Estimation of Genetic Parameters for Early Growth Traits in Luzhong Mutton Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Fixed Effects Analysis
3.2. Variance Components and Genetic Parameter Estimation
3.3. Correlation Analysis of Early Growth Traits
3.4. Correlation Analysis of Early Growth Traits
4. Discussion
4.1. Comparison of Different Animal Models
4.2. Estimation of Heritability
4.3. Genetic and Phenotypic Correlations
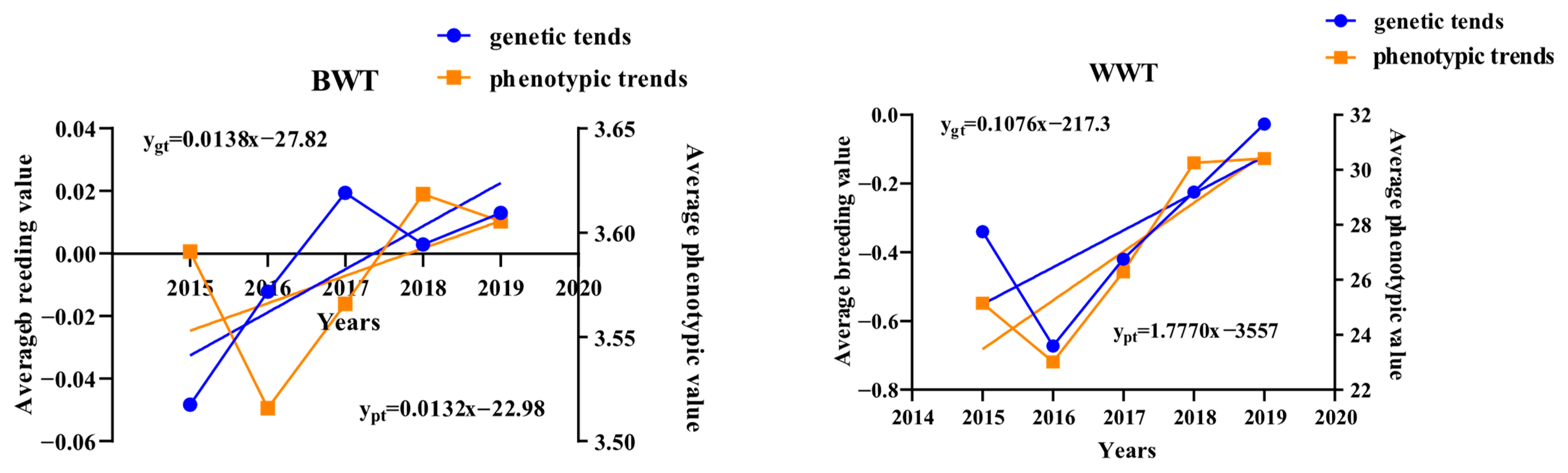
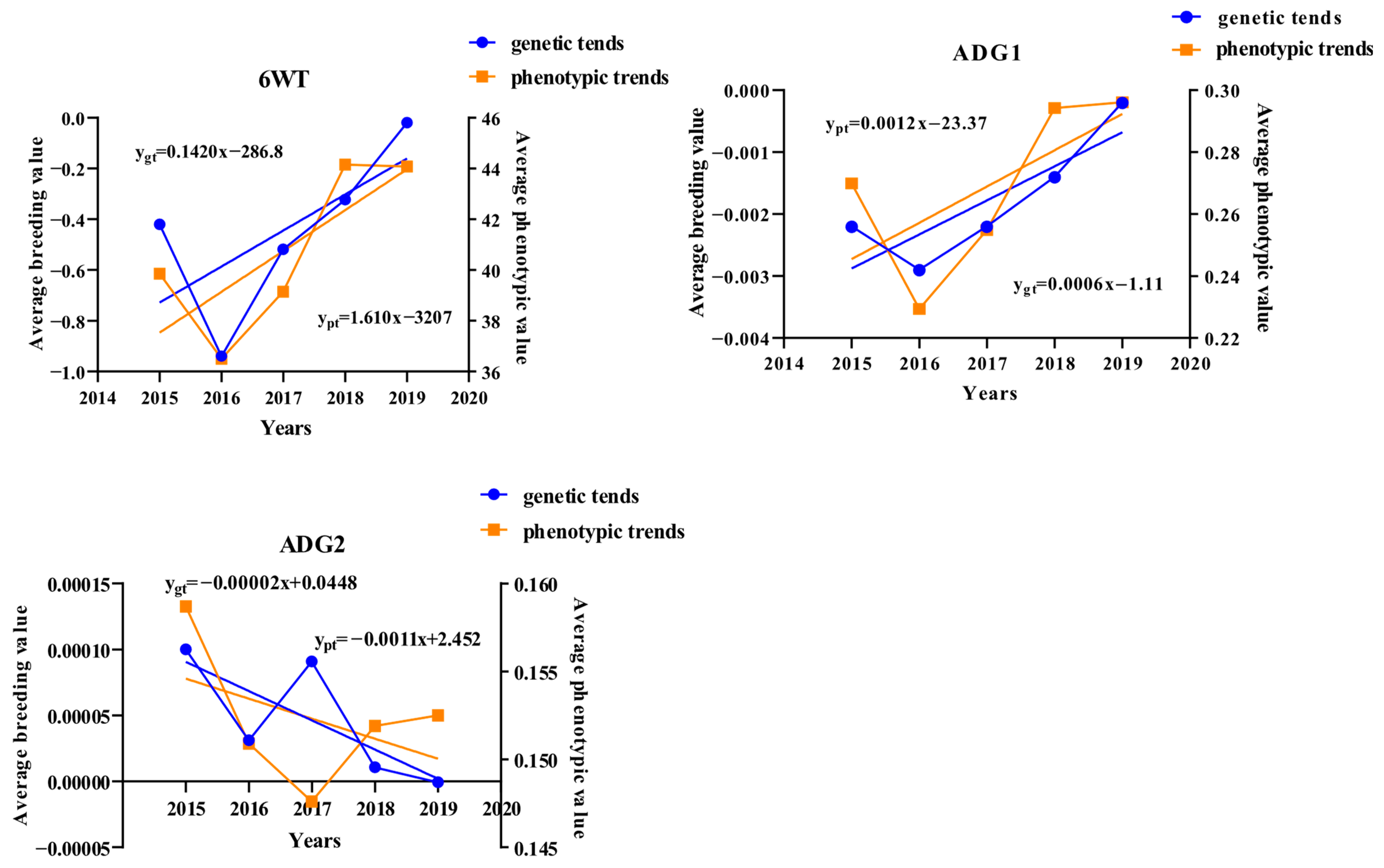
4.4. Genetic and Phenotypic Trends
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Crops and Livestock Products, Food and Agricultural Organization of the United Nations, Statistics Division (FAOSTAT). Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 1 March 2024).
- Balasundaram, B.; Thiruvenkadan, A.K.; Murali, N.; Muralidharan, J.; Cauveri, D.; Peters, S.O. Genetic Parameters of Growth Traits and Quantitative Genetic Metrics for Selection and Conservation of Mecheri Sheep of Tamil Nadu. Animals 2023, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.S.; Mackay, T.F. Introduction to Quantitative Genetics, 4th ed.; Longman: London, UK, 1996. [Google Scholar]
- Alemayehu, A.; Mirkena, T.; Melesse, A.; Getachew, T.; Haile, A. Genetic parameters and trends of growth traits in community-based breeding programs of Abera sheep in Ethiopia. Acta Agric. Scand. Sect. A—Anim. Sci. 2022, 71, 1–11. [Google Scholar] [CrossRef]
- Hizli, H.; Takma, C.; Yazgan, E. Comparison of different models for estimation of direct and maternal genetic parameters on body weights in Awassi sheep. Arch. Anim. Breed. 2022, 65, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Shahrebabak, M.M.; Vatankhah, M.; Shahrebabak, H.M. Direct and maternal (co)variance components, genetic parameters, and annual trends for growth traits of Makooei sheep in Iran. Trop. Anim. Health Prod. 2013, 45, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Mohammadi, H. Comparison of different models for estimation of genetic parameters of early growth traits in the Mehraban sheep. J. Anim. Breed. Genet. 2008, 125, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chu, M.; Pan, L.; Wang, X.; He, X.; Zhang, R.; Tao, L.; La, Y.; Ma, L.; Di, R. Polymorphism Detection of GDF9 Gene and Its Association with Litter Size in Luzhong Mutton Sheep (Ovis aries). Animals 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.; Jensen, J.; Labouriau, R.; Christensen, O.; Sahana, G. DMU—A Package for Analyzing Multivariate Mixed Models in Quantitative Genetics and Genomics. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Vancover, BC, Canada, 17–22 August 2014. [Google Scholar]
