Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Animals and Sample Collection
2.3. Trait Analysis
2.4. Genomic DNA Extraction, PCR Amplification, and Identification
2.5. Statistical Analysis
3. Results
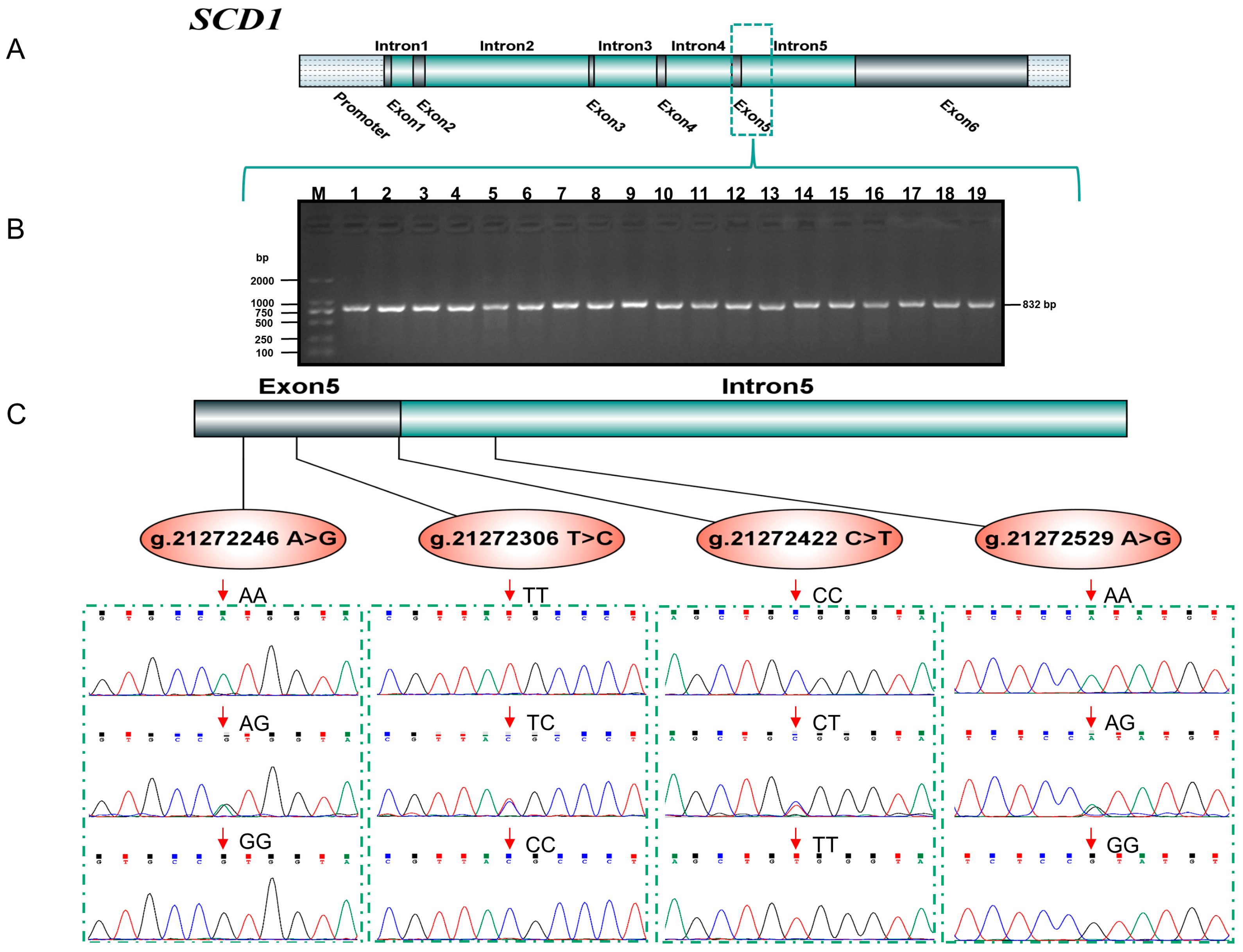
3.1. SNP Detection and Genotyping of the SCD1 Gene
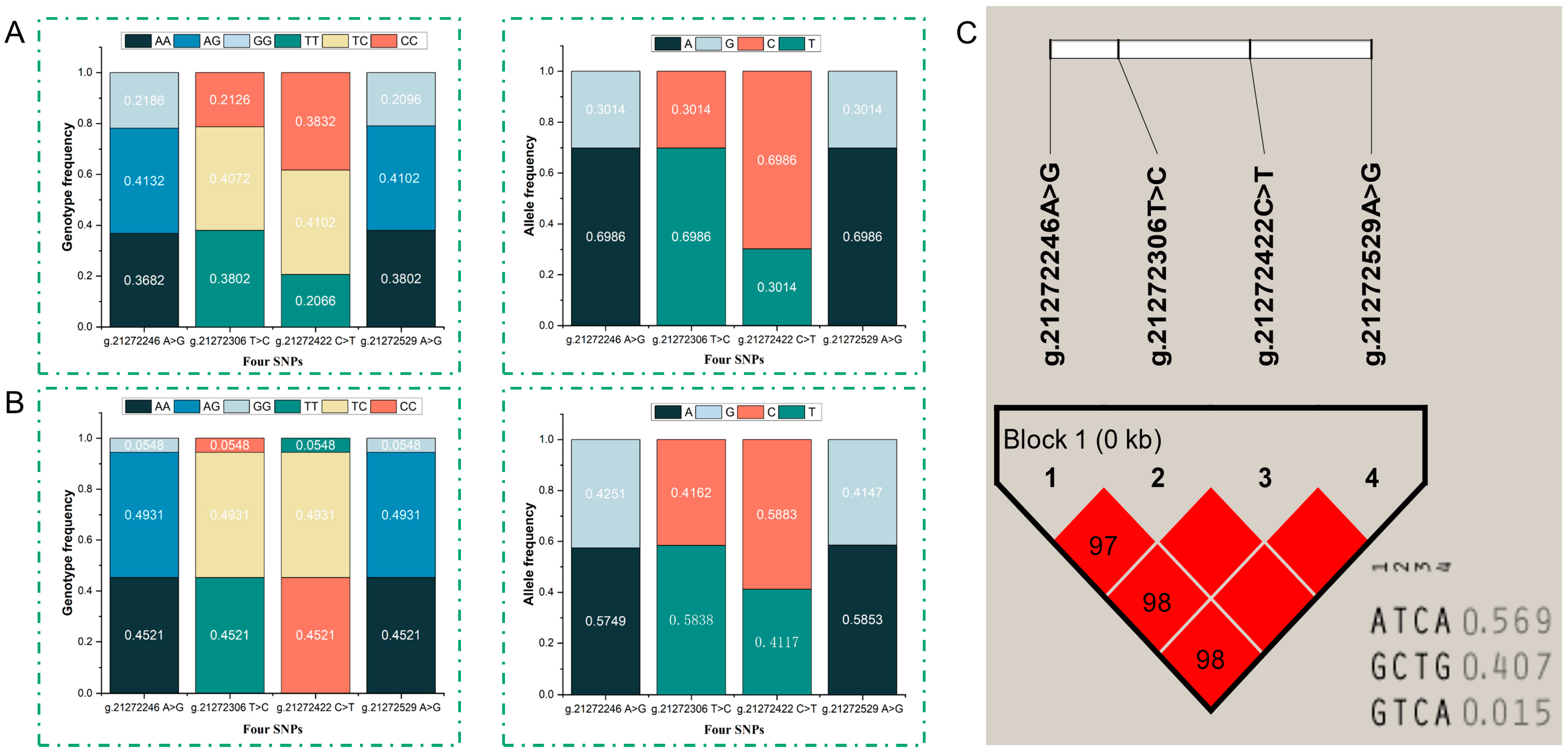
3.2. Analysis of Population Genetic Polymorphism, Linkage, and Haplotypes
3.3. Association Analyses of SCD1 Gene Polymorphisms with Carcass and Meat Quality Traits in Chinese Simmental Cattle
3.4. Association Analyses of SCD1 Gene Polymorphisms with Adipogenesis Traits and Fatty Acid Composition
3.5. Association Analysis of the SNPs in the SCD1 Gene with Milk Production Traits in Chinese Holstein Cows
3.6. Association Analysis of Haplotypes and Carcass Traits, Meat Quality, Adipogenesis Traits, and Fatty Acid Composition in Chinese Simmental Cattle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kęsek-Woźniak, M.M.; Wojtas, E.; Zielak-Steciwko, A.E. Impact of SNPs in ACACA, SCD1, and DGAT1 Genes on Fatty Acid Profile in Bovine Milk with Regard to Lactation Phases. Animals 2020, 10, 997. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, G.; Yang, W.; Jin, W.; Gong, J.; Xu, X.; Niu, X. Animal-SNPAtlas: A comprehensive SNP database for multiple animals. Nucleic Acids Res. 2022, 51, D816–D826. [Google Scholar] [CrossRef] [PubMed]
- di Martino, O.; Troiano, A.; Addi, L.; Guarino, A.; Calabrò, S.; Tudisco, R.; Murru, N.; Cutrignelli, M.I.; Infascelli, F.; Calabrò, V. Regulation of Stearoyl Coenzyme A Desaturase 1 Gene Promoter in Bovine Mammary Cells. Anim. Biotechnol. 2015, 26, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Piórkowska, K.; Małopolska, M.; Ropka-Molik, K.; Szyndler-Nędza, M.; Wiechniak, A.; Żukowski, K.; Lambert, B.; Tyra, M. Evaluation of SCD, ACACA and FASN Mutations: Effects on Pork Quality and Other Production Traits in Pigs Selected Based on RNA-Seq Results. Animals 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Aldai, N.; Vinsky, M.; Dugan, M.E.R.; McAllister, T.A. Association analyses of single nucleotide polymorphisms in bovine stearoyl-CoA desaturase and fatty acid synthase genes with fatty acid composition in commercial cross-bred beef steers. Anim. Genet. 2012, 43, 93–97. [Google Scholar] [CrossRef]
