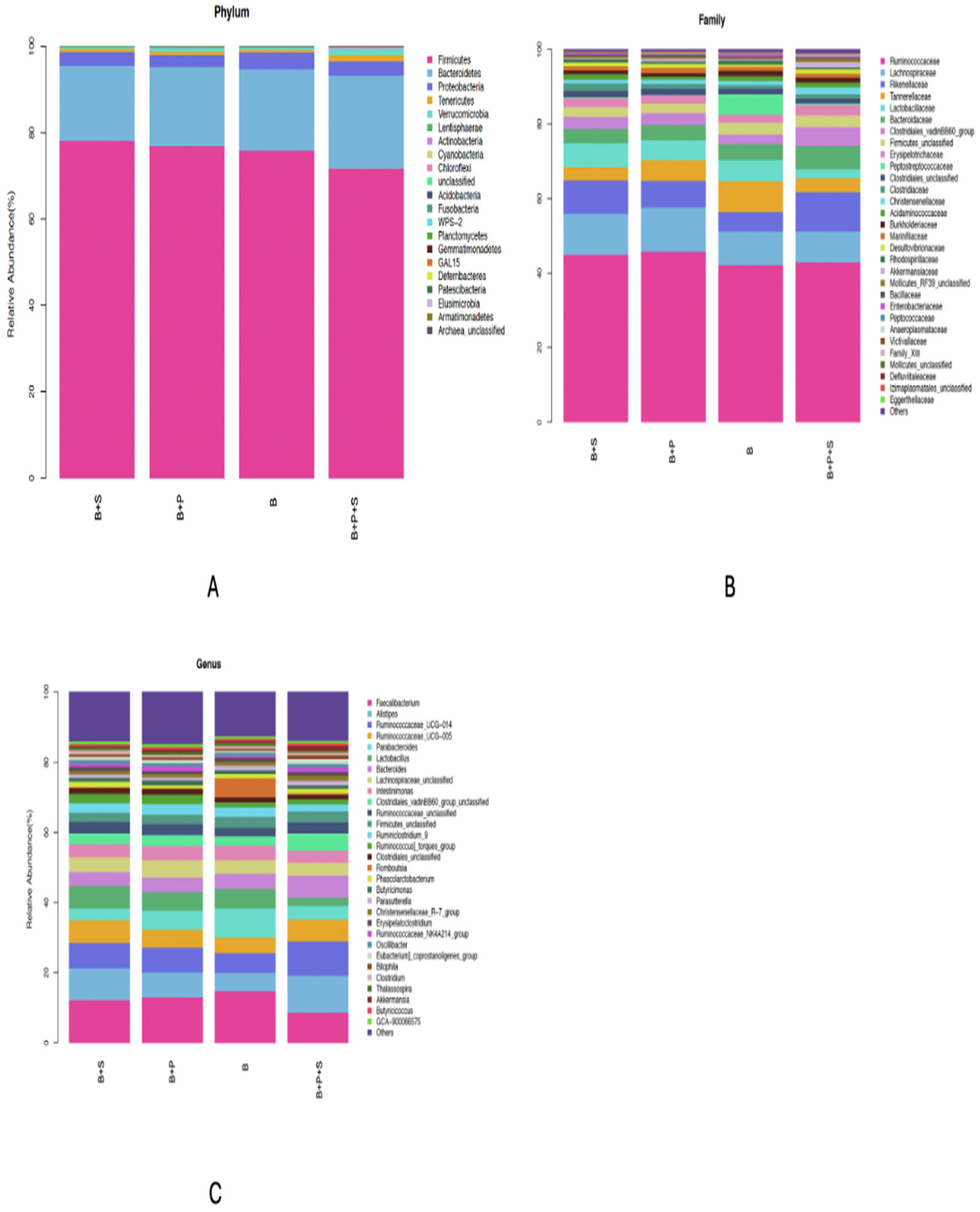
The effects of the dietary supplementation of P. pentosaceus GT001 on the phylum, family and genus taxa levels in the microbial content of the cecum are presented in Figure 3A–C. The estimation of the phylum level is shown in Figure 3A, where Proteobacteria, Bacteroidetes and Firmicutes were the most dominant phyla of the cecal community, accounting for about 3.32%, 19.06% and 75.61%, respectively. Rikenellaceae, Lachnospiraceae and Ruminococcaceae were the dominant families in the cecal community at the family level, as presented in Figure 3B. Rikenellaceae accounted for 8.02%, Lachnospiraceae recorded 10.05% and Ruminococcaceae accounted for the highest, which was 43.93%. In terms of the genus taxonomy, Ruminococcaceae_UCG-005, Ruminococcaceae_UCG-014, Alistipes and Faecalibacterium accounted for around 5.60%, 7.43%, 8.02%, and 12.12% of the genera in the cecal community, respectively, as presented in Figure 3C.