- Willham, R.L. The role of maternal effects in animal breeding. 3. Biometrical aspects of maternal effects in animals. J. Anim. Sci. 1972, 35, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1973; pp. 199–213. [Google Scholar]
- Gowane, G.R.; Chopra, A.; Prince, L.L.; Paswan, C.; Arora, A.L. Estimates of (co)variance components and genetic parameters for body weights and first greasy fleece weight in Bharat Merino sheep. Animal 2010, 4, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Dahiya, S.P.; Magotra, A.; Bangar, Y.C. Evaluating animal models comprising direct and maternal effects associated with growth rates and the Kleiber ratio in Harnali sheep. Zygote 2022, 30, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Li, G.Y.; Zhang, J.; Wang, X.J.; Zhang, A.W.; Zhao, J.T.; Wang, L.J.; Yang, J.F.; Luo, T.Z.; Shen, K.Z. Estimates of (co)variance components and phenotypic and genetic parameters of growth traits and wool traits in Alpine Merino sheep. J. Anim. Breed. Genet. 2022, 139, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Gemeda, D.; Schoeman, S.J.S.; Cloete, W.P.; Jordaan, G.F. Genetic parameters for early growth traits in a Merino lambs estimated using multitrait analysis. Eth. J. Anim. Prod. 2003, 3, 1–11. [Google Scholar]
- Shokrollahi, B.; Baneh, H. (Co)variance components and genetic parameters for growth traits in Arabi sheep using different animal models. Genet. Mol. Res. 2012, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- El Fadili, M.; Michaux, C.; Detilleux, J.; Leroy, P.L. Genetic parameters for growth traits of the Moroccan Timahdit breed of sheep. Small Rumin. Res. 2000, 37, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, E.L.; Mattos, E.C.; Eler, J.P.; Barreto Neto, A.D.; Ferraz, J.B. Estimation of genetic parameters and genetic changes for growth characteristics of Santa Ines sheep. Genet. Mol. Res. 2016, 15, 15038910. [Google Scholar] [CrossRef]
- Mandal, A.; Gayari, I.; Baneh, H.; Notter, D.R. Genetic analysis of body weight and growth curve parameters in Muzaffarnagari sheep of India. J. Anim. Breed. Genet. 2024, 141, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Haile, A.; Hilali, M.; Hassen, H.; Lobo, R.N.B.; Rischkowsky, B. Estimates of genetic parameters and genetic trends for growth, reproduction, milk production and milk composition traits of Awassi sheep. Animal 2019, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Tesema, Z.; Deribe, B.; Lakew, M.; Getachew, T.; Tilahun, M.; Belayneh, N.; Kefale, A.; Shibesh, M.; Zegeye, A.; Yizengaw, L.; et al. Genetic and non-genetic parameter estimates for growth traits and Kleiber ratios in Dorper × indigenous sheep. Animal 2022, 16, 100533. [Google Scholar] [CrossRef] [PubMed]
