Hypothalamic Neuromodulation and Control of the Dermal Surface Temperature of Livestock during Hyperthermia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Search Methodology
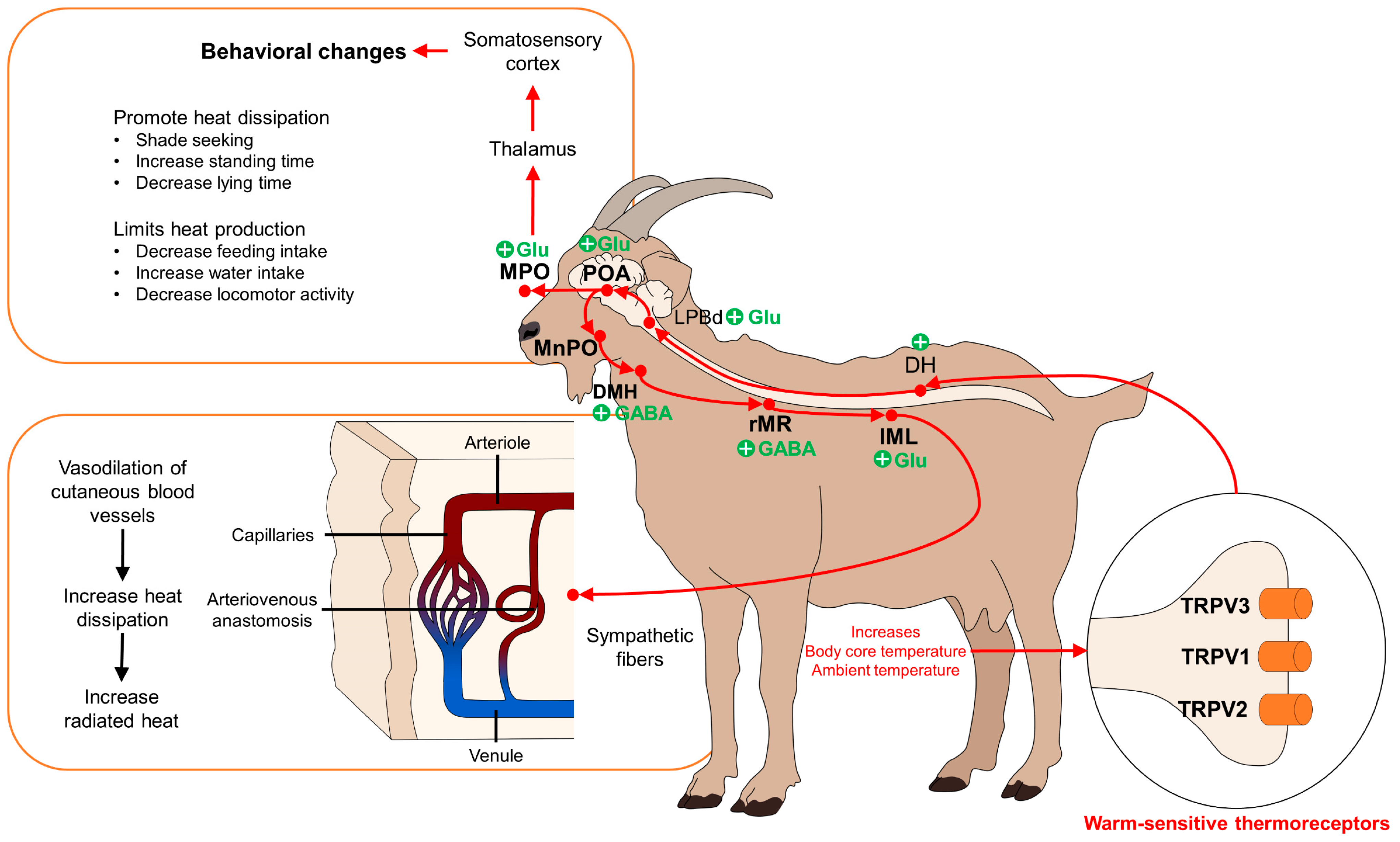
3. Hypothalamic Control of Hyperthermia: Peripheral Perception of Thermal Stimuli and Supraspinal Processing
3.1. Peripheral Thermal Receptors
3.2. Hypothalamic Modulation of Hyperthermia
4. Response to Hyperthermia: Systemic and Local Changes
4.1. Vasomotor Response to Hyperthermia: Vasodilation
4.2. Sweating
4.3. Endocrine and Immunological Response Associated with Hyperthermia
5. Behavioral Changes Observed in Domestic Animals Experiencing Hyperthermia
Management of Heat Stress in Livestock Production Systems
6. Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of Heat Stress on Global Cattle Production during the 21st Century: A Modelling Study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef]
- Fuquay, J.W. Heat Stress as It Affects Animal Production. J. Anim. Sci. 1981, 52, 164–174. [Google Scholar] [CrossRef]
- North, M.A.; Franke, J.A.; Ouweneel, B.; Trisos, C.H. Global Risk of Heat Stress to Cattle from Climate Change. Environ. Res. Lett. 2023, 18, 094027. [Google Scholar] [CrossRef]
- Napolitano, F.; De Rosa, G.; Chay-Canul, A.; Álvarez-Macías, A.; Pereira, A.M.F.; Bragaglio, A.; Mora-Medina, P.; Rodríguez-González, D.; García-Herrera, R.; Hernández-Ávalos, I.; et al. The Challenge of Global Warming in Water Buffalo Farming: Physiological and Behavioral Aspects and Strategies to Face Heat Stress. Animals 2023, 13, 3103. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Marx, J.O.; Gordon, C.J.; David, J.M. Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comp. Med. 2018, 68, 425–438. [Google Scholar] [CrossRef]
- Vilela, R.A.; Lourenço Junior, J.d.B.; Jacintho, M.A.C.; Barbosa, A.V.C.; Pantoja, M.H.d.A.; Oliveira, C.M.C.; Garcia, A.R. Dynamics of Thermolysis and Skin Microstructure in Water Buffaloes Reared in Humid Tropical Climate—A Microscopic and Thermographic Study. Front. Vet. Sci. 2022, 9, 871206. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Ghezzi, M.D.; Mora-Medina, P.; Hernández-Ávalos, I.; Jacome, J.; Castellón, A.; Falcón, I.; Reséndiz, F.; Romero, N.; Ponce, R.; et al. Anatomical, Physiological, and Behavioral Mechanisms of Thermoregulation in Elephants. J. Anim. Behav. Biometeorol. 2022, 10, 2233. [Google Scholar] [CrossRef]
- Mota-rojas, D.; Wang, D.; Titto, C.G.; Gómez-prado, J.; Fuente, V.C.; Ghezzi, M.; Boscato-funes, L.; Barrios-garcía, H.; Torres-bernal, F.; Casas-alvarado, A.; et al. Pathophysiology of Fever and Application of Infrared Thermography ( IRT ) in the Detection of Sick Domestic Animals: Recent Advances. Animals 2021, 11, 2316. [Google Scholar] [CrossRef]
- Shelton, D.S.; Alberts, J.R. Development of Behavioral Responses to Thermal Challenges. Dev. Psychobiol. 2018, 60, 5–14. [Google Scholar] [CrossRef]
- Athaíde, L.G.; Joset, W.C.L.; de Almeida, J.C.F.; Pantoja, M.H.d.A.; Noronha, R.d.P.P.; Bezerra, A.S.; Barbosa, A.V.C.; Martorano, L.G.; da Silva, J.A.R.; Lourenço Júnior, J.d.B. Thermoregulatory and Behavioral Responses of Buffaloes With and Without Direct Sun Exposure During Abnormal Environmental Condition in Marajó Island, Pará, Brazil. Front. Vet. Sci. 2020, 7, 522551. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K. Central Mechanisms for Thermoregulation in a Hot Environment. Ind. Health 2006, 44, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat Stress in Lactating Dairy Cows: A Review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. A Thermosensory Pathway Mediating Heat-Defense Responses. Proc. Natl. Acad. Sci. USA 2010, 107, 8848–8853. [Google Scholar] [CrossRef] [PubMed]
- Dimicco, J.A.; Zaretsky, D.V. The Dorsomedial Hypothalamus: A New Player in Thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R47–R63. [Google Scholar] [CrossRef] [PubMed]
- Autio, E.; Heiskanen, M.-L.; Mononen, J. Thermographic Evaluation of the Lower Critical Temperature in Weanling Horses. J. Appl. Anim. Welf. Sci. 2007, 10, 207–216. [Google Scholar] [CrossRef]
- Kang, H.; Zsoldos, R.R.; Sole-Guitart, A.; Narayan, E.; Cawdell-Smith, A.J.; Gaughan, J.B. Heat Stress in Horses: A Literature Review. Int. J. Biometeorol. 2023, 67, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S.; Godyń, D.; Hoffmann, G. The Physiological and Productivity Effects of Heat Stress in Cattle—A Review. Ann. Anim. Sci. 2019, 19, 579–593. [Google Scholar] [CrossRef]
- Tansey, E.A.; Johnson, C.D. Recent Advances in Thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef]
- Rosania, K. Blocking the Body’s Response to Cold. Lab Anim. 2012, 41, 89. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Flonta, M.-L. Cold Current in Thermoreceptive Neurons. Nature 2001, 413, 480. [Google Scholar] [CrossRef]