- Matosinho, C.G.R.; Fonseca, P.A.d.S.; Peixoto, M.G.C.D.; Rosse, I.C.; Lopes, F.C.F.; Zózimo, T.; Filho, A.E.V.; Bruneli, F.Â.T.; Carvalho, M.R.S.; Gama, M.A.S. Phenotypic variation in milk fatty acid composition and its association with stearoyl-CoA desaturase 1 (SCD1) gene polymorphisms in Gir cows. J. Anim. Breed. Genet. 2023, 140, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Conte, G.; Castiglioni, B.; Chessa, S.; Macciotta, N.P.P.; Serra, A.; Buccioni, A.; Pagnacco, G.; Secchiari, P. Stearoyl-coenzyme A desaturase gene polymorphism and milk fatty acid composition in Italian Holsteins. J. Dairy Sci. 2007, 90, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, S.; Cui, K.; Shafique, L.; Rehman, S.U.; Luo, C.; Wang, Z.; Ruan, J.; Qian, Q.; Liu, Q. Fatty acid biosynthesis and transcriptional regulation of Stearoyl-CoA Desaturase 1 (SCD1) in buffalo milk. BMC Genet. 2020, 21, 23. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, Z.; Jiang, P.; Yu, H.; Xiao, H.; Yang, R. Identification of the bovine HSL gene expression profiles and its association with fatty acid composition and fat deposition traits. Meat Sci. 2017, 131, 107–118. [Google Scholar] [CrossRef]
- GB/T 17238-2008; Fresh and Frozen Beef, Cuts. National Standards of People’s Republic of China: Taipei City, Taiwan, 2015.
- He, W.; Fang, X.; Lu, X.; Liu, Y.; Li, G.; Zhao, Z.; Li, J.; Yang, R. Function Identification of Bovine ACSF3 Gene and Its Association With Lipid Metabolism Traits in Beef Cattle. Front. Vet. Sci. 2022, 8, 766765. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, Z.; Yu, X.; Li, J.; Lu, C.; Yang, R. Bovine lipid metabolism related gene GPAM: Molecular characterization, function identification, and association analysis with fat deposition traits. Gene 2017, 608, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, P.; Yu, X.; Mi, J.; Bai, Z.; Zhang, X.; Liu, Y.; Fang, X.; Yang, R.; Zhao, Z. Analysis of the Bovine DLK1 Gene Polymorphism and Its Relation to Lipid Metabolism in Chinese Simmental. Animals 2020, 10, 923. [Google Scholar] [CrossRef]
- Gill, J.L.; Bishop, S.C.; McCorquodale, C.; Williams, J.L.; Wiener, P. Association of selected SNP with carcass and taste panel assessed meat quality traits in a commercial population of Aberdeen Angus-sired beef cattle. Genet. Sel. Evol. 2009, 41, 36. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.D.; Beha, A.; Tennagels, N.; Tschank, G.; Herling, A.W.; Quint, M.; Gerl, M.; Metz-Weidmann, C.; Haun, G.; Korn, M. Gene expression profiling in skeletal muscle of Zucker diabetic fatty rats: Implications for a role of stearoyl-CoA desaturase 1 in insulin resistance. Diabetologia 2005, 48, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Maharani, D.; Park, H.-B.; Lee, J.-B.; Yoo, C.-K.; Lim, H.-T.; Han, S.-H.; Lee, S.-S.; Ko, M.-S.; Cho, I.-C.; Lee, J.-H. Association of the gene encoding stearoyl-CoA desaturase (SCD) with fatty acid composition in an intercross population between Landrace and Korean native pigs. Mol. Biol. Rep. 2012, 40, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, F.; Tsuchimura, M.; Oyama, K.; Matsuhashi, T.; Maruyama, S.; Mannen, H.; Sasazaki, S. Effect of DNA markers on the fertility traits of Japanese Black cattle for improving beef quantity and quality. Arch. Anim. Breed. 2020, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Felista, W.M.; Shedrach, B.P.; John, R.O.; Oyelola, A.A.; Edward, C.; Christopher, P.G.; Bunmi, S.M.-A.; Robert, T.K.; Aduli, E.O.M.-A. Single Nucleotide Polymorphisms in the Fatty Acid Binding Protein 4, Fatty Acid Synthase and Stearoyl-CoA Desaturase Genes Influence Carcass Characteristics of Tropical Crossbred Beef Steers. Agriculture 2022, 12, 1171. [Google Scholar] [CrossRef]
- Kovalchuk, S.N.; Arkhipova, A.L.; Klimov, E.A. Development of real-time PCR assay for genotyping SNP rs41255693 in cattle SCD gene. AIMS Agric. Food 2020, 5, 14–19. [Google Scholar] [CrossRef]
- Raschia, M.A.; Nani, J.P.; Maizon, D.O.; Beribe, M.J.; Amadio, A.F.; Poli, M.A. Single nucleotide polymorphisms in candidate genes associated with milk yield in Argentinean Holstein and Holstein x Jersey cows. J. Anim. Sci. Technol. 2018, 60, 31. [Google Scholar] [CrossRef]
- Kucharski, M.; Kaczor, U. Stearoyl-CoA desaturase—The lipid metabolism regulator. Postepy higieny i medycyny doswiadczalnej (Online) 2014, 68, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Lunt, D.K.; Chung, K.Y.; Choi, C.B.; Tume, R.K.; Zembayashi, M. Adiposity, fatty acid composition, and delta-9 desaturase activity during growth in beef cattle. Anim. Sci. J. 2006, 77, 478–486. [Google Scholar] [CrossRef]
