Element Concentrations and Histopathology of Liver and Kidney in West Greenland Ringed Seals (Pusa hispida)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
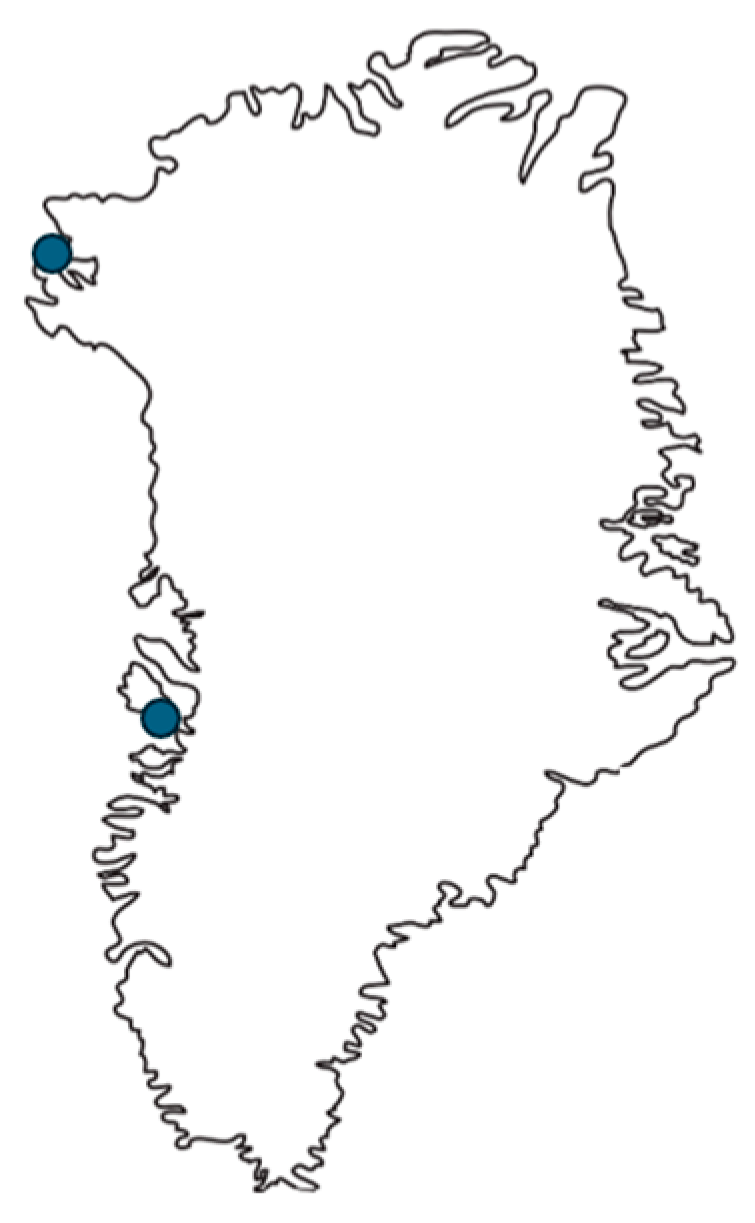
2.1. Sampling and Age Estimation
2.2. Metal Analyses
2.3. Histology
2.4. Statistics
3. Results
3.1. Age and Sex Distribution
3.2. Metals
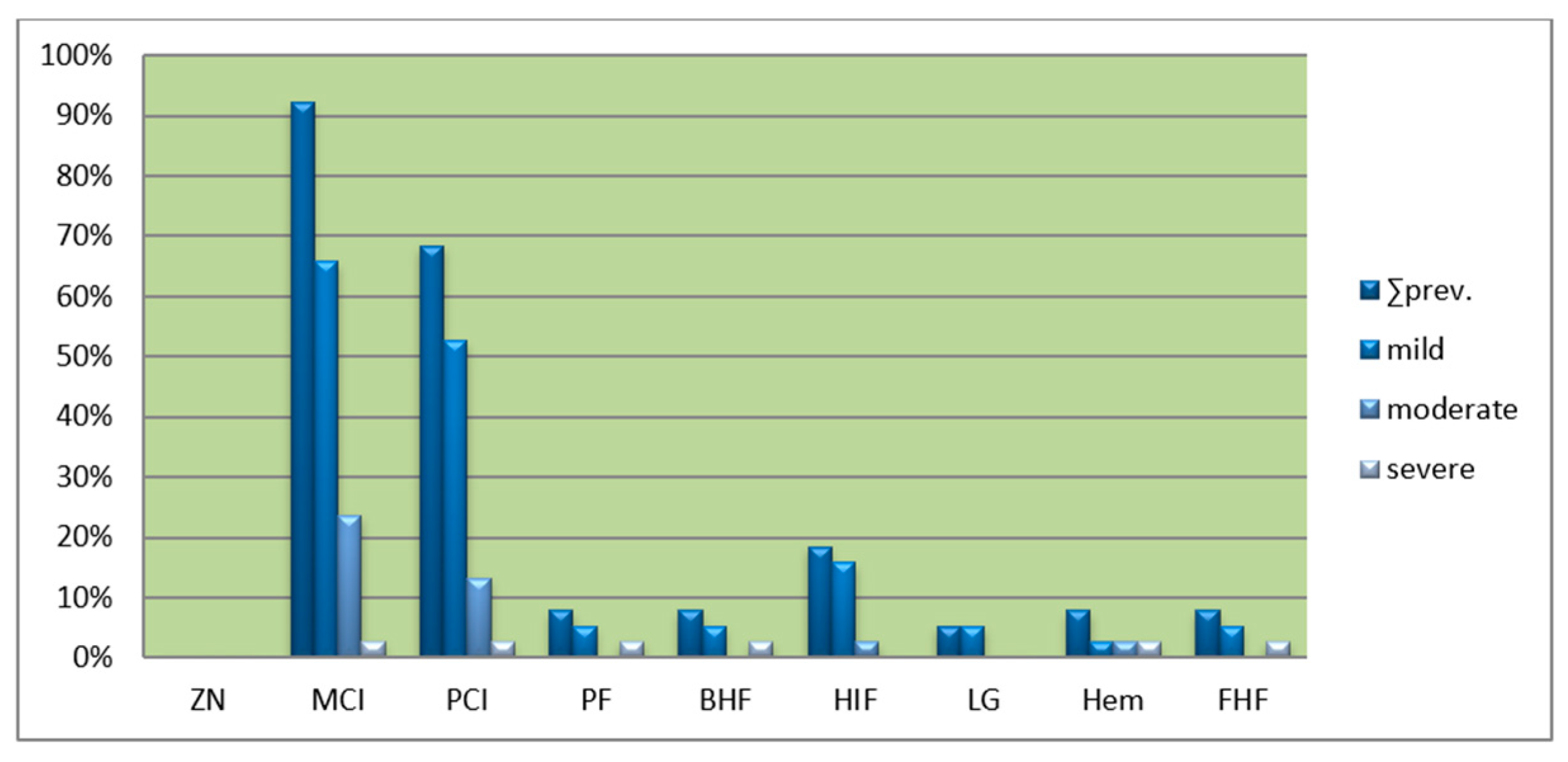
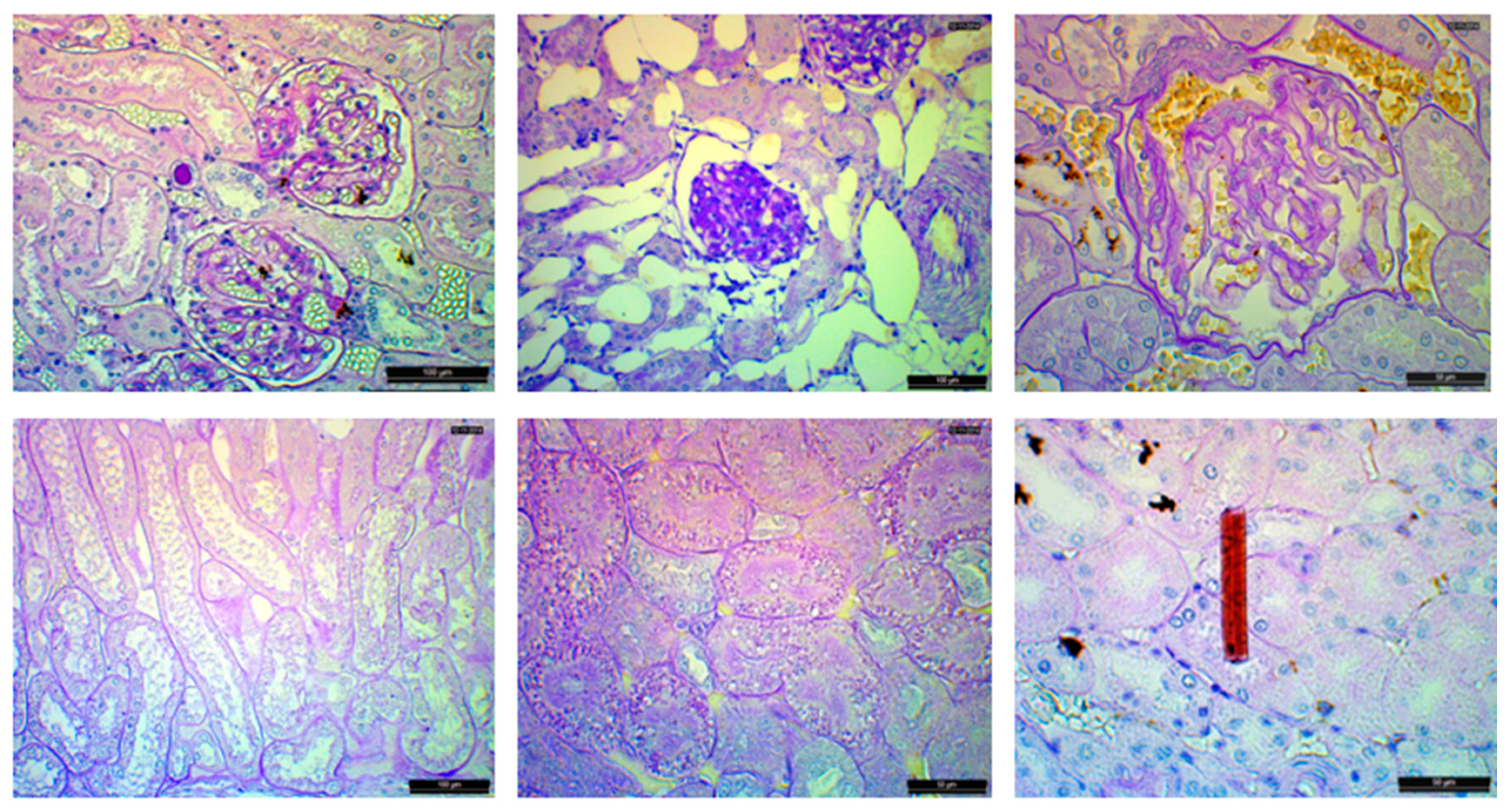
3.3. Liver Histology
3.4. Kidney Histology
3.5. Histology vs. Heavy Metals, Selenium and Age
4. Discussion
4.1. Heavy Metals
4.2. Liver Histopathology
4.3. Kidney Histopathology
4.4. Histopathology vs. Metals and Age
4.5. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AMAP. AMAP Assessment 2002. Human Health in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2003; pp. xiii, 137. [Google Scholar]
- AMAP. AMAP Assessment 2002: Heavy Metals in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2005; pp. xvi+265. Available online: https://www.amap.no/documents/doc/amapassessment-2002-heavy-metals-in-the-arctic/97 (accessed on 10 May 2024).
- Bard, S.M. Global transport of anthropogenic contaminants and the consequences for the Arctic marine ecosystem. Mar. Pollut. Bull. 1999, 38, 356–379. [Google Scholar] [CrossRef]
- Muir, D.; Wagemann, R.; Hargrave, B.; Thomas, D.; Peakall, D.; Norstrom, R. Arctic marine ecosystem contamination. Sci. Total Environ. 1992, 122, 75–134. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; Debes, F.; Choi, A.L.; Budtz-Jørgensen, E. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol. Teratol. 2014, 43, 39–44. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.L.; Mogensen, U.B.; Bjerve, K.S.; Debes, F.; Weihe, P.; Grandjean, P.; Budtz-Jørgensen, E. Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicol. Teratol. 