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion Channels and Thermosensitivity: TRP, TREK, or Both? Int. J. Mol. Sci. 2019, 20, 2371. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Siemens, J. TRP Ion Channels in Thermosensation, Thermoregulation and Metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Simon, S.A. TRPV1 Receptors and Signal Transduction. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; Chapter 5; ISBN 0849340489. Available online: https://www.ncbi.nlm.nih.gov/books/NBK5260/ (accessed on 16 April 2024).
- Desai, B.N.; Clapham, D.E. TRP Channels and Mice Deficient in TRP Channels. Pflügers Arch.—Eur. J. Physiol. 2005, 451, 11–18. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral Thermosensation in Mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Togashi, K.; Hara, Y.; Tominaga, T.; Higashi, T.; Konishi, Y.; Mori, Y.; Tominaga, M. TRPM2 Activation by Cyclic ADP-Ribose at Body Temperature Is Involved in Insulin Secretion. EMBO J. 2006, 25, 1804–1815. [Google Scholar] [CrossRef]
- Nozadze, I.; Tsiklauri, N.; Gurtskaia, G.; Tsagareli, M.G. Role of Thermo TRPA1 and TRPV1 Channels in Heat, Cold, and Mechanical Nociception of Rats. Behav. Pharmacol. 2016, 27, 29–36. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, F.; Liu, S.; Colton, C.K.; Wang, C.; Cui, Y.; Cao, X.; Zhu, M.X.; Sun, C.; Wang, K.; et al. Heteromeric Heat-Sensitive Transient Receptor Potential Channels Exhibit Distinct Temperature and Chemical Response. J. Biol. Chem. 2012, 287, 7279–7288. [Google Scholar] [CrossRef]
- Lei, J.; Yoshimoto, R.U.; Matsui, T.; Amagai, M.; Kido, M.A.; Tominaga, M. Involvement of Skin TRPV3 in Temperature Detection Regulated by TMEM79 in Mice. Nat. Commun. 2023, 14, 4104. [Google Scholar] [CrossRef]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP Channel Trio Mediates Acute Noxious Heat Sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Paricio-Montesinos, R.; Schwaller, F.; Udhayachandran, A.; Rau, F.; Walcher, J.; Evangelista, R.; Vriens, J.; Voets, T.; Poulet, J.F.A.; Lewin, G.R. The Sensory Coding of Warm Perception. Neuron 2020, 106, 830–841.e3. [Google Scholar] [CrossRef]
- Yahiro, T.; Kataoka, N.; Nakamura, Y.; Nakamura, K. The Lateral Parabrachial Nucleus, but Not the Thalamus, Mediates Thermosensory Pathways for Behavioural Thermoregulation. Sci. Rep. 2017, 7, 5031. [Google Scholar] [CrossRef]
- Childs, C. Body temperature and clinical thermometry. Handb. Clin. Neurol. 2018, 157, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Du, X.; Zhang, W.; Gao, C.; Xie, H.; Xiao, Y.; Jia, X.; Liu, J.; Xu, J.; Fu, X.; et al. Parabrachial Neuron Types Categorically Encode Thermoregulation Variables during Heat Defense. Sci. Adv. 2020, 6, eabb9414. [Google Scholar] [CrossRef] [PubMed]
- Bienboire-Frosini, C.; Wang, D.; Marcet-Rius, M.; Villanueva-García, D.; Gazzano, A.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Lezama-García, K.; Verduzco-Mendoza, A.; et al. The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period. Animals 2023, 13, 2173. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Cooke, E.K.; Leib, D.E.; Lin, Y.-C.; Daly, G.E.; Zimmerman, C.A.; Knight, Z.A. Warm-Sensitive Neurons That Control Body Temperature. Cell 2016, 167, 47–59.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A Hypothalamic Circuit That Controls Body Temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef]
- Kaiyala, K.J.; Ogimoto, K.; Nelson, J.T.; Muta, K.; Morton, G.J. Physiological Role for Leptin in the Control of Thermal Conductance. Mol. Metab. 2016, 5, 892–902. [Google Scholar] [CrossRef]
- Keringer, P.; Furedi, N.; Gaszner, B.; Miko, A.; Pakai, E.; Fekete, K.; Olah, E.; Kelava, L.; Romanovsky, A.A.; Rumbus, Z.; et al. The Hyperthermic Effect of Central Cholecystokinin Is Mediated by the Cyclooxygenase-2 Pathway. Am. J. Physiol.-Endocrinol. Metab. 2022, 322, E10–E23. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yahiro, T.; Fukushima, A.; Kataoka, N.; Hioki, H.; Nakamura, K. Prostaglandin EP3 Receptor–Expressing Preoptic Neurons Bidirectionally Control Body Temperature via Tonic GABAergic Signaling. Sci. Adv. 2022, 8, eadd5463. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Hanani, M. The Actions of Hyperthermia on the Autonomic Nervous System: Central and Peripheral Mechanisms and Clinical Implications. Auton. Neurosci. 2012, 168, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. Thermoregulation: Some Concepts Have Changed. Functional Architecture of the Thermoregulatory System. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R37–R46. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, M.; Kim, D.