- Kim, H.J.; Sharma, A.; Lee, S.H.; Lee, D.H.; Lim, D.J.; Cho, Y.M.; Yang, B.S.; Lee, S.H. Genetic association of PLAG1, SCD, CYP7B1 and FASN SNPs and their effects on carcass weight, intramuscular fat and fatty acid composition in Hanwoo steers (Korean cattle). Anim. Genet. 2016, 48, 251. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Utsugi, T.; Oyama, K.; Mannen, H.; Kobayashi, M.; Tanabe, Y.; Ogino, A.; Tsuji, S. Genotype of stearoyl-coA desaturase is associated with fatty acid composition in Japanese Black cattle. Mamm. Genome 2004, 15, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Gutowska, I.; Wiercioch, M.; Łukomska, A.; Tarnowski, M.; Drozd, A.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Sodium Orthovanadate Changes Fatty Acid Composition and Increased Expression of Stearoyl-Coenzyme A Desaturase in THP-1 Macrophages. Biol. Trace Elem. Res. 2019, 193, 152–161. [Google Scholar] [CrossRef]
- Safina, N.Y.; Shakirov, S.K.; Ravilov, R.K.; Sharafutdinov, G.S. Associations of the SCD1 gene SNP with fatty acids composition of Holstein cows. In BIO Web of Conferences; EDP Sciences: Ulis, France, 2020; Volume 27, p. 00060. [Google Scholar] [CrossRef]
- Hilmia, N.; Noor, R.R.; Sumantri, C.; Gurnadi, R.E.; Priyanto, R. Polymorphism of stearoyl-CoA desaturase (SCD1) gene in Indonesian local cattle. J. Indones. Trop. Anim. Agric. 2017, 42, 1–5. [Google Scholar] [CrossRef]
- Macciotta, N.P.P.; Mele, M.; Conte, G.; Serra, A.; Cassandro, M.; Dal Zotto, R.; Borlino, A.C.; Pagnacco, G.; Secchiari, P. Association between a polymorphism at the stearoyl CoA desaturase locus and milk production traits in Italian Holsteins. J. Dairy Sci. 2008, 91, 3184–3189. [Google Scholar] [CrossRef]
- Alim, M.A.; Fan, Y.P.; Wu, X.P.; Xie, Y.; Zhang, Y.; Zhang, S.L.; Sun, D.X.; Zhang, Y.; Zhang, Q.; Liu, L.; et al. Genetic effects of stearoyl-coenzyme A desaturase (SCD) polymorphism on milk production traits in the Chinese dairy population. Mol. Biol. Rep. 2012, 39, 8733–8740. [Google Scholar] [CrossRef]
- Schennink, A.; Heck, J.M.; Bovenhuis, H.; Visker, M.H.; van Valenberg, H.J.; van Arendonk, J.A. Milk fatty acid unsaturation: Genetic parameters and effects of stearoyl-CoA desaturase (SCD1) and acyl CoA: Diacylglycerol acyltransferase 1 (DGAT1). J. Dairy Sci. 2008, 91, 2135–2143. [Google Scholar] [CrossRef]
| Primer Names | Primer Sequences (5′-3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|
| SCD1-1 | F: TAGTGGGTGACACATTCATAGC R: GGATTGCCTGGGAGGATGA | 833 | 55 |
| SCD1-2 | F: AATCATCCTCCCAGGCAATCC R: GCGTAAGAGGTTCAGCCAATG | 702 | 55 |
| SCD1-3 | F: CGGGTTTGAGGACACGTCT R: TTTATTCGTTGCCAACAAGGG | 599 | 55 |