2014, 42, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Basu, N.; Braune, B.; O’Hara, T.; Scheuhammer, T.M.; Sonne, C.; Andersen, M.; Andreasen, C.; Andriashek, D.; Asmund, G.; et al. What are the toxicological effects of mercury in Arctic biota? Sci. Total Environ. 2013, 443, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Sonne, C.; Aspholm, O.; Dietz, R.; Andersen, S.; Berntssen, M.H.; Hylland, K. Metal concentrations and metallothionein binding capacity in liver, kidney and brain tissues of three Arctic seal species. Sci. Total Environ. 2009, 407, 6166–6172. [Google Scholar] [CrossRef]
- Routti, H.; Letcher, R.J.; Born, E.W.; Branigan, M.; Dietz, R.; Evans, T.J.; Fisk, A.T.; Peacock, E.; Sonne, C. Spatio-temporal trends of selected trace elements in liver tissue from polar bears (Ursus maritimus) from Alaska, Canada and Greenland. J. Environ. Monitor. 2011, 13, 2260–2267. [Google Scholar] [CrossRef]
- Sonne, C. Health effects from long-range transported contaminants in Arctic top predators: An integrated review based on studies of polar bears and relevant model species. Environ. Int. 2010, 36, 461–491. [Google Scholar] [CrossRef]
- Letcher, R.J.; Bustnes, J.O.; Dietz, R.; Jenssen, B.M.; Jørgensen, E.H.; Sonne, C.; Verreault, J.; Vijayan, M.M.; Gabrielsen, G.W. Effects Assessment of Persistent Organohalogen Contaminants in Arctic Wildlife and Fish. Sci. Total Environ. 2010, 408, 2995–3043. [Google Scholar] [CrossRef]
- Dietz, R.; Rigét, F.F.; Sonne, C.; Letcher, R.J.; Born, E.W.; Muir, D.C.G. Seasonal and temporal trends in polychlorinated biphenyls and organochlorine pesticides in East Greenland polar bears (Ursus maritimus), 1990–2001. Sci. Total Environ. 2004, 331, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Outridge, P.M.; Hobson, K.A. Anthropogenic contributions to mercury levels in present-day Arctic animals—A review. Sci. Total Environ. 