-Y.; Son, C.; Ahn, B.H.; Heo, G.; Park, J.; Kim, M.; Park, H.-E.; Koo, D.-J.; et al. A Forebrain Neural Substrate for Behavioral Thermoregulation. Neuron 2022, 110, 266–279.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.A.; Teo, C.F.; Åkerblom, M.; Chen, C.; Tynan-La Fontaine, M.; Greiner, V.J.; Diaz, A.; McManus, M.T.; Jan, Y.N.; Jan, L.Y. Thermoregulation via Temperature-Dependent PGD2 Production in Mouse Preoptic Area. Neuron 2019, 103, 309–322.e7. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.J.; Shaker, J.R.; Cone, A.L.; Ndiokho, I.B.; Bruchas, M.R. Parabrachial Opioidergic Projections to Preoptic Hypothalamus Mediate Behavioral and Physiological Thermal Defenses. eLife 2021, 10, e60779. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Central Circuitries for Body Temperature Regulation and Fever. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef] [PubMed]
- Yahiro, T.; Kataoka, N.; Nakamura, K. Two Ascending Thermosensory Pathways from the Lateral Parabrachial Nucleus That Mediate Behavioral and Autonomous Thermoregulation. J. Neurosci. 2023, 43, 5221–5240. [Google Scholar] [CrossRef]
- Morrison, S.F. Central Neural Pathways for Thermoregulation. Front. Biosci. 2011, 16, 74. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K.; Madden, C.J. Central Control of Thermogenesis in Mammals. Exp. Physiol. 2008, 93, 773–797. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.A.; Gibson, B.M.; Simmons, G.H.; Halliwill, J.R.; Minson, C.T. Cholinergic Nerve Contribution to Cutaneous Active Vasodilation during Exercise Is Similar to Whole Body Passive Heating. J. Appl. Physiol. 2023, 134, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Christen, S.; Declachaux, A.; Dischl, B.; Golay, S.; Liaudet, L.; Feihl, F.; Waeber, N. Dose-Dependent Vasodilatory Effects of Acetylcholine and Local Warming on Skin Microcirculation. J. Cardiovasc. Pharmacol. 2004, 44, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Kourosh-Arami, M.; Komaki, A.; Hajizadeh, S. Relative Contribution of Central and Peripheral Factors in Superficial Blood Flow Regulation Following Cold Exposure. Physiol. Pharmacol. 2020, 24, 89–100. [Google Scholar] [CrossRef]
- Francisco, M.A.; Minson, C.T. Cutaneous active vasodilation as a heat loss thermoeffector. Handb. Clin. Neurol. 2018, 156, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Ogi, A.; Villanueva-García, D.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Lendez, P.; Ghezzi, M. Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications. Animals 2023, 14, 142. [Google Scholar] [CrossRef]
- da Silva, W.C.; da Silva, J.A.R.; Martorano, L.G.; da Silva, É.B.R.; Sousa, C.E.L.; Neves, K.A.L.; de Araújo, C.V.; Joaquim, L.A.; Rodrigues, T.C.G.d.C.; Belo, T.S.; et al. Thermographic Profiles in Livestock Systems under Full Sun and Shaded Pastures during an Extreme Climate Event in the Eastern Amazon, Brazil: El Niño of 2023. Animals 2024, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive Physiological Indicators of Heat Stress in Cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.B. Thermoregulation. In Textbook of Veterinary Physiology; Klein, B.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 559–567. [Google Scholar]
- Rodríguez-González, D.; Guerrero-Legarreta, I.; Cruz Monterrosa, R.G.; Napolitano, F.; Gonçalves-Titto, C.; El-Aziz, A.H.A.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Oliva-Domínguez, A.; Mota-Rojas, D. Assessment of Thermal Changes in Water Buffalo Mobilized from the Paddock and Transported by Short Journeys. Front. Vet. Sci. 2023, 10, 1184577. [Google Scholar] [CrossRef]
- Johnson, J.M.; Minson, C.T.; Kellogg, D.L. Cutaneous Vasodilator and Vasoconstrictor Mechanisms in Temperature Regulation. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2014; pp. 33–89. [Google Scholar]
- Hodges, G.J.; Johnson, J.M. Adrenergic Control of the Human Cutaneous Circulation. Appl. Physiol. Nutr. Metab. 2009, 34, 829–839. [Google Scholar] [CrossRef]
- Holowatz, L.A. Aging and the Control of Human Skin Blood Flow. Front. Biosci. 2010, 15, 718. [Google Scholar] [CrossRef] [PubMed]
- Holowatz, L.A.; Kenney, W.L. Peripheral Mechanisms of Thermoregulatory Control of Skin Blood Flow in Aged Humans. J. Appl. Physiol. 2010, 109, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific Findings Related to Changes in Vascular Microcirculation Using Infrared Thermography in the River Buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Lezama-García, K.; Martínez-Burnes, J.; Pérez-Jiménez, J.C.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Mota-Rojas, D. Relation between the Dam’s Weight on Superficial Temperature of Her Puppies at Different Stages of the Post-Partum. Vet. Sci. 2022, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R. The Vascularity and Possible Thermoregulatory Function of the Horns in Goats. Physiol. Zool. 1966, 39, 127–139. [Google Scholar] [CrossRef]