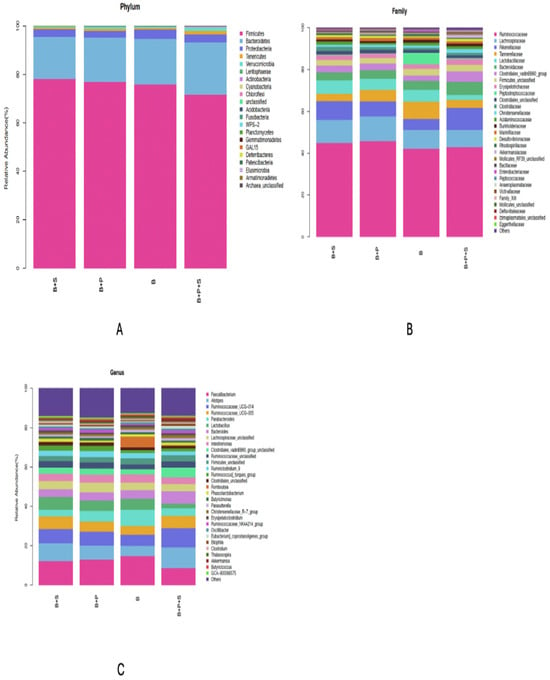
Figure 3.
Effect of dietary inclusion of P. pentosaceus GT001 on the relative abundance percentage of cecal microbiota communities at the (A) phylum, (B) family and (C) genus levels. B—basal diet (corn and soybean-based); B + S—basal diet + Salmonella typhimurium challenged; B + P—basal diet + P. pentosaceus − not Salmonella typhimurium challenged; B + P + S—basal diet + P. pentosaceus + Salmonella typhimurium challenged.
Some significant differences were observed in the relative abundance at the phylum, family and genus levels (Table 13). The abundance of the Cyanobacteria phylum was similar between the B and B + P treatments, but both were higher compared to the Salmonella-challenged groups (B + S and B + P + S) (p = 0.033). The B + P + S treatment recorded the highest significance for the Actinobacteria phylum, while the other treatments were similar (p = 0.002). Proteobacteria at the phylum level was similar among the dietary treatments (p = 0.615). Significance was noted among the treatments in terms of Clostridiaceae and Lactobacillaceae at the family level. Clostridiaceae was higher in the probiotic-supplemented group (B + P), but similarities were observed among B, B + S and B + P + S (p = 0.054). No significant differences were noted among the treatments with regard to Peptostreptococcaceae and Bacteroidaceae at the family level. At the genus level, the control and probiotic-supplemented groups (B and B + P) recorded the highest significant values in terms of Lactobacillus and GCA-900066575 compared to the Salmonella-challenged groups (B + S and B + P + S) (p < 0.05). Also, the control treatment (B) was significantly higher compared to B + S, B + P and B + P + S in regards to Escherichia-Shigella and CHKCI001 genus. Clostridiales_unclassified, Anaerofustis, CHKCI002, Papillibacter and GCA-900066225 genus were similar among the dietary treatments (p > 0.05).

Table 13.
Effect of dietary inclusion of Pediococcus pentosaceus GT001 on the bacterial taxonomy within the composition of the cecum microbiota of broiler chickens.
4. Discussion
4.1. Growth Performance
The use of probiotics as a feed additive is widely accepted as a preventive measure in reducing pathogen infection and enhancing the growth performance of poultry [15]. To assess the effectiveness of P. pentosaceus GT001 on the growth performance, this study noted the decreased body weight of the Salmonella-infected birds. Salmonella infection resulting from the damage of the internal mucosal affects feed absorption and reduces intestinal motility [16,17]. The positive effects of P. pentosaceus GT001 as a probiotic on the body weight changes of broilers are in agreement with previous studies by Sikandar et al. [18] and Chang et al. [19]. In the present study, the FCR was similar between the Salmonella-infected birds and the probiotic-supplemented birds. This is in agreement with the findings of Mountzouris et al. [20], who reported similarity in terms of the FCR of Salmonella-infected and probiotic birds. On the contrary, previous studies have reported superior FCRs in probiotic supplemented birds [18,19]. These inconsistent outcomes may potentially stem from variations in the type, quantity, or dosage of Salmonella delivered, which resulted in varying degrees of intestinal environment stability [21]. Additionally, the high mortality in the Salmonella-challenged birds in the present study is attributable to the Salmonella infection at day 3.