2009, 407, 6120–6131. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.C.; Sangalang, G.; Uthe, J.F.; Ronald, K. Steroidogenesis Invitro in Harp Seal (Pagophilus groenlandicus) without and with Methyl Mercury Treatment In vivo. Environ. Physiol. Biochem. 1975, 5, 428–439. [Google Scholar] [PubMed]
- Rolland, R.M. A review of chemically-induced alterations in thyroid and vitamin A status from field studies of wildlife and fish. J. Wildl. Dis. 2000, 36, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Rigét, F.U.; Dietz, R.; Johansen, P.; Asmund, G. Lead, cadmium, mercury and selenium in Greenland marine biota and sediments during AMAP phase 1. Sci. Total Environ. 2000, 245, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Paludan-Müller, P.; Agger, C.T.; Nielsen, C.O. Cadmium, mercury, zinc and selenium in ringed seals (Phoca hispida) from Greenland and Svalbard. NAMMCO Sci. Publ. 1998, 1, 243–272. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Letcher, R.J.; Desforges, J.-P.; Eulaers, I.; Sonne, C.; Wilson, S.; Andersen-Ranberg, E.; Basu, N.; Barst, B.D.; Bustnes, J.O.; et al. Current state of knowledge on biological effects from contaminants on arctic wildlife and fish. Sci. Total Environ. 2019, 696, 133792. [Google Scholar] [CrossRef]
- Desforges, J.-P.; Hall, A.; McConnell, B.; Rosing-Asvid, A.; Barber, J.L.; Brownlow, A.; De Guise, S.; Eulaers, I.; Jepson, P.D.; Letcher, R.J.; et al. Predicting global killer whale population collapse from PCB pollution. Science 2018, 361, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Letcher, R.J.; Aars, J.; Andersen, M.; Boltunov, A.; Born, E.W.; Ciesielski, T.M.; Das, K.; Dastnai, S.; Derocher, A.E.; et al. A risk assessment review of mercury exposure in Arctic marine and terrestrial mammals. Sci. Total Environ. 2022, 829, 154445. [Google Scholar] [CrossRef]