- Algra, M.; de Keijzer, L.; Arndt, S.S.; van Eerdenburg, F.J.C.M.; Goerlich, V.C. Evaluation of the Thermal Response of the Horns in Dairy Cattle. Animals 2023, 13, 500. [Google Scholar] [CrossRef]
- ICVGAN. Nómina Anatómica Veterinaria, 6th ed.; Editorial Committee: Hanover, Germany; Ghent, Belgium; Columbia, MO, USA; Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Mincu, M.; Nicolae, I.; Gavojdian, D. Infrared Thermography as a Non-Invasive Method for Evaluating Stress in Lactating Dairy Cows during Isolation Challenges. Front. Vet. Sci. 2023, 10, 1236668. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Bolaños, J.; Ceballes-Serrano, C.C.; Velásquez-Mejía, D.; Riaño-Rojas, J.C.; Giraldo, C.E.; Carmona, J.U.; Ceballos-Márquez, A. Application of Udder Surface Temperature by Infrared Thermography for Diagnosis of Subclinical Mastitis in Holstein Cows Located in Tropical Highlands. J. Dairy Sci. 2021, 104, 10310–10323. [Google Scholar] [CrossRef]
- Samara, E.M.; Ayadi, M.; Aljumaah, R.S. Feasibility of Utilising an Infrared-Thermographic Technique for Early Detection of Subclinical Mastitis in Dairy Camels (Camelus dromedarius). J. Dairy Res. 2014, 81, 38–45. [Google Scholar] [CrossRef]
- Uddin, J.; McNeill, D.M.; Phillips, C.J.C. Infrared Thermography as a Tool for the Measurement of Negative Emotions in Dairy Cows. Int. J. Biometeorol. 2023, 67, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.T.; Berry, D.P.; Esmonde, H.; McGovern, F.; Creighton, P.; McHugh, N. Infrared Thermography as a Tool to Detect Hoof Lesions in Sheep. Transl. Anim. Sci. 2019, 3, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef] [PubMed]
- de Lima, V.; Piles, M.; Rafel, O.; López-Béjar, M.; Ramón, J.; Velarde, A.; Dalmau, A. Use of Infrared Thermography to Assess the Influence of High Environmental Temperature on Rabbits. Res. Vet. Sci. 2013, 95, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, V.; Luzi, F.; Mazzola, S.; Bariffi, G.; Zappaterra, M.; Nanni Costa, L.; Padalino, B. The Use of Infrared Thermography (IRT) as Stress Indicator in Horses Trained for Endurance: A Pilot Study. Animals 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Muns, R.; Wang, D.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Mota-Rojas, D. Assessment of Pain and Inflammation in Domestic Animals Using Infrared Thermography: A Narrative Review. Animals 2023, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, V.; Luzi, F.; Farish, M.; Nanni Costa, L. The Use of Thermography to Assess the Teeth Temperature during Resection by Grinding in Piglets. In Proceedings of the 2012 International Conference on Quantitative InfraRed Thermography, Naples, Italy, 11–14 June 2012; QIRT Council: Naples, Italy, 2012; pp. 1–2. [Google Scholar]
- Van der Saag, D.; Lomax, S.; Windsor, P.A.; Taylor, C.; White, P.J. Evaluating Treatments with Topical Anaesthetic and Buccal Meloxicam for Pain and Inflammation Caused by Amputation Dehorning of Calves. PLoS ONE 2018, 13, e0198808. [Google Scholar] [CrossRef] [PubMed]
- Eicher, S.D.; Cheng, H.W.; Sorrells, A.D.; Schutz, M.M. Short Communication: Behavioral and Physiological Indicators of Sensitivity or Chronic Pain Following Tail Docking. J. Dairy Sci. 2006, 89, 3047–3051. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Wilson, T.E.; Crandall, C.G. Neural Control and Mechanisms of Eccrine Sweating during Heat Stress and Exercise. J. Appl. Physiol. 2006, 100, 1692–1701. [Google Scholar] [CrossRef]
- Hafez, E.S.E.; Badreldin, A.L.; Shafei, M.M. Skin Structure of Egyptian Buffaloes and Cattle with Particular Reference to Sweat Glands. J. Agric. Sci. 1955, 46, 19–30. [Google Scholar] [CrossRef]
- McCutcheon, L.J.; Geor, R.J. Thermoregulation and exercise-associated heat illnesses. In Equine Sports Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2014; pp. 901–918. [Google Scholar]
- Kingston, J.K.; Geor, R.J.; McCutcheon, L.J. Rate and Composition of Sweat Fluid Losses Are Unaltered by Hypohydration during Prolonged Exercise in Horses. J. Appl. Physiol. 1997, 83, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.J.; Lund, R.J. Thermoregulation: Base Mechanisms and Hyperthermia. Vet. Clin. N. Am. Equine Pract. 1998, 14, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Debbarma, D.; Uppal, V.; Bansal, N.; Gupta, A. Histomorphometrical Study on Regional Variation in Distribution of Sweat Glands in Buffalo Skin. Dermatol. Res. Pract. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.V.; Silva, L.K.X.; Kahwage, P.R.; Lourenço Júnior, J.B.; Sousa, J.S.; Silva, A.G.M.; Franco, I.M.; Martorano, L.G.; Garcia, A.R. Assessment of Surface Temperatures of Buffalo Bulls (Bubalus bubalis) Raised under Tropical Conditions Using Infrared Thermography. Arq. Bras. De Med. Vet. E Zootec. 2016, 68, 422–430. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Koenneker, K.; Schulze, M.; Pieper, L.; Jung, M.; Schmicke, M.; Beyer, F. Comparative Assessment of the Stress Response of Cattle to Common Dairy Management Practices. Animals 2023, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Afsal, A.; Sejian, V.; Bagath, M.; Krishnan, G.; Devaraj, C.; Bhatta, R. Heat Stress and Livestock Adaptation: Neuroendocrine Regulation. Int. J. Vet. Anim. Med. 2018, 1, 108. [Google Scholar]