4.2. Serum Biochemistry Activities
There was an enhancement in all the lipid profile and liver function indices measured. The probiotic P. pentosaceus GT001-supplemented groups had better values than the Salmonella-challenged group. Our results were in accordance with the results of the study performed by de Azevedo et al. [22], which showed that P. pentosaceus stimulates the immune system, decreases the HDL, total cholesterol and TG levels, increases LDL and improves the digestion of protein. The purpose of HDL is to convey any residual cholesterol that is not being used to the liver. The leftover cholesterol will be used as a constituent in the production of bile salt and steroid hormones, while the remaining inactive cholesterol will be defecated. Alterations in the activities of AST and ALT are also specific indicators that can be utilized to ascertain the organism’s hepatocyte activity as well as specific indicators of hepatocyte damage [23]. In the current study, the AST and ALT activity levels obtained among the treatment groups were within the normal range, though the probiotic-supplemented groups were significantly high. The range of AST and ALT activities among the treatments during the study indicates that feeding P. pentosaceus GT001 to broilers resulted in normal liver function.
4.3. Serum Antioxidant Activities
The P. pentosaceus GT001-supplemented groups showed significant responses in all the antioxidant activity indices measured. There was an increase in the serum T-AOC, SOD, CAT and GHS-Px activities, which is similar to the observation in the research by Mohamed et al. [24] and Zhang et al. [25] when probiotic was fed to broiler chickens. The serum MDA activity was decreased in the P. pentosaceus GT001-supplemented group in the current the study. Mohamed et al. [24] and Wang et al. [26] previously reported a reduced MDA in probiotic-fed birds. This observation may arise from the enhanced serum SOD activities of the birds. It is known that alterations in the activity of a number of antioxidant enzymes can be employed to evaluate an animal’s overall antioxidant state and degree of oxidative stress. According to Yang et al. [27], the T-AOC reflects the organism’s level of antioxidants and SOD catalyzes the conversion of the superoxide anion into hydrogen peroxide. Contrary to the current study, Abudabos et al. [28] and Erdoğan et al. [29] reported comparable values among treatments, indicating that either probiotics or Salmonella had no effect on the overall antioxidant capacity or oxidative stress. Factors including the type of challenge, dosage and the probiotic strain could account for this observed variation.
4.4. Cytokines and Immunological Activities in the Serum
Immunological indices serve as the first line of defense against invasive pathogens since many microbial pathogens first come into touch with their hosts via mucosal surfaces, particularly in the alimentary canal [30]. Therefore, regular and moderate use of probiotics can have a significant influence on the immune system and boost the immunoglobulin concentration. The immunoglobulin (IgG, IgA and IgM) levels in the current study were improved in the probiotic group and probiotic + challenged group. The P. pentosaceus augmented endogenous interferon and cytokine production, boosting humoral as well as cell-mediated immunity [31,32]. A similar observation was noted in the serum cytokines indices. The increase levels of the anti-inflammatory cytokines IL-10 and IL-6 may be due to the suppression of the stress-related inflammatory response when the probiotic was the supplement in the diet [33]. In accordance with the present study, Masuda et al. [34] observed highly induced cytokines when the P. pentosaceus strain was supplemented in a diet.
4.5. Digestive Enzymes
The administration of probiotic P. pentosaceus GT001 enhanced the lipase and amylase activities in both the serum and intestine in this study, in accordance with the previous studies by Mohamed et al. [35] and Wang and Gu [36]. The authors noted the improved amylase and lipase activities of the broiler diet supplemented with probiotics. The digestion of protein, carbohydrate, and fats is improved by higher lipase and amylase activity, which may have contributed to the improvement in growth observed in this study.
4.6. Organs and Meat Quality
A significant rise was noted in the weights of the bursa of Fabricius and spleen, unlike the other organs measured, in which no differences in weight were observed in the birds fed with P. pentosaceus GT001. Given that T and B lymphocyte maturation sites are located in the bursa of Fabricius in birds, the size and mass of this organ can reveal crucial general information about the maturation and development of the immune system [37]. According to Mohamed et al. [35] and Park and Kim [37], birds fed a meal supplemented with probiotics showed more weight in the bursa of Fabricius than the control groups. As a result, from our study, the apparent expanded bursa of Fabricius in the groups administered with P. pentosaceus GT001 may be a favorable signal for the immune system development of the birds. Thus, the apparent larger bursa of Fabricius in the P. pentosaceus GT001-fed groups in our study may have a positive impact on the immune system development of the birds at the start of the study.