- Das, K.; Debacker, V.; Bouquegneau, J.M. Metallothioneins in marine mammals. Cell Mol. Biol. 2000, 46, 283–294. [Google Scholar]
- Arctic Monitoring and Assessment Programme (AMAP). AMAP Assessment Report 2009: Human Health in the Arctic, Oslo 2009. Available online: https://www.amap.no/documents/doc/amap-assessment-2009-human-health-in-the-arctic/98 (accessed on 10 May 2024).
- Grandjean, P.; Satoh, H.; Murata, K.; Eto, K. Adverse effects of methylmercury: Environmental health research implications. Environ. Health Perspect. 2010, 118, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012, 120, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Eto, K. The Pathology of Minamata Disease. A Tragic Story of Water Pollution; Kyushu University Press: Fukuoka, Japan, 1999. [Google Scholar]
- Dietz, R.; Nielsen, C.; Hansen, M.; Hansen, C. Organic Mercury in Greenland Birds and Mammals. Sci. Total Environ. 1990, 95, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Miyazaki, N.; Kunito, T.; Tanabe, S. Trace elements and butyltins in a Dall’s porpoise (Phocoenoides dalli) from the Sanriku coast of Japan. Chemosphere 2005, 63, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Aschner, J.L. Mercury neurotoxicity: Mechanisms of blood-brain barrier transport. Neurosci. Biobehav. Rev. 1990, 14, 169–176. [Google Scholar] [CrossRef]
- WHO. Environmental Health Criteria 101: Methylmercury; World Health Organization: Geneva, Switzerland, 1993; 144p. [Google Scholar]
- ATSDR. Toxicological Profile for Mercury. Agency for Toxic Substances and Disease Registry; US Department of Health and Human Services: Atlanta, GA, USA, 1999; 617p.