- Ochoa-Amaya, J.E.; Malucelli, B.E.; Cruz-Casallas, P.E.; Nasello, A.G.; Felicio, L.F.; Carvalho-Freitas, M.I.R. Acute and Chronic Stress and the Inflammatory Response in Hyperprolactinemic Rats. Neuroimmunomodulation 2010, 17, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Muneyyirci-Delale, O. Stress-Induced Hyperprolactinemia: Pathophysiology and Clinical Approach. Obstet. Gynecol. Int. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Arsenault, R.; Napper, S.; Griebel, P. Models and Methods to Investigate Acute Stress Responses in Cattle. Animals 2015, 5, 1268–1295. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of Animals to Heat Stress. Animal 2018, 12, S431–S444. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.-H.; Xu, H.-J.; Yong, Y.-H.; An, L.-L.; Jiao, P.-R.; Liao, M. Heat Stress Upregulation of Toll-like Receptors 2/4 and Acute Inflammatory Cytokines in Peripheral Blood Mononuclear Cell (PBMC) of Bama Miniature Pigs: An in Vivo and in Vitro Study. Animal 2014, 8, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The Impact of Heat Stress on the Immune System in Dairy Cattle: A Review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef] [PubMed]
- El Sabry, M.; Almasri, O. Space Allowance: A Tool for Improving Behavior, Milk and Meat Production, and Reproduction Performance of Buffalo in Different Housing Systems—A Review. Trop. Anim. Health Prod. 2022, 54, 7–10. [Google Scholar] [CrossRef]
- Galloso-Hernández, M.A.; Rodríguez-Estévez, V.; Alvarez-Díaz, C.A.; Soca-Pérez, M.; Dublin, D.; Iglesias-Gómez, J.; Simon Guelmes, L. Effect of Silvopastoral Systems in the Thermoregulatory and Feeding Behaviors of Water Buffaloes Under Different Conditions of Heat Stress. Front. Vet. Sci. 2020, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Habeeb, A.; Ghezzi, M.D.; Kanth, P.; Napolitano, F.; Lendez, P.A.; Cuibus, A.; Ceriani, M.C.; Sarubbi, J.; Braghieri, A.; et al. Termorregulación del búfalo de agua: Mecanismos neurobiológicos, cambios microcirculatorios y aplicaciones prácticas de la termografía infrarroja. In El Búfalo de Agua en Latinoamérica, Hallazgos Recientes; Napolitano, F., Mota-Rojas, D., Guerrero-Legarreta, I., Orihuela, A., Eds.; BM Editores: Ciudad de México, México, 2020; pp. 923–958. [Google Scholar]
- Amit, M.D.; Dash, S.S.; Sahu, S.; Sarangi, A.; Singh, M. Effect of Microclimate on Feeding, Drinking and Physiological Parameters of Buffalo: A Review. Pharma Innov. J. 2021, 10, 2416–2419. [Google Scholar]
- Galloso-Hernández, M.A.; Soca-Pérez, M.; Dublin, D.; Alvarez-Díaz, C.A.; Iglesias-Gómez, J.; Díaz-Gaona, C.; Rodríguez-Estévez, V. Thermoregulatory and Feeding Behavior under Different Management and Heat Stress Conditions in Heifer Water Buffalo (Bubalus bubalis) in the Tropics. Animals 2021, 11, 1162. [Google Scholar] [CrossRef]
- Garcia, A.R.; Silva, L.K.X.; Barros, D.V.; Junior, J.d.B.L.; Martorano, L.G.; Lisboa, L.S.S.; da Silva, J.A.R.; de Sousa, J.S.; da Silva, A.O.A. Key Points for the Thermal Comfort of Water Buffaloes in Eastern Amazon. Ciência Rural 2023, 53, e20210544. [Google Scholar] [CrossRef]
- Joksimovic-Todorovic, M.; Davidovic, V.; Hristov, S.; Stankovic, B. Effect of Heat Stress on Milk Production in Dairy Cows. Biotechnol. Anim. Husb. 2011, 27, 1017–1023. [Google Scholar] [CrossRef]
- Wright, P. Why Do Elephants Flap Their Ears? South Afr. J. Zool. 1984, 19, 266–269. [Google Scholar] [CrossRef]
- Phillips, P.; Heath, J. Heat Loss in Dumbo: A Theoretical Approach. J. Therm. Biol. 2001, 26, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mondal, T. Heat Stress and Thermoregulatory Responses of Goats: A Review. Biol. Rhythm Res. 2021, 52, 407–433. [Google Scholar] [CrossRef]
- Kamal, R.; Dutt, T.; Patel, M.; Dey, A.; Bharti, P.K.; Chandran, P.C. Heat Stress and Effect of Shade Materials on Hormonal and Behavior Response of Dairy Cattle: A Review. Trop. Anim. Health Prod. 2018, 50, 701–706. [Google Scholar] [CrossRef]