- Habtegiorgis, K.; Haile, A.; Getachew, T.; Kirmani, M.A.; Gemiyo, D. Analysis of genetic parameters and genetic trends for early growth and reproductive traits of Doyogena sheep managed under community-based breeding program. Heliyon 2022, 8, e09749. [Google Scholar] [CrossRef] [PubMed]
- Behrem, S. Estimation of genetic parameters for pre-weaning growth traits in Central Anatolian Merino sheep. Small Rumin. Res. 2021, 197, 106319. [Google Scholar] [CrossRef]
- Sharif, N.; Ali, A.; Dawood, M.; Khan, M.I.; Do, D.N. Environmental Effects and Genetic Parameters for Growth Traits of Lohi Sheep. Animals 2022, 12, 3590. [Google Scholar] [CrossRef] [PubMed]
- Jawasreh, K.; Ismail, Z.B.; Iya, F.; Castaneda-Bustos, V.J.; Valencia-Posadas, M. Genetic parameter estimation for pre-weaning growth traits in Jordan Awassi sheep. Vet. World 2018, 11, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, B.; Muralidharan, J.; Murali, N.; Cauveri, D.; Raja, A.; Okpeku, M.; Thiruvenkadan, A.K. Development of selection strategies for genetic improvement in production traits of Mecheri sheep based on a Bayesian multi trait evaluation. PLoS ONE 2023, 18, e0289460. [Google Scholar] [CrossRef] [PubMed]
- Boujenane, I.; Diallo, I.T. Estimates of genetic parameters and genetic trends for pre-weaning growth traits in Sardi sheep. Small Rumin. Res. 2017, 146, 61–68. [Google Scholar] [CrossRef]
- Lalit; Malik, Z.S.; Dalal, D.S.; Dahiya, S.P.; Patil, C.S.; Dahiya, R. Genetic analysis of growth traits in Harnali sheep. Vet. World 2016, 9, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, S.; Lemma, S.; Komen, H.; Van Arendonk, J.A.M. Estimates of genetic parameters and genetic trends for live weight and fleece traits in Menz sheep. Small Rumin. Res. 2007, 70, 145–153. [Google Scholar] [CrossRef]
- Illa, S.K.; Gollamoori, G.; Lopes, F.B.; Nath, S.; Thiruvenkadan, A.K. Multi trait genetic evaluation of growth traits in Nellore sheep raised on pasture in semi-arid regions of India using Bayesian approach. Small Rumin. Res. 2020, 192, 106224. [Google Scholar] [CrossRef]
- Arthy, V.; Venkataramanan, R.; Sivaselvam, S.N.; Sreekumar, C.; Balasubramanyam, D. Genetic evaluation of growth in farmers’ flocks of Madras Red sheep under long-term selection in a group breeding scheme. Trop. Anim. Health Prod. 2018, 50, 1463–1471. [Google Scholar] [CrossRef]