- Thévenod, F.; Friedmann, J.M.; Katsen, A.D.; Hauser, I.A. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J. Biol. Chem. 2000, 275, 1887–1896. [Google Scholar] [CrossRef]
- NOAH. Praktisk Viden om Miljøgifte; Fremad: Copenhagen, Denmark, 1974; pp. 174–175. [Google Scholar]
- Elinder, C.-G.; Nordberg, M. Metallothionein. In Cadmium and Health; Friberg, L., Elinder, C.-G., Kjellström, T., Nordberg, G.F., Eds.; A Toxicological and Epidemiological Apprasial Volume I; CRC Press, Inc.: Boca Raton, FL, USA, 1985; pp. 66–75. [Google Scholar]
- Dudley, R.E.; Svoboda, D.J.; Klaassen, C.D. Acute Exposure to Cadmium Causes Severe Liver Injury in Rats. Toxicol. Appl. Pharmacol. 1982, 65, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.E.; Gammal, L.M.; Klaassen, C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985, 77, 414–426. [Google Scholar] [CrossRef]
- Ando, M.; Sayato, Y.; Osawa, T. Studies on the Disposition of Calcium in Bones of Rats after Continuous Oral Administration of Cadmium. Toxicol. Appl. Pharmacol. 1978, 46, 625–632. [Google Scholar] [CrossRef]
- Wang, C.; Brown, S.; Bhattacharyya, M. Effect of Cadmium on Bone Calcium and 45Ca in Mouse Dams on a Calcium-Deficient Diet: Evidence of Itai-Itai-like Syndrome. Toxicol. Appl. Pharmacol. 1994, 127, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A.; Liu, J.; Waalkes, M.P. Cadmium and cancer of prostate and testis. BioMetals 2004, 17, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, P.R.; Almeida AP de Malm, O. A greater capacity of metallothionein synthesis: Would it be sufficient as explanation for the absence of pathological effects in marine mammals with extremely high Cd concentrations? A review. Orbital 2018, 10, 355–363. [Google Scholar] [CrossRef]
- Beyer, W.N.; Meador, J.P. (Eds.) Environmental Contaminants in Biota: Interpreting Tissue Concentrations; CRC Press: Boca Raton, FL, USA, 2011; pp. 278–297. [Google Scholar]
- Ralston, N.V.C.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.L.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Decataldo, A.; Di Leo, A.; Giandomenico, S.; Cardellicchio, N. Association of metals (mercury, cadmium and zinc) with metallothionein-like proteins in storage organs of stranded dolphins from the Mediterranean sea (Southern Italy). J. Environ. Monit. 2004, 6, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lailson-Brito, J.; Cruz, R.; Dorneles, P.R.; Andrade, L.; Azevedo, A.D.; Fragoso, A.B.; Vidal, L.G.; Costa, M.B.; Bisi, T.L.; Almeida, R.; et al. Mercury-selenium relationships in liver of guiana dolphin: The possible role of Kupffer cells in the detoxification process by tiemannite formation. PLoS ONE 2012, 7, e42162. [Google Scholar] [CrossRef]
- Dietz, R.; Heide-Jørgensen, M.-P.; Härkönen, T.; Teilmann, J.; Valentin, N. Age determination of european harbour seal, Phoca Vitulina L. Sarsia 1991, 76, 17–21. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Lyon, H.; Andersen, A.P.; Hasselager, E.; Høyer, P.E.; Møller, M.; Prentø, P.; Van Deurs, B. Theory and Strategy in Histochemistry; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Luna, L.G. Manual of Histological Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw Hill: New York, NY, USA, 1960. [Google Scholar]
- Saxena, R. “Special Stain and HE” Chapter 12: “Special Stains in Interpretation of Liver Biopsies”, 2nd ed.; Dako education guide; Dako North America: Carpinteria, CA, USA, 2010; pp. 109–110. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; 718p. [Google Scholar]
- Wagemann, R.; Innes, S.; Richard, P. Overview and regional and temporal differences of heavy metals in Arctic whales and ringed seals in the Canadian Arctic. Sci. Total Environ. 1996, 186, 41–66. [Google Scholar] [CrossRef]