- Berihulay, H.; Abied, A.; He, X.; Jiang, L.; Ma, Y. Adaptation Mechanisms of Small Ruminants to Environmental Heat Stress. Animals 2019, 9, 75. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Tait, L.A.; Eigenberg, R.; Bryden, W.L. Effect of Shade on Respiration Rate and Rectal Temperature of Angus Heifers. Anim. Prod. Aust. 2004, 25, 69–72. [Google Scholar]
- Kamal, R.; Dutt, T.; Patel, B.H.M.; Singh, G.; Chandran, P.C.; Dey, A.; Barari, S.K. Effect of Shade Materials on Rectal Temperature, Respiration Rate and Body Surface Temperature of Crossbred Calves during Rainy Season. Indian J. Anim. Sci. 2016, 86, 75–81. [Google Scholar] [CrossRef]
- Taylor, D.; Brown, W.; Price, I.; Trotter, M.; Lamb, D.; Hinch, G. Gps Tracking of Sheep to Investigate Shelter and Shade Use in Relation to Weather Conditions. In Proceedings of the 10th International Conference Precision Agriculture, Denver, CO, USA, 18–21 July 2010; pp. 247–248. [Google Scholar]
- Knight, M.I.; Linden, N.P.; Butler, K.L.; Rice, M.; Ponnampalam, E.N.; Behrendt, R.; Jongman, E.C. The Effect of Shade on Sheep Grazing Pasture during Summer Conditions. J. Vet. Behav. 2023, 64–65, 16–24. [Google Scholar] [CrossRef]
- Tucker, C.B.; Rogers, A.R.; Schütz, K.E. Effect of Solar Radiation on Dairy Cattle Behaviour, Use of Shade and Body Temperature in a Pasture-Based System. Appl. Anim. Behav. Sci. 2008, 109, 141–154. [Google Scholar] [CrossRef]
- Herbut, P.; Hoffmann, G.; Angrecka, S.; Godyń, D.; Vieira, F.M.C.; Adamczyk, K.; Kupczyński, R. The Effects of Heat Stress on the Behaviour of Dairy Cows—A Review. Ann. Anim. Sci. 2021, 21, 385–402. [Google Scholar] [CrossRef]
- Allen, J.D.; Hall, L.W.; Collier, R.J.; Smith, J.F. Effect of Core Body Temperature, Time of Day, and Climate Conditions on Behavioral Patterns of Lactating Dairy Cows Experiencing Mild to Moderate Heat Stress. J. Dairy Sci. 2015, 98, 118–127. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S. The Effect of Heat Stress on Time Spent Lying by Cows in a Housing System. Ann. Anim. Sci. 2018, 18, 825–833. [Google Scholar] [CrossRef]
- Hut, P.R.; Scheurwater, J.; Nielen, M.; van den Broek, J.; Hostens, M.M. Heat Stress in a Temperate Climate Leads to Adapted Sensor-Based Behavioral Patterns of Dairy Cows. J. Dairy Sci. 2022, 105, 6909–6922. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.R.d.; Nguyen, T.; Sujani, S.; White, R.R. Open-Source Wearable Sensors for Behavioral Analysis of Sheep Undergoing Heat Stress. Appl. Sci. 2023, 13, 9281. [Google Scholar] [CrossRef]
- Li, F.K.; Yang, Y.; Jenna, K.; Xia, C.H.; Lv, S.J.; Wei, W.H. Effect of Heat Stress on the Behavioral and Physiological Patterns of Small-Tail Han Sheep Housed Indoors. Trop. Anim. Health Prod. 2018, 50, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Poullet, N.; Beramice, D.; Dantec, L.; Canario, L.; Gourdine, J.-L. Behavior Comparison During Chronic Heat Stress in Large White and Creole Pigs Using Image-Analysis. Front. Anim. Sci. 2021, 2, 784376. [Google Scholar] [CrossRef]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation Mechanisms and Perspectives for Validating Thermal Windows in Pigs with Hypothermia and Hyperthermia: An Overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef] [PubMed]
- Arp, S.; Owens, F.; Armbruster, S.; Schmidt, D. Effect of Animal Density, Coat Color and Heat Stress on Performance of Feedlot Steers. Anim. Sci. Res. Rep. 1983, 114, 79–81. [Google Scholar]
- Anzures-Olvera, F.; Véliz, F.G.; de Santiago, A.; García, J.E.; Mellado, J.; Macías-Cruz, U.; Avendaño-Reyes, L.; Mellado, M. The Impact of Hair Coat Color on Physiological Variables, Reproductive Performance and Milk Yield of Holstein Cows in a Hot Environment. J. Therm. Biol. 2019, 81, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral Thermoregulation in Mammals: A Review. Front. Biosci. 2011, 16, 1428. [Google Scholar] [CrossRef]
- Fundora, O.; Sánchez, R.R.R. Datos Preliminares de La Conducta Alimentaria de Búfalos de Río En Pastoreo. Rev. Cuba. De Cienc. Agrícol. 2001, 35, 15–17. [Google Scholar]
- Spiers, D.E.; Spain, J.N.; Sampson, J.D.; Rhoads, R.P. Use of Physiological Parameters to Predict Milk Yield and Feed Intake in Heat-Stressed Dairy Cows. J. Therm. Biol. 2004, 29, 759–764. [Google Scholar] [CrossRef]
- Chen, L.; Thorup, V.M.; Kudahl, A.B.; Østergaard, S. Impact of Heat Stress on Feed Intake, Milk Yield, Milk Composition, and Feed Efficiency in Dairy Cows: A Meta-Analysis. J. Dairy Sci. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.A.; Stone, A.E. Graduate Student Literature Review: Heat Abatement Strategies Used to Reduce Negative Effects of Heat Stress in Dairy Cows. J. Dairy Sci. 2020, 103, 9667–9675. [Google Scholar] [CrossRef] [PubMed]
- Galloso-Hernández, M.A. Potencial de Los Sistemas Silvopastoriles Para La Producción Bufalina En Ambientes Tropicales; Universidad de Córdoba, UCO Press, Spain. 2021.