- Magotra, A.; Bangar, Y.C.; Chauhan, A.; Yadav, A.S.; Malik, Z.S. Impact of mother genetic and resource environment on her offspring’s growth features in Munjal sheep. Zygote 2022, 30, 495–500. [Google Scholar] [CrossRef]
- Gowane, G.R.; Chopra, A.; Prince, L.L.; Mishra, A.K.; Arora, A.L. Genetic analysis for growth traits of prolific Garole × Malpura (GM) sheep. Trop. Anim. Health Prod. 2011, 43, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Koçak, S.; Çinkaya, S.; Tekerli, M.; Demirtaş, M.; Bozkurt, Z.; Çelikeloğlu, K.; Hacan, Ö.; Erdoğan, M. Estimation of (Co) Variance Components and Genetic Parameters for Pre- and Post-Weaning Growth Traits in Dağlıç Sheep. Animals 2023, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Bangar, Y.C.; Magotra, A.; Yadav, A.S. Estimates of covariance components and genetic parameters for growth, average daily gain and Kleiber ratio in Harnali sheep. Trop. Anim. Health Prod. 2020, 52, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, N.; Pollott, G.E. The impact of data structure on genetic (co)variance components of early growth in sheep, estimated using an animal model with maternal effects. J. Anim. Sci. 2003, 81, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Oyieng, E.; Mrode, R.; Ojango, J.M.K.; Ekine-Dzivenu, C.C.; Audho, J.; Okeyo, A.M. Genetic parameters and genetic trends for growth traits of the Red Maasai sheep and its crosses to Dorper sheep under extensive production system in Kenya. Small Rumin. Res. 2022, 206, 106588. [Google Scholar] [CrossRef]
- Hanford, K.J.; Van Vleck, L.D.; Snowder, G.D. Estimates of genetic parameters and genetic change for reproduction, weight, and wool characteristics of Columbia sheep. J. Anim. Sci. 2002, 80, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Khan, N.N.; Ganai, N.A.; Shanaz, S.; Rather, M.A.; Alam, S. Multivariate quantitative genetic analysis of body weight traits in Corriedale sheep. Trop. Anim. Health Prod. 2021, 53, 197. [Google Scholar] [CrossRef]
- Areb, E.; Getachew, T.; Kirmani, M.A.; Abate, Z.; Haile, A. Estimation of (co)variance components, genetic parameters, and genetic trends of growth traits in community-based breeding programs of Bonga sheep. Animal 2021, 15, 100202. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Ghafouri-Kesbi, F. Estimation of genetic parameters for growth-related traits and evaluating the results of a 27-year selection program in Baluchi sheep. Small Rumin. Res. 2015, 130, 8–14. [Google Scholar] [CrossRef]