Probiotics have been used to improve meat quality but have shown inconsistent results [38,39,40]. No significant differences were noted among the treatment groups in terms of the breast pH and drip loss measured after 48 h post slaughter. Drip loss is a significant variable in the assessment of meat quality because water loss can result in the loss of some nutrients in the fluid, which can affect the meat’s softness, flavor, and juiciness [41]. When choosing poultry meat, consumers consider color to be one of the most important quality factors.
Numerous factors, like diet and genetics, affect the color of meat [42]. However, the present study did not observe any influence of P. pentosaceus GT001 on meat color. Contrary to our current study, Park and Kim [37] and Macelline et al. [38] reported enhanced meat quality when the diet of broiler chickens was supplemented with probiotics. These inconsistent results could be due to variations in the type and dosage of probiotic used [21].
4.7. Intestinal Length and pH
Healthier small intestinal pH values were observed in the P. pentosaceus GT001-fed group, while a similar length of small intestine was noted among all the treatment groups. Numerous theories have been proposed to explain the beneficial effects of probiotics in reducing the pH of the gut, which leads to a decrease in the stability of the dangerous bacteria in the intestines [43]. Probiotics can lower the pH of the digestive system and disrupt the ideal pH range 7 for Salmonella environments [43]. As a result, birds’ performance, feed conversion, and growth rate can all be improved. This finding is in accordance with that reported by Chen et al. [44], who reported that P. pentosaceus supplementation significantly decreased the pH value in the gut.
4.8. Intestinal Morphology
The birds fed P. pentosaceus GT001 had longer duodenal, jejunal and ilea villi and crypts compared to the Salmonella-infected birds in the present study. Therefore, the decline in production performance in the birds challenged with Salmonella can be explained by decreased villus height since a longer villus is a sign of a healthy gut. Research [45] shows that shorter villi and deeper crypts result in less disease resistance, increased mucus secretion in the GIT, poor nutrient absorption, the presence of toxins, and generally poorer broiler performance. Shalaei et al. [46] reported that birds fed a diet containing probiotic had higher villi height in the duodenum than birds fed other additives.
4.9. Salmonella Load and Total Viable Count
The small intestinal digesta content of the Salmonella load was below a detectable range after 14 days infection. The Salmonella load in the birds in the probiotic + challenge group was similar to that of the probiotic and control. Probiotics have been shown by researchers [20,37] to competitively exclude pathogens in the chicken gut. According to the competitive exclusion theory of Patterson and Burkholder [47], beneficial gut bacteria compete with pathogenic bacteria for receptor sites and nutrition before producing antimicrobial chemicals such as bacteriocins to reduce their burden in the intestine. Probiotics also enhance the function of the mucosal barrier, which reduces the load of Salmonella in the gut [48]. According to these findings, P. pentosaceus GT001 may be used as a probiotic to treat Salmonella infections in poultry. Consistent with the current study, several researchers [19,49] also reported a reduced Salmonella load in the GIT when the broiler diet was supplemented with probiotics. Ultimately, the findings demonstrated that probiotic P. pentosaceus GT001 effectively improved the microbiota and Salmonella load of birds that were exposed to Salmonella.
4.10. Cecum Microbial Ecology
Dietary inclusion of P. Pentosaceus GT001 in this study changed the broiler microbiota and enhanced the immunity against Salmonella infection. When Zhang et al. [50] supplemented broiler chicks’ diets with probiotics, they observed no discernible changes in the microbiota’s alpha diversity (Shannon, Simpson and Chao1) at 42 days of age in the cecum. The microbiome community’s diversity is described by the Simpson and Shannon indices, but the richness of the microbial diversity is reflected by the Chao1 index and observed species [51].
At the phylum level, the majority of the broiler microbiota was made up of Firmicutes and Bacteroidetes, accounting for about 95% of the total cecum microbiota. These organisms are involved in energy production and metabolism, specifically the fermentation of microbes and the digestion of starch [52]. Also, in the broilers infected with Salmonella, the greater presence of Proteobacteria points to gastrointestinal dysbiosis and imbalance, but the abundance of Proteobacteria was similar among the treatments. The relative abundance of Cyanobacteria phyla was increased in the B and B + P groups. Contrary to our study, Mohamed et al. [24] and Trela et al. [53] reported a decrease in Cyanobacteria in the microbial content of the cecum when birds were fed probiotics. Some organisms within the phylum Cyanobacteria are capable of producing neurotoxins that can lead to diseases and death [54]. This study observed that the level of the families of Rikenellaceae, Lachnospiraceae and Ruminococcaceae dominated the cecum, amounting for a total of 62%. Because the families Ruminococcaceae, Rikenellaceae and Lachnospiraceae are associated with the production of short-chain fatty acids, they enhance the feed conversion ratio [55]. This could explain why the feed conversion was similar across the dietary treatments, leading to the enhanced growth performance of the Salmonella-challenged bird plus probiotic supplementation. Zhou et al. [56] also reported that these three families are the most abundant in the small intestine, which is in consistent with our findings.