- Sonne, C.; Leifsson, P.S.; Dietz, R. Liver and renal lesions in mercury-contaminated narwhals (Monodon monoceros) from North West Greenland. Toxicol. Environ. Chem. 2013, 95, 1–14. [Google Scholar] [CrossRef]
- Koeman, J.H.; Peeters, W.H.M.; Koudstaal-Hol, C.H.M.; Tjioe, P.S.; de Goeij, J.J.M. Mercury-selenium correlations in marine mammals. Nature 1973, 245, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.T.; Nielsen, C.O.; Dietz, R.; Hansen, M.M. Zinc, Cadmium, Mercury and Selenium in Minke Whales, Belugas and Narwhals from West Greenland. Polar Biol. 1990, 10, 529–539. [Google Scholar] [CrossRef]
- Aubail, A.; Teilmann, J.; Dietz, R.; Rigét, F.; Harkonen, T.; Karlsson, O.; Rosing-Asvid, A.; Caurant, F. Investigation of mercury concentrations in fur of phocid seals using stable isotopes as tracers of trophic levels and geographical regions. Polar Biol. 2011, 34, 1411–1420. [Google Scholar] [CrossRef]
- Dietz, R.; Riget, F.; Johansen, P. Lead, cadmium, mercury and selenium in Greenland marine animals. Sci. Total Environ. 1996, 186, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Wagemann, R.; Snow, N.B.; Lutz, A.; Scott, D.P. Heavy metals in tissues and organs of the narwhal (Monodon monoceros). Can. J. Fish. Aquat. Sci. 1983, 40, 206–214. [Google Scholar] [CrossRef]
- Wagemann, R.; Stewart, R.E.A.; Lockhart, W.L.; Stewart, B.E.; Povoledo, M. Trace metals and methyl mercury: Associations and transfer in harp seal (Phoca groenlandica) mothers and their pups. Mar. Mammal Sci. 1988, 4, 339–355. [Google Scholar] [CrossRef]
- Wagemann, R.; Stewart, B.P.; Desjardins, C. Heavy metals and selenium in tissues of beluga whales, delphinapterus leucas, from Canadian Arctic and the st. Lawrence Estuary. Can. Bull. Fish. Aquat. Sci. 1990, 224, 191–206. [Google Scholar]
- Dyrssen, D.; Wedborg, M. The sulfur–mercury (II) system in natural waters. Water Air Soil Pollut. 1991, 56, 507–519. [Google Scholar] [CrossRef]
- Yang, J.; Kunito, T.; Tanabe, S.; Miyazaki, N. Mercury and its relation with selenium in the liver of Dall’s porpoises (Phocoenoides dalli) off the Sanriku coast of Japan. Environ. Pollut. 2007, 148, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Levander, O.A.; Burk, R.F. Selenium. In Present Knowledge in Nutrition; Ekhard, E., Ziegler, L.J., Filer, L.J., Eds.; International Life Sciences Institute-Nutrition Foundation: Washington, DC, USA, 1996. [Google Scholar]
- Behne, D.; Pfeifer, H.; Rothlein, D.; Kyriakopoulos, A. Cellular and subcellular distribution of selenium and selenium-containing proteins in the rat. In Trace Elements in Man and Animals 10; Roussel, A.M., Favier, A.E., Anderson, R.A., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; pp. 29–34. [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Blackwell, J.L.; Raymond, L.J. Importance of Molar Ratios in Selenium-Dependent Protection Against Methylmercury Toxicity. Biol. Trace Elem. Res. 2007, 119, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Ranberg, E.; Lehnert, K.; Leifsson, P.S.; Dietz, R.; Andersen, S.; Siebert, U.; Measures, L.; Sonne, C. Morphometric, molecular and histopathologic description of hepatic infection by Orthosplanchnus arcticus (Trematoda: Digenea: Brachycladiidae) in ringed seals (Pusa hispida) from Northwest Greenland. Polar Biol. 2018, 41, 1019–1025. [Google Scholar] [CrossRef]
- Woshner, V.M. Concentrations and Interactions of Selected Elements in Tissues of Four Marine Mammal Species Harvested by Inuit Hunters in Arctic Alaska, with an Intensive Histologic Assessment, Emphasizing the Beluga Whale. Ph.D. Thesis, University of Illinois, Champaign, IL, USA, 2000. [Google Scholar]
- Blakley, B.; Sisodia, C.; Mukkur, T. The effect of methylmercury, tetraethyl lead, and sodium arsenite on the humoral immune response in mice. Toxicol. Appl. Pharmacol. 1980, 52, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Baatrup, E.; Ole, T.-U.; Nielsen, H.L.; Wilsky, K. Mercury-selenium interactions in relation to histochemical staining of mercury in the rat liver. Histochem. J. 1989, 21, 89–98. [Google Scholar] [CrossRef]