- Mader, T.L.; Davis, M.S. Effect of Management Strategies on Reducing Heat Stress of Feedlot Cattle: Feed and Water Intake1. J. Anim. Sci. 2004, 82, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal Biology in River Buffalo in the Humid Tropics: Neurophysiological and Behavioral Responses Assessed by Infrared Thermography. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Napolitano, F.; Pacelli, C.; Grasso, F.; Braghieri, A.; De Rosa, G. The Behaviour and Welfare of Buffaloes (Bubalus bubalis) in Modern Dairy Enterprises. Animal 2013, 7, 1704–1713. [Google Scholar] [CrossRef]
- Sullivan, K.F.; Mader, T.L. Managing Heat Stress Episodes in Confined Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.S.C.; Moura, G.A.B.; Fonsêca, V.F.C.; Gebremedhin, K.G.; Milan, H.M.; Chiquitelli Neto, M.; Simão, B.R.; Campanelli, V.P.C.; Pacheco, R.D.L. Economically Sustainable Shade Design for Feedlot Cattle. Front. Vet. Sci. 2023, 10, 1110671. [Google Scholar] [CrossRef]
- Dean, L.; Tarpoff, A.J.; Nickles, K.; Place, S.; Edwards-Callaway, L. Heat Stress Mitigation Strategies in Feedyards: Use, Perceptions, and Experiences of Industry Stakeholders. Animals 2023, 13, 3029. [Google Scholar] [CrossRef]
- Mader, T.L.; Grffin, D. Management of Cattle Exposed to Adverse Environmental Conditions. Vet. Clin. Food Anim. Pract. 2015, 31, 247–258. [Google Scholar] [CrossRef]
- St. Pierre, M.; Reeh, P.W.; Zimmermann, K. Differential Effects of TRPV Channel Block on Polymodal Activation of Rat Cutaneous Nociceptors in Vitro. Exp. Brain Res. 2009, 196, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S. Heat Stress Biomarker Amino Acids and Neuropeptide Afford Thermotolerance in Chicks. J. Poult. Sci. 2019, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress Biomarkers and Proteomics Alteration to Thermal Stress in Ruminants: A Review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.I.; Leite, P.G.; Lopes Neto, J.P.; Furtado, D.A.; Lopes, F.F.d.M. Estimation of Rectal Temperature of Goats Based on Surface Temperature. Eng. Agrícola 2021, 41, 591–598. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of Environmental Factors on Infrared Eye Temperature Measurements in Cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.J.; Bench, C.; Chabot, J.B.; Colyn, J.; Liu, T.; Okine, E.K.; Stewart, M.; Webster, J.R. The Non-Invasive and Automated Detection of Bovine Respiratory Disease Onset in Receiver Calves Using Infrared Thermography. Res. Vet. Sci. 2012, 93, 928–935. [Google Scholar] [CrossRef]

| Species Studied | Physiological Response and Behavioral Modifications | References | |
|---|---|---|---|
| Vasodilation, sweating | |||
| Water Buffaloes | The number of glands per cm2 of skin is lower in buffalo (394 gland/cm2) than in cattle (2633 gland/cm2). This difference makes animals resort to polypnea as a heat-related tolerance response that also needs to be enhanced by providing shade. | [7] | |
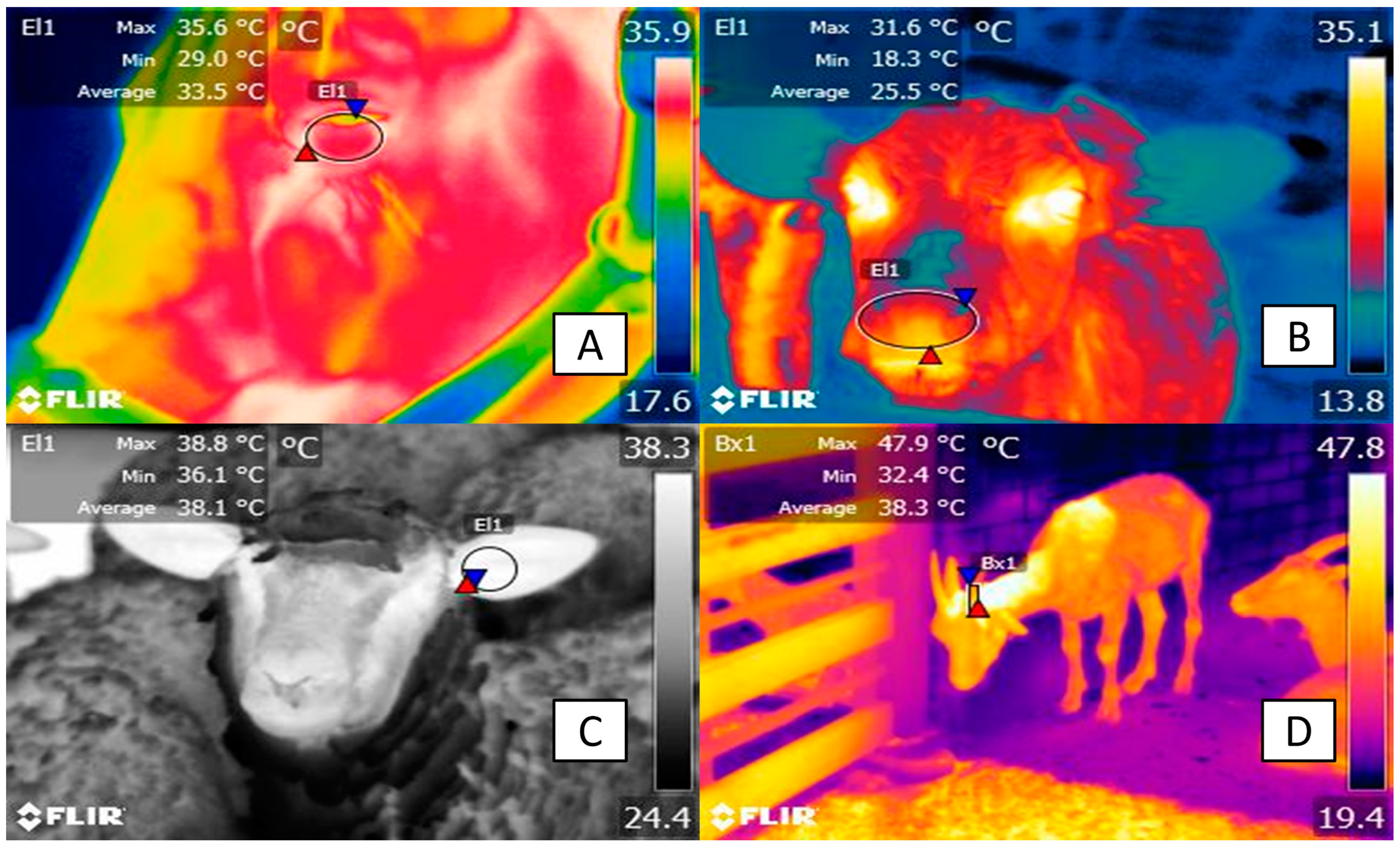
| Water buffalo | After 2 h of transport, increases in the surface temperature above 4 °C were recorded with IRT for thermal windows: the head regions, such as the lacrimal caruncle, the periocular subregion, the lower eyelid, the ear canal, the nasal passages, and the parietal subregion, as well as body regions, such as the abdominal and lumbar regions. | [62] | |
| Review study | Peripheral vasodilation enhances heat dissipation and, therefore, helps to reduce core temperature. | [63,64] | |
| Review study | Sympathetic cholinergic nerves, vasoactive intestinal peptide, substance P, histamine, prostaglandins, and TRPV1 activation participate in vasodilation. | [65,66] | |
| Cow | The horns have a thermoregulatory function. In a temperate climate zone, the temperature in the horns increased by 0.18 °C for each unit of the heat load index. | [71] | |