- Besufkad, S.; Goshme, S.; Bisrat, A.; Abebe, A.; Abebe, A.; Getachew, T.; Areaya, A.; Zewdie, T.; Gizaw, S. Estimation of genetic parameters for growth traits and kleiber ratio in dorper sheep breed. Heliyon 2024, 10, e24971. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, C.M.; Ilatsia, E.D.; Kosgey, I.S.; Kahi, A.K. Direct and maternal (co)variance components, genetic parameters and annual trends for growth traits of Dorper sheep in semi-arid Kenya. Trop. Anim. Health Prod. 2010, 42, 473–481. [Google Scholar] [CrossRef]

| Traits | BWT (kg) | WWT (kg) | 6WT (kg) | ADG1 (kg) | ADG2 (kg) |
|---|---|---|---|---|---|
| N | 2464 | 2923 | 2428 | 2424 | 1836 |
| Mean ± Standard error | 3.589 ± 0.009 | 27.395 ± 0.101 | 41.231 ± 0.114 | 0.276 ± 0.001 | 0.152 ± 0.001 |
| Standard Deviation | 0.435 | 5.472 | 5.593 | 0.049 | 0.027 |
| Min | 2.500 | 11.200 | 28.500 | 0.120 | 0.050 |
| Max | 4.400 | 37.500 | 54.000 | 0.372 | 0.259 |
| Traits | Birth Year | Birth Season | Sex | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F | p | Mean Square | F | p | Mean Square | F | p | |
| BWT | 0.934 ** | 5.37 | 0.0003 | 0.707 ** | 407.00 | 0.0068 | 33.107 ** | 190.44 | <0.0001 |
| WWT | 5746.681 ** | 292.03 | <0.0001 | 649.839 ** | 33.02 | <0.0001 | 1933.506 ** | 98.25 | <0.0001 |
| 6WT | 3770.473 ** | 181.93 | <0.0001 | 1193.097 ** | 57.57 | <0.0001 | 461.788 ** | 22.28 | <0.0001 |
| ADG1 | 0.282 ** | 165.42 | <0.0001 | 0.040 ** | 23.49 | <0.0001 | 0.122 ** | 71.44 | <0.0001 |
| ADG2 | 0.007 ** | 6.42 | <0.0001 | 0.005 ** | 4.20 | 0.0057 | 0.008 ** | 7.07 | 0.0079 |
| Traits | Model | σa2 | σm2 | σa,m | σc2 | σe2 | σp2 | σa2/σp2 | σm2/σp2 | σc2/σp2 | σe2/σp2 | hT2 | −2lnL | AIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BWT | 1 | 0.036 | 0.138 | 0.174 | 0.209 ± 0.035 | 0.791 | 0.209 | −1834.122 | −1830.12 | |||||
| 2 | 0.034 | 0.003 | 0.134 | 0.171 | 0.200 ± 0.059 | 0.015 ± 0.042 | 0.785 | 0.200 | −1813.992 | −1807.99 | ||||
| 3 | 0.031 | 0.032 | 0.122 | 0.185 | 0.168 ± 0.062 | 0.170 ± 0.055 | 0.661 | 0.253 | −1813.832 | −1807.83 | ||||
| 4 | 0.028 | 0.036 | −0.014 | 0.128 | 0.177 | 0.156 ± 0.057 | 0.201 ± 0.100 | 0.723 | 0.136 | −1839.796 | −1831.80 | |||
| 5 | 0.027 | 0.017 | 0.005 | 0.128 | 0.176 | 0.152 ± 0.094 | 0.096 ± 0.047 | 0.026 ± 0.147 | 0.726 | 0.200 | −1811.112 | −1803.11 | ||
| 6 | 0.025 | 0.031 | −0.012 | 0.003 | 0.128 | 0.176 | 0.143 ± 0.078 | 0.177 ± 0.209 | 0.020 ± 0.160 | 0.727 | 0.132 | −1830.646 | −1820.65 | |