- Corcoran, G.; Fix, L.; Jones, D.; Moslen, M.; Nicotera, P.; Oberhammer, F.; Buttyan, R. Apoptosis: Molecular control point in toxicity. Toxicol. Appl. Pharmacol. 1994, 128, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sonne-Hansen, C.; Dietz, R.; Leifsson, P.; Hyldstrup, L.; Riget, F. Cadmium toxicity to ringed seals (Phoca hispida): An epidemiological study of possible cadmium-induced nephropathy and osteodystrophy in ringed seals (Phoca hispida) from Qaanaaq in Northwest Greenland. Sci. Total Environ. 2002, 295, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Jubb, K.V.F.; Kennedy, P.C.; Palmer, N. Pathology of Domestic Animals, 4th ed.; Academic Press, Inc.: San Diego, CA, USA, 1993; 747p. [Google Scholar]
- Sonne, C.; Dietz, R.; Leifsson, P.S.; Asmund, G.; Born, E.W.; Kirkegaard, M. Are liver and renal lesions in East Greenland Polar Bears (Ursus maritimus) associated with high mercury levels? Environ. Health 2007, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Druet, P.; Bernard, A.; Hirsch, F.; Weening, J.J.; Gengoux, P.; Mahieu, P.; Birkeland, S. Immunologically mediated glomerulonephritis induced by heavy metals. Arch. Toxicol. 1982, 50, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Yasutake, A.; Miyamoto, K.-I.; Tokunaga, H.; Otsuka, Y. Chronic effects of methylmercury in rats. II. Pathological aspects. Tohoku J. Exp. Med. 1997, 182, 197–205. [Google Scholar] [CrossRef]
- Sato, S.; Kitamura, H.; Ghazizadeh, M.; Adachi, A.; Sasaki, Y.; Ishizaki, M.; Inoue, K.; Wakamatsu, K.; Sugisaki, Y. Occurrence of hyaline droplets in renal biopsy specimens: An ultrastructural study. Med. Mol. Morphol. 2005, 38, 63–71. [Google Scholar] [CrossRef]
- WHO. Cadmium. Environmental Health Criteria; World Health Organization: Geneva, Switzerland, 1992; Volume 134. [Google Scholar]
- Zachary, J.F.; McGavin, M.D. Pathologic Basis of Veterinary Disease; Elsevier Health Sciences: Gainesville, FL, USA, 2013. [Google Scholar]
- Fischbach, F.; Gregory, D.; Harrison, P.M.; Hoy, T.; Williams, J. On the Structure of Hemosiderin and its Relationship to Ferritin. J. Ultrastruct. Res. 1971, 37, 495–503. [Google Scholar] [CrossRef]
- Dewailly, É.; Ayotte, P.; Bruneau, S.; Lebel, G.; Levallois, P.; Weber, J.P. Exposure of the Inuit population of Nunavik (Arctic Québec) to lead and mercury. Arch. Environ. Health Int. J. 2001, 56, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Mulvad, G.; Pedersen, H.; Hansen, J.; Dewailly, E.; Jul, E.; Pedersen, M.; Bjerregaard, P.; Malcom, G.; Deguchi, Y.; Middaugh, J. Exposure of Greenlandic Inuit to organochlorines and heavy metals through the marine food-chain: An international study. Sci. Total Environ. 1996, 186, 137–139. [Google Scholar] [CrossRef]
- Weihe, P.; Hansen, J.C.; Murata, K.; Debes, F.; Jørgensen, P.J.; Steuerwald, U.; White, R.F.; Grandjean, P. Neurobehavioral performance of Inuit children with increased prenatal exposure to methylmercury. Int. J. Circumpolar Health 2002, 61, 41–49. [Google Scholar] [CrossRef]


| Liver | |||
| Histopathological change | Mild | Moderate | Severe |
| MCI = mononuclear cell infiltration | sum < 9 | 9 ≤ sum < 20 | sum ≥ 20 |
| PCI = portal cell infiltration (PA = portal area) | ≤25% of PAs | ≤50% of PAs | >50% of Pas |
| PF = portal fibrosis | ≤25% of PAs | ≤50% of PAs | >50% of Pas |
| FHF = focal hepatic fibrosis (LS = liver sample) | ≤25% of LS | ≤50% of LS | >50% of LS |
| Hem = hemosiderosis | ≤25% of LS | ≤50% of LS | >50% of LS |
| BHF = bile duct hyperplasia/fibrosis | ≤25% of PAs | ≤50% of PAs | >50% of Pas |
| LG = lipid granuloma | sum < 7 | 9 ≤ sum < 15 | sum ≥ 15 |
| HIF = hepatic intracellular fat | sum < 7 | 7 ≤ sum < 15 | sum ≥ 15 |