| Cow | Arrangement of the blood vessels to thermoregulate is very important. For example, the parietal region is irrigated by the superficial temporal artery (temporalis superficialis), transversa faciei, auricularis rostralis, palpebralis inferior lateralis, and palpebralis superior lateralis, blood vessels that provide circulation to the horns, which can favor thermoregulation. | [72] | |
| Cattle | Stress-associated hyperthermia in lactating dairy cows during isolation challenges | [73] | |
| Sheep | hoof thermoregulation. A difference of 8.5 °C was determined between healthy and diseased hooves (diagnostic sensitivity of 92% and specificity of 91%). | [77] | |
| Sheep | The recording of thermal stress during shearing. Ocular surface IRT was positively correlated with rectal temperature and cortisol levels. | [78] | |
| Horses | Sweating dissipates 70% of the heat loss during exercise due to evaporation. Sweat rate (L/h/m2) in horses is three times greater in horses compared to humans with a similar exercise intensity. | [74,75] | |
| Water buffalo | The number of sweat glands/cm2 in the head, neck, and tail regions was 20–30% higher compared to the thorax and abdomen region. This difference might impact not only the effectiveness of thermoregulation strategies in animals but also make it evident that animals use specific anatomical regions to thermoregulate. | [90] | |
| Endocrine and immunological response | |||
| Cattle | In hyperthermia due to acute heat stress, the concentrations of antidiuretic hormone, prolactin, glucocorticoids, and catecholamines increase, while those of aldosterone decrease. During chronic heat stress, levels of cortisol, growth hormone, and thyroxine may remain unchanged or may even decrease. | [60,94] | |
| Miniature pigs | Blood cortisol levels, which are associated with greater production of cytokines such as interleukin-4 (IL-4), IL-5, IL-6, and IL-12. | [99] | |
| Thermoregulatory behaviors | |||
| Cows, goats, elephants | Behaviors that promote heat dissipation (e.g., shade-seeking, changes in body posture) or those that limit heat production (e.g., reducing feed intake and locomotor activity). | [84,85,86,87]. | |
| Cows | Adaptative behaviors (increase time standing and reduce rumination time) from an average ambient temperature of 12 °C and a temperature–humidity index of 56. | [121] | |
| Ewes and Rams | Higher temperatures environments (35 °C) increased the total time of standing (355 vs. 486 min) and decreased eating (932 vs. 727 min). Likewise, the lying behavior of ewes and rams increased by up to 45.10 ± 2.42% during severe heat stress. | [123] | |
| Cows | An average rectal temperature of 39 °C, reduced dry matter intake (DMI) by 14.6 kg/day, and reduced and milk yields by 11.8 kg/day were recorded. | [106] | |
| Water Buffaloes | Wallowing helps to decrease body temperature by increasing heat dissipation via evaporation, as shown in a study assessing the thoracoabdominal surface temperature. | [135] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarubbi, J.; Martínez-Burnes, J.; Ghezzi, M.D.; Olmos-Hernandez, A.; Lendez, P.A.; Ceriani, M.C.; Hernández-Avalos, I. Hypothalamic Neuromodulation and Control of the Dermal Surface Temperature of Livestock during Hyperthermia. Animals 2024, 14, 1745. https://doi.org/10.3390/ani14121745
Sarubbi J, Martínez-Burnes J, Ghezzi MD, Olmos-Hernandez A, Lendez PA, Ceriani MC, Hernández-Avalos I. Hypothalamic Neuromodulation and Control of the Dermal Surface Temperature of Livestock during Hyperthermia. Animals. 2024; 14(12):1745. https://doi.org/10.3390/ani14121745
Chicago/Turabian StyleSarubbi, Juliana, Julio Martínez-Burnes, Marcelo Daniel Ghezzi, Adriana Olmos-Hernandez, Pamela Anahí Lendez, María Carolina Ceriani, and Ismael Hernández-Avalos. 2024. "Hypothalamic Neuromodulation and Control of the Dermal Surface Temperature of Livestock during Hyperthermia" Animals 14, no. 12: 1745. https://doi.org/10.3390/ani14121745