| WWT | 1 | 7.599 | 8.309 | 15.907 | 0.478 ± 0.027 | 0.522 | 0.478 | 10,801.161 | 10,805.16 | |||||
| 2 | 7.551 | 0.063 | 8.295 | 15.909 | 0.475 ± 0.046 | 0.004 ± 0.030 | 0.521 | 0.475 | 10,801.139 | 10,807.14 | ||||
| 3 | 7.560 | 0.051 | 8.298 | 15.909 | 0.475 ± 0.045 | 0.003 ± 0.029 | 0.522 | 0.477 | 10,801.145 | 10,807.15 | ||||
| 4 | 8.544 | 4.372 | −5.310 | 8.026 | 15.633 | 0.547 ± 0.031 | 0.280 ± 0.047 | 0.513 | 0.177 | 10,750.433 | 10,758.43 | |||
| 5 | 7.373 | 0.267 | 0.045 | 8.248 | 15.932 | 0.463 ± 0.122 | 0.017 ± 0.081 | 0.003 ± 0.083 | 0.518 | 0.471 | 10,801.139 | 10,809.14 | ||
| 6 | 8.538 | 4.023 | −5.250 | 0.296 | 8.024 | 15.631 | 0.546 ± 0.127 | 0.257 ± 0.121 | 0.019 ± 0.113 | 0.513 | 0.171 | 10,750.416 | 10,760.42 | |
| 6WT | 1 | 8.944 | 8.861 | 17.805 | 0.502 ± 0.031 | 0.498 | 0.502 | 9257.474 | 9261.47 | |||||
| 2 | 8.977 | 0.003 | 8.833 | 17.813 | 0.504 ± 0.054 | 0.0002 ± 0.034 | 0.496 | 0.504 | 9257.733 | 9263.73 | ||||
| 3 | 8.969 | 0.009 | 8.834 | 17.812 | 0.504 ± 0.052 | 0.0005 ± 0.032 | 0.496 | 0.504 | 9257.622 | 9263.62 | ||||
| 4 | 11.434 | 3.442 | −5.857 | 8.483 | 17.502 | 0.653 ± 0.031 | 0.197 ± 0.053 | 0.485 | 0.250 | 9208.665 | 9216.66 | |||
| 5 | 8.926 | 0.032 | 0.002 | 8.852 | 17.811 | 0.501 ± 0.126 | 0.002 ± 0.078 | 0.0001 ± 0.081 | 0.497 | 0.502 | 9257.994 | 9265.99 | ||
| 6 | 11.409 | 3.441 | −5.817 | 0.034 | 8.458 | 17.524 | 0.651 ± 0.139 | 0.196 ± 0.114 | 0.002 ± 0.106 | 0.483 | 0.251 | 9208.734 | 9218.73 | |
| ADG1 | 1 | 6.13 × 10−4 | 7.89 × 10−4 | 1.40 × 10−3 | 0.437 ± 0.028 | 0.563 | 0.437 | −13,536.32079 | −13,532.32 | |||||
| 2 | 6.06 × 10−4 | 6.700 × 10−6 | 7.89 × 10−4 | 1.40 × 10−3 | 0.432 ± 0.051 | 0.005 ± 0.035 | 0.563 | 0.432 | −13,534.7641 | −13,528.76 | ||||
| 3 | 5.93 × 10−4 | 3.22 × 10−5 | 7.82 × 10−4 | 1.41 × 10−3 | 0.421 ± 0.052 | 0.023 ± 0.036 | 0.556 | 0.433 | −13,532.18564 | −13,526.19 | ||||
| 4 | 7.28 × 10−4 | 3.77 × 10−4 | −5.10 × 10−4 | 7.76 × 10−4 | 1.371 × 10−3 | 0.531 ± 0.035 | 0.275 ± 0.052 | 0.565 | 0.111 | −13,586.49552 | −13,578.50 | |||
| 5 | 6.04 × 10−4 | 1.47 × 10−5 | 8.000 × 10−7 | 7.85 × 10−4 | 1.405 × 10−3 | 0.430 ± 0.140 | 0.010 ± 0.100 | 0.001 ± 0.103 | 0.559 | 0.435 | −13,534.12069 | −13,526.12 | ||
| 6 | 7.20 × 10−4 | 3.74 × 10−4 | −5.02 × 10−4 | 1.300 × 10−6 | 7.78 × 10−4 | 1.371 × 10−3 | 0.525 ± 0.141 | 0.273 ± 0.136 | 0.001 ± 0.133 | 0.567 | 0.112 | −13,586.32817 | −13,576.33 | |
| ADG2 | 1 | 1.16 × 10−5 | 7.25 × 10−4 | 7.36 × 10−4 | 0.016 ± 0.039 | 0.984 | 0.016 | −11,269.75683 | −11,265.76 | |||||