| ITO = Ito/Stellate cell abundance | sum < 20 * | 20 ≤ sum < 30 * | sum ≥ 30 * |
| Kidney | |||
| GBMT = glomerular (incl. capsule) basement membrane thickening | <20% of Gl. | <50% of Gl. | ≥50% Gl. |
| GA = glomerular atrophy, Gl = Glomeruli | <10% of Gl. | <25% of Gl. | ≥25% Gl. |
| GMD = glomerular mesangial deposits | <10% of Gl. | <25% Gl. | ≥25% Gl. |
| GHC = glomerular hyper-cellularity | <10% of Gl. | <25% Gl. | ≥25% Gl. |
| DT = dilated tubules | ≤50% | <75% | ≥75% |
| THD = intracellular hyaline droplets (PT = proximal tubules) | ≤25% of PT | ≤50% of PT | >50% of PT |
| MCI = mononuclear cell infiltration | sum < 9 | 9 ≤ sum < 20 | sum ≥ 20 |
| THC = tubular hyaline casts (CT = collecting tubules) | <10% of CT | <25% CT | ≥25% CT |
| IF = interstitial fibrosis (KS = kidney sample) | <20% of KS | <50% of KS | ≥50% KS |
| GS = glomerular sclerosis | <20% of Gl. | <50% of Gl. | ≥50% Gl. |
| TN = tubular necrosis (Tub. = tubules) | <20% of tub. | <50% of tub. | ≥50% tub. |
| Qaanaaq 2008 | Qeqertarsuaq 2008 | |||||||
|---|---|---|---|---|---|---|---|---|
| n | mean | SD | Range | n | Mean | SD | Range | |
| Age (years) | 19 | 1.7 | 2.80 | 0–11 | 18 | 0.53 | 0.95 | 0–3 |
| Length (cm) | 19 | 134.2 | 13.17 | 108.7–152.8 | 18 | 111.65 | 8.13 | 97–128 |
| Weight | 0 | N/A | N/A | N/A | 18 | 28.48 | 6.90 | 21–46 |
| Cd-L (μg/g ww) | 19 | 7.80 | 8.95 | 0.013–38.79 | 18 | 11.58 | 6.32 | 0.11–25.45 |
| Hg-L (μg/g ww) | 19 | 3.73 | 5.01 | 0.275–23.29 | 18 | 1.78 | 1.70 | 0.45–8.00 |
| Se-L (μg/g ww) | 19 | 2.43 | 1.66 | 0.89–8.12 | 18 | 1.54 | 0.71 | 0.49–3.78 |
| Molar Hg-L/Se-L | 19 | 0.49 | 0.21 | 0.12–1.13 | 18 | 0.46 | 0.16 | 0.20–0.83 |
| Hepatic Histologic Change | |||||||
|---|---|---|---|---|---|---|---|
| MCI | PCI | Ito cell abund. | Hem. | FHF | PF | BHF | |
| Age | 0.22 | 0.02 * | 0.75 | 0.803 | 0.04 * | 1.187 × 10-04 *** | 2.303 × 10-06 *** |
| Hg | 0.42 | 0.20 | 0.80 | 2.4 × 10-05 *** (−) | 0.19 | 0.85 | 0.96 |
| Cd | 0.56 | 0.56 | 0.51 | 8.7 × 10-07 *** (−) | 0.17 | 0.03 * | 0.08 |
| Se | 0.66 | 0.60 | 0.64 | 4.4 × 10-04 *** (−) | 0.06 | 0.42 | 0.28 |
| Renal Histologic Change | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GBMT | GA | GMD | GHC | DT | THD | MCI | THC | GS | |
| Age | 0.07 | 0.02 | 0.52 | 0.04 * (+) | 2.0 × 10-03 ** (+) | 0.08 | 0.73 | 0.04 * | 0.04 * |
| Hg | 0.99 | 0.11 | 0.99 | 0.09 | 0.63 | 0.09 | 0.17 | 0.76 | 0.29 |
| Cd | 0.08 | 0.91 | 0.82 | 0.09 | 0.12 | 0.33 | 0.56 | 0.17 | 0.87 |
| Se | 0.977 | 0.108 | 0.859 | 0.42 | 0.58 | 0.13 | 0.27 | 0.92 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen-Ranberg, E.U.; Leifsson, P.S.; Rigét, F.F.; Søndergaard, J.; Andersen, S.; Alstrup, A.K.O.; Dietz, R.; Sonne, C. Element Concentrations and Histopathology of Liver and Kidney in West Greenland Ringed Seals (Pusa hispida). Animals 2024, 14, 1739. https://doi.org/10.3390/ani14121739
Andersen-Ranberg EU, Leifsson PS, Rigét FF, Søndergaard J, Andersen S, Alstrup AKO, Dietz R, Sonne C. Element Concentrations and Histopathology of Liver and Kidney in West Greenland Ringed Seals (Pusa hispida). Animals. 2024; 14(12):1739. https://doi.org/10.3390/ani14121739
Chicago/Turabian StyleAndersen-Ranberg, Emilie U., Pall S. Leifsson, Frank F. Rigét, Jens Søndergaard, Steen Andersen, Aage Kristian Olsen Alstrup, Rune Dietz, and Christian Sonne. 2024. "Element Concentrations and Histopathology of Liver and Kidney in West Greenland Ringed Seals (Pusa hispida)" Animals 14, no. 12: 1739. https://doi.org/10.3390/ani14121739
APA StyleAndersen-Ranberg, E. U., Leifsson, P. S., Rigét, F. F., Søndergaard, J., Andersen, S., Alstrup, A. K. O., Dietz, R., & Sonne, C. (2024). Element Concentrations and Histopathology of Liver and Kidney in West Greenland Ringed Seals (Pusa hispida). Animals, 14(12), 1739. https://doi.org/10.3390/ani14121739