In terms of the cecum community, the key bacteria genera observed in this study were Ruminococcaceae_UCG-005, Ruminococcaceae_UCG-014, Alistipes and Faecalibacterium and they constituted about 33.17%. In accordance with research by Mohamed et al. [24] and Zhu et al. [57], Ruminococcaceae_UCG-014, Alistipes and Faecalibacterium are the most abundant genera of bacteria in the microbial community of the cecum. These genera are members of the Firmicutes family, which has been associated with enhanced weight gain in chickens and an increase in the rate of nutrient absorption by creating compounds that the gut wall can absorb as an energy source [58]. This assertion can be associated with the enhanced performance in the Salmonella-challenged birds + probiotic supplementation treatment. The relative abundance of the genera Escherichia-Shigella was inhibited by Pediococcus pentosaceus GT00l in the cecal community, while Clostridiales_unclassified was similar among the treatments. In accordance with this study, Mohamed et al. [24] observed a dramatic decrease in the relative abundance of genera of the Escherichia–Shigella when the broiler diet was supplemented with either probiotic or antibiotic. According to Ma et al. [59], Escherichia–Shigella is an opportunistic pathogenic bacterium that can cause intestinal destruction and measure pro-inflammatory activities through a variety of means, including the spread of virulence factors. This increases the host’s risk of infection. In the gut, Clostridium_unclassified can cooperate and compete with other bacteria to proliferate. According to Bertoluzzi et al. [60], Clostridia_unclassified generates some toxins that might lead to severe illnesses in poultry. Bacteriocins, which Pediococcus pentosaceus is able to produce, have been reported to exhibit antibacterial activity in a number of models [61]. Pediococcus pentosaceus is a lactic acid bacterium that also has the ability to create organic acid. According to Cui et al. [62], lactic acid can prevent pathogenic bacteria from colonizing the GIT. In the B and B + P groups, the relative abundance of the Lactobacillus genus was increased. One of the most important bacteria probiotics is Lactobacillus due to it suppression of pathogens, promotion of the growth of beneficial bacteria and enhancement of growth performance. It also assists in the maintenance of the microbial balance in the intestine [63].
5. Conclusions
In conclusion, this study demonstrates P. pentosaceus GT001 to be a probiotic and feed additive that has health benefits and could be incorporated in the broiler diet. Dietary inclusion of P. pentosaceus GT001 as a probiotic led to significant changes in the growth performance, immune function, digestive enzymes and intestinal morphology of Salmonella typhimurium-challenged broiler chickens. Additionally, the inclusion of P. pentosaceus GT001 modulated the community structure in the cecum and reduced the Salmonella load in the small intestine of the Salmonella typhimurium-challenged birds. The inclusion of P. pentosaceus GT001 in the poultry diet has the potential to reduce the Salmonella load and produce healthier broiler birds.
Author Contributions
Investigation, data collection, methodology, statistical analysis, writing—original draft, G.Z.B.; writing—review and editing, methodology, data collection, L.A., P.A. and A.D.O.-O.; investigation, data collection, A.A.-A.K., V.K.L. and C.C.; statistical analysis, K.O.A. and T.M.M.; investigation, project administration funding acquisition, supervision and writing—reviewing and editing, Z.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Innovation Research 2035 Pilot Program of Southwest University (SWU-XDPY22005), Fundamental Research Funds for National Key R&D Program of China (2022YFD1300501-3), the National Natural Science Foundation of China (32372894), the Natural Science Foundation Project of Chongqing (cstc2021jcyj-msxmX0966).