| 2 | 2.19 × 10−5 | 4.92 × 10−5 | 6.75 × 10−4 | 7.46 × 10−4 | 0.029 ± 0.049 | 0.066 ± 0.041 | 0.905 | 0.029 | −11,277.21275 | −11,271.21 | ||||
| 3 | 1.20 × 10−5 | 3.76 × 10−5 | 6.93 × 10−4 | 7.43 × 10−4 | 0.016 ± 0.056 | 0.051 ± 0.049 | 0.933 | 0.041 | −11,275.12621 | −11,269.13 | ||||
| 4 | 3.92 × 10−5 | 6.04 × 10−5 | −2.51 × 10−5 | 6.74 × 10−4 | 7.48 × 10−4 | 0.052 ± 0.046 | 0.081 ± 0.092 | 0.900 | 0.042 | −11,279.22475 | −11,271.22 | |||
| 5 | 2.42 × 10−5 | 4.06 × 10−5 | 5.50 × 10−6 | 6.77 × 10−4 | 7.48 × 10−4 | 0.032 ± 0.041 | 0.054 ± 0.126 | 0.007 ± 0.132 | 0.906 | 0.060 | −11,278.20752 | −11,270.21 | ||
| 6 | 3.61 × 10−5 | 6.66 × 10−5 | −1.99 × 10−5 | 5.90 × 10−6 | 6.62 × 10−4 | 7.51 × 10−4 | 0.048 ± 0.050 | 0.089 ± 0.195 | 0.008 ± 0.157 | 0.882 | 0.053 | −11,275.76864 | −11,265.77 |
| Model | BWT | WWT | 6WT | ADG1 | ADG2 |
|---|---|---|---|---|---|
| 2:1 | −20.130 ns | 0.022 ns | −0.259 ns | −1.557 ns | 7.456 ** |
| 3:1 | −20.290 ns | 0.016 ns | −0.148 ns | −4.135 ns | 5.369 * |
| 4:1 | 5.674 ns | 50.728 ** | 48.809 ** | 50.175 ** | 9.468 ** |
| 5:1 | −23.010 ns | 0.022 ns | −0.519 ns | −2.200 ns | 8.451 * |
| 6:1 | −3.4756 ns | 50.745 ** | 48.740 ** | 50.007 ** | 6.012 ns |
| 4:2 | 25.804 ** | 50.706 ** | 49.068 ** | 51.731 ** | 2.012 ns |
| 5:2 | −2.880 ns | 0.000 ns | −0.261 ns | −0.643 ns | 0.995 ns |
| 6:2 | 16.654 ** | 50.723 ** | 48.999 ** | 51.564 ** | −1.444 ns |
| 4:3 | 25.964 ** | 50.712 ** | 48.957 ** | 54.310 ** | 4.099 * |
| 5:3 | −2.720 ns | 0.006 ns | −0.372 ns | 1.935 ns | 3.081 ns |
| 6:3 | 16.815 ** | 50.729 ** | 48.888 ** | 54.143 ** | 0.642 ns |
| 6:4 | −9.150 ns | 0.017 ns | −0.069 ns | −0.167 ns | −3.456 ns |
| 6:5 | 19.535 ** | 50.723 ** | 49.260 ** | 52.207 ** | −2.439 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Li, X.; He, J.; Zhang, M.; Liu, G.; Wei, C.; Zhang, G.; Zhang, W.; Nie, F.; Wang, M.; et al. Estimation of Genetic Parameters for Early Growth Traits in Luzhong Mutton Sheep. Animals 2024, 14, 1754. https://doi.org/10.3390/ani14121754
Ren Y, Li X, He J, Zhang M, Liu G, Wei C, Zhang G, Zhang W, Nie F, Wang M, et al. Estimation of Genetic Parameters for Early Growth Traits in Luzhong Mutton Sheep. Animals. 2024; 14(12):1754. https://doi.org/10.3390/ani14121754
Chicago/Turabian StyleRen, Yifan, Xue Li, Junmin He, Menghua Zhang, Guifen Liu, Chen Wei, Guoping Zhang, Wenhao Zhang, Fumei Nie, Ming Wang, and et al. 2024. "Estimation of Genetic Parameters for Early Growth Traits in Luzhong Mutton Sheep" Animals 14, no. 12: 1754. https://doi.org/10.3390/ani14121754