Institutional Review Board Statement
This study was conducted in accordance with the guidelines approved by the Ghanaian Institutional Animal Care and Use Committee (IACUC), a committee of the Council for Scientific and Industrial Research (CSIR), (RPN 008/CSIR-IACUC/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request, the corresponding author may supply the data supporting the study’s findings.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thung, T.Y.; Mahyudin, N.A.; Basri, D.F.; Radzi, C.W.M.; Nakaguchi, Y.; Nishibuchi, M.; Radu, S. Prevalence and antibiotic resistance of Salmonella enteritidis and Salmonella typhimurium in raw chicken meat at retail markets in Malaysia. Poult. Sci. 2016, 95, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Kellermann, A.; Santos, L.R.D.; Bessa, M.C.; Nascimento, V.P.D. Salmonella spp. in raw broiler parts: Occurrence, antimicrobial resistance profile and phage typing of the Salmonella enteritidis isolates. Braz. J. Microbiol. 2007, 38, 296–299. [Google Scholar] [CrossRef]
- Antunes, P.; Réu, C.; Sousa, J.C.; Peixe, L.; Pestana, N. Incidence of Salmonella from poultry products and their susceptibility to antimicrobial agents. Int. J. Food Microbiol. 2003, 82, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Saleem, G.; Ramzaan, R.; Khattak, F.; Akhtar, R. Effects of acetic acid supplementation in broiler chickens orally challenged with Salmonella pullorum. Turk. J. Vet. Anim. Sci. 2016, 40, 434–443. [Google Scholar] [CrossRef]
- Chang, C.H.; Teng, P.Y.; Lee, T.Y.; Yu, B. The effects of the supplementation of multi-strain probiotics on intestinal microbiota, metabolites and inflammation of young SPF chickens challenged with Salmonella enterica subsp. enterica. Anim. Sci. J. 2019, 90, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.; Alyemni, A.; Al Marshad, B.A. Bacillus subtilis PB6 based-probiotic (CloSTATTM) improves intestinal morphological and microbiological status of broiler chickens under Clostridium perfringens challenge. Int. J. Agric. Biol. 2013, 15, 978–982. [Google Scholar]
- Rajput, I.R.; Li, W.F. Potential role of probiotics in mechanism of intestinal immunity. Pak. Vet. J. 2012, 32, 3. [Google Scholar]
- Gong, L.; He, H.; Li, D.; Cao, L.; Khan, T.A.; Li, Y.; Pan, L.; Yan, L.; Ding, X.; Sun, Y.; et al. A new isolate of Pediococcus pentosaceus (SL001) with antibacterial activity against fish pathogens and potency in facilitating the immunity and growth performance of grass carps. Front. Microbiol. 2019, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, K.; Lee, Y.S.; Palaniyandi, S.A.; Yang, S.H.; Suh1, J.-W. Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front. Microbiol. 2015, 6, 1–14. [Google Scholar]
- De Souza de Azevedo, P.O.; Mendonca, C.M.N.; Moreno, A.C.R.; Bueno, A.V.I.; de Almeida, S.R.Y.; Seibert, L. Antibacterial and Antifungal Activity of Crude and Freeze-Dried Bacteriocin-Like Inhibitory Substance Produced by Pediococcus Pentosaceus. Sci Rep. 2020, 10, 12291. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Fact. 2021, 20, 45. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Erdogmuş, S.Z.; Gülmez, N.; Fındik, A.; Şah, H.; Gülmez, M. Efficacy of probiotics on health status and growth performance of Eimeria tenella infected broiler chickens. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 311–320. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Peña-González, E.M.; Janacua-Vidales, H.; Santana, V.; Ortega, J.A. Meat Quality and Lipid Oxidation of Pork after Dietary Supplementation with Oregano Essential Oil. World Appl. Sci. J. 2013, 21, 665–673. [Google Scholar]
- Dersjant-Li, Y.; Romero, L.F.; Wealleans, A.; Awati, A. Analysis of eight trial studies confirmed beneficial effect of a combination of enzymes and direct fed microbials on weight gain and feed utilisation efficiency in broilers. In Proceedings of the International Poultry Scientific Forum, Atlanta, GA, USA, 27–28 January 2014; Volume 69. [Google Scholar]
- Sikandar, A.; Cheema, A.H.; Adil, M.; Younus, M.; Zaneb, H.; Zaman, A.; Tipu, M.Y.; Masood, S. Ovine paratuberculosis—A histopathological study from Pakistan. J. Anim. Plant Sci. 2013, 23, 749–753. [Google Scholar]
- Vandeplas, S.; Dauphin, R.D.; Thiry, C. Efficiency of a Lactobacillus plantarum-xylanase combination on growth performances, microflora populations, and nutrient digestibilities of broilers infected with Salmonella typhimurium. Poult. Sci. 2009, 88, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Adil, M.; Ali, H.M.; Muhammad, N.; Rehman, T.; Rehman, A.; Rehman, H.F. Effects of Bacillus subtilis on performance, immune system and gut in Salmonella-challenged broilers. S. Afr. J. Anim. Sci. 2020, 50, 2221–4062. [Google Scholar] [CrossRef]
- Chang, C.H.; Teng, P.Y.; Tzu Tai Lee, T.T.; Yu, B. Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. Enterica. Asian-Australas. J. Anim. Sci. 2020, 33, 1797–1808. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Balaskas, C.; Xanthakos, I.; Tzivinikou, A.; Fegeros, K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009, 50, 467–478. [Google Scholar] [CrossRef]
- Malago, J.J.; Koninkx, J.F.; Ovelgönne, H.H.; van Asten, F.J.; Swennenhuis, J.F.; van Dijk, J.E. Expression levels of heat shock proteins in enterocyte-like Caco-2 cells after exposure to Salmonella enteritidis. Cell Stress Chaperon. 2003, 8, 194–203. [Google Scholar] [CrossRef]
- de Azevedo, P.O.S.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Antimicrobial activity of bacteriocin-like inhibitory substance produced by Pediococcus pentosaceus: From shake flasks to bioreactor. Mol. Biol. 2019, 46, 461–469. [Google Scholar] [CrossRef]
- Begum, S.A.; Upadhyaya, T.N.; Baruah, G.K. Hematobiochemical alterations of acute chlorpyriphos intoxication in indigenous chicken. J. Vet. World. 2015, 8, 750–754. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Sun, W.; Bumbie, G.Z.; Elokil, A.A.; Mohammed, K.A.F.; Zebin, R.; Hu, P.; Wu, L.; Tang, Z. Feeding Bacillus subtilis ATCC19659 to Broiler Chickens Enhances Growth Performance and Immune Function by Modulating Intestinal Morphology and Cecum Microbiota. Front. Microbiol. 2022, 12, 798350. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, K.; Zhang, J.; Xu, W.; Huang, Q.; Wang, T. Dietary effects of Bacillus subtilis fmbj on the antioxidant capacity of broilers at an early age. Poult. Sci. 2017, 96, 3564–3573. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Xu, H.; Mei, X.; Gong, L.; Wang, B.; Li, W.; Jiang, S. Direct-fed glucose oxidase and its combination with B. amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow feathered broilers. Poult. Sci. 2018, 97, 3540–3549. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from Bacillus amyloliquefaciens C-1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Alyemni, A.H.; Zakaria, H.A.H. Effect of Two Strains of Probiotics on the Antioxidant Capacity, Oxidative Stress, and Immune Responses of Salmonella-Challenged Broilers. Braz. J. Poult. Sci. 2015, 18, 175–180. [Google Scholar] [CrossRef]
- Erdoğan, Z.; Erdoğan, S.; Aslantaş, Ö.; Çelik, S. Effects of dietary supplementation of synbiotics and phytobiotics on performance, caecal coliform population and some oxidant/antioxidant parameters of broilers. J. Anim. Physiol. Anim. Nutr. 2010, 94, e40–e48. [Google Scholar] [CrossRef]
- Muir, W.I.; Bryden, W.L.; Husband, A.J. Evaluation of the efficacy of intraperitoneal immunization in reducing Salmonella typhimurium infection in chickens. Poult. Sci. 1998, 77, 1874–1883. [Google Scholar] [CrossRef]
- Alkhalf, A.; Alhaj, M.; Al-Homidan, I. Influence of probiotic supplementation on immune response of broiler chicks. Egypt. Poult. Sci. 2010, 30, 271–280. [Google Scholar]
- Dhama, K.; Singh, S.D. Probiotics improving poultry health and production: An overview. Poult. Punch. 2010, 26, 41. [Google Scholar]
- Wang, L.; Li, L.; Lv, Y.; Chen, Q.; Feng, J.; Zhao, X. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Sci. Rep. 2018, 8, 2229. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Kimura, M.; Okada, S.; Yasui, H. Pediococcus pentosaceus Sn26 Inhibits IgE Production and the Occurrence of Ovalbumin-Induced Allergic Diarrhea in Mice. Biosci. Biotechnol. Biochem. 2010, 74, 329–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohamed, E.A.; Mohamed, T.E.; Heba, M.S.; Amira, M.E.; Mohamed, M.S.; Gehan, B.A.Y.; Ayman, E.T.; Soliman, M.S.; Ahmed, E.A.; Attalla, F.E.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Q. Effect of probiotic on growth performance and digestive enzyme activity of Arbor Acres broilers. Res. Vet. Sci. 2010, 89, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, I.H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014, 93, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Macelline Shemil Priyan, W.H.D.; Cho, H.M.; Awanthika, T.H.K.; Wickramasuriya, S.S.; Jayasena, D.D.; Tharangani, H.R.M.; Song, Z.; Heo, J.M. Determination of The Growth Performances and Meat Quality of Broilers Fed Saccharomyces cerevisiae as a Probiotic in Two Different Feeding Intervals. Korean J. Poult. Sci. 2017, 44, 161–172. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhou, T.X.; Ao, X.; Kim, I.H. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize-soybean meal based diets. Livest. Sci. 2012, 150, 419–424. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, Y.B. Effect of the feeding probiotics, illite, activated carbon, and hardwood vinegar on the meat quality and shelf-life in chicken thigh. Korean J. Food Sci. Anim. Resour. 2008, 28, 480–485. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Dong, X.; Yao, Z.; Xu, B.; Zhen, S.; Li, C.; Li, X. Effects of pre chilling parameters on water-holding capacity of chilled pork and optimization of pre chilling parameters using response surface methodology. J. Anim. Sci. 2012, 90, 2836–2841. [Google Scholar] [CrossRef]
- Fletcher, D.L. Broiler breast meat color variation, pH, and texture. Poult. Sci. 1999, 78, 1323–1327. [Google Scholar] [CrossRef]
- Angel, R.; Dalloul, R.A.; Doerr, J. Performance of broiler chickens fed diets supplemented with a direct-fed microbial. Poult. Sci. 2005, 84, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, L.; Qiu, H. Isolation and Probiotic Potential of Lactobacillus salivarius and Pediococcus pentosaceus in Specific Pathogen Free Chickens. Braz. J. Poult. Sci. 2017, 19, 325–332. [Google Scholar] [CrossRef]
- Sen, S.; Ingale, S.; Kim, Y.; Kim, J.; Kim, K.; Lohakare, J.; Kim, E.; Kim, H.; Ryu, M.; Kwon, I. Effect of supplementation of Bacillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012, 93, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Shalaei, M.; Hosseini, S.M.; Zergani, E. Effect of different supplements on eggshell quality, some characteristics of gastrointestinal tract and performance of laying hens. Vet. Res. Forum. 2014, 5, 277–286. [Google Scholar] [PubMed]
- Patterson, A.; Burkholder, M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Applegate, T.J.; Selvara, R.K. Effect of Bacillus subtilis and Bacillus licheniformis probiotic supplementation on cecal Salmonella load in broilers challenged with salmonella. J. Appl. Poult. Res. 2020, 29, 808–816. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Yu, Y. Effects of Bacillus Coagulans on growth performance, antioxidant capacity, immunity function, and gut health in broilers. Poult. Sci. 2021, 100, 10116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef]
- Mohd Shaufi, M.A.; Sieo, C.C.; Chong, C.W.; Gan, H.M.; Ho, Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015, 7, 4. [Google Scholar] [CrossRef]
- Trela, J.; Kieronczyk, B.; Hautekiet, V.; Józefiak, D. Combination of Bacillus licheniformis and salinomycin: Effect on the growth performance and git microbial populations of broiler chickens. Animals 2020, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2004, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Heckert, R.A.; Estevez, I.; Russek-Cohen, E. Effects of density and perch availability on the immune status of broilers. Poult. Sci. 2002, 81, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lan, F.; Li, X. The spatial and temporal characterization of gut microbiota in broilers. Front. Vet. Sci. 2021, 8, 7122. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Liu, H. Soybean oligosaccharide, stachyose, and raffinose in broilers diets: Effects on odor compound concentration and microbiota in cecal digesta. Poult. Sci. 2020, 99, 3532–3539. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Park, J.W.; Kim, I.H. Effects of Bacillus subtilis, Kefir and β-glucan supplementation on growth performance, blood characteristics, meat quality and intestine microbiota in broilers. Korean J. Poult. Sci. 2016, 43, 159–167. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Zhang, H.; Wang, J.; Zhang, W.; Gao, J. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018, 8, 15358. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Serpa Vieira, B.; de Paula Dorigam, J.C.; Menconi, A.; Sokale, A.; Doranalli, K. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganisms 2019, 7, 71. [Google Scholar] [CrossRef]
- Nobutani, K.; Sawada, D.; Fujiwara, S.; Kuwano, Y.; Nishida, K.; Nakayama, J. The effects of administration of the Lactobacillus gasseri strain CP 2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 2017, 122, 212–224. [Google Scholar] [CrossRef]
- Cui, Y.L.; Run, S.C.; Wan, F.C. Bacteriostasis of Bacillus coagulans TBC 169 to enteropathogenic bacteria. Chin. J. Microecol. 2015, 7, 333–338. [Google Scholar]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ramasamy, K.; Sieo, C.C.; Ho, Y.W. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE 2017, 12, e0175959. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).