| SCD1-4 | F: TGTGCAGCATCCAGTTCTTG R: AAGGCGGAAGACAGGGAAG | 781 | 54 |
| SCD1-5 | F: ATCTCTAGCTCCTACACAACCA R: AGCCCTCTAAAGTCACTCATCT | 704 | 54 |
| SCD1-6 | F: AGGTTAGCAGAAGGTCAGAGG R: AAGACCACAACAGCCAGACT | 746 | 55 |
| SCD1-7 | F: GCATTCCACTCACCACATAACC R: TTGTGCCTCTCCTCGCTATG | 936 | 55 |
| SCD1-8 | F: TCCTTGCTCCACCACTTCC R: CCACCCAGATGACCCTACTC | 832 | 55 |
| SCD1-9 | F: CATTCATTCAACAGCAACAGGT R: CAGGAGAGAAAGGGAGCATACT | 1002 | 54 |
| SCD1-10 | F: CTCCCTTTCTCTCCTGACTCTG R: CCATCACTGCCTCTGAATACAC | 1002 | 55 |
| SCD1-11 | F: TCACTGAACCACTGTTTCTCTT R: AAGGCATCCAGATAAGTTGTCA | 1203 | 51 |
| SCD1-12 | F: ATGCTGACAACTTATCTGGATG R: CAGGGCAATCAGATTCACTTT | 981 | 51 |
| Primer Names | SNP | Mutation Region | Number | Variation ID | Gene Position |
|---|---|---|---|---|---|
| SCD1-7 | A>G | intron3 | 2 | rs41255689 | 26:21270336 |
| A>G | intron4 | rs41255690 | 26:21270739 | ||
| SCD1-8 | A>G | exon5 | 4 | rs41255691 | 26:21272246 |
| T>C | exon5 | rs41255692 | 26:21272306 | ||
| C>T | exon5 | rs41255693 | 26:21272422 | ||
| A>G | intron5 | rs383175036 | 26:21272529 | ||
| SCD1-9 | G>T | exon6 | 3 | rs41255694 | 26:21275659 |
| G>C | exon6 | rs41255695 | 26:21275732 | ||
| C>A | exon6 | rs41255696 | 26:21275851 | ||
| SCD1-10 | C>T | exon6 | 2 | rs41255697 | 26:21276141 |
| A>G | exon6 | rs41255698 | 26:21276672 | ||
| SCD1-11 | A>G | exon6 | 6 | rs41255700 | 26:21277095 |
| C>T | exon6 | rs41255701 | 26:21277195 | ||
| G>A | exon6 | rs41255702 | 26:21277296 | ||
| T>G | exon6 | rs41255703 | 26:21277378 | ||
| G>A | exon6 | rs41255704 | 26:21277585 | ||
| G>A | exon6 | rs382676818 | 26:21277770 |
| SNP | g.21272246 A>G | g.21272306 T>C | g.21272422 C>T | g.21272529 A>G | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene position | 26:21272246 | 26:21272306 | 26:21272422 | 26:21272529 | ||||||||||||
| Mutation type | synonymous mutation | synonymous mutation | missense mutation | synonymous mutation | ||||||||||||
| Breed | Chinese Simmental steer n = 334 | Chinese Holstein cattle n = 73 | Chinese Simmental steer n = 334 | Chinese Holstein cattle n = 73 | Chinese Simmental steer n = 334 | Chinese Holstein cattle n = 73 | Chinese Simmental steer n = 334 | Chinese Holstein cattle n = 73 | ||||||||
| Genotype frequency | AA (123) | 0.368 | AA (33) | 0.452 | TT (127) | 0.380 | TT (33) | 0.452 | CC (128) | 0.383 | TT (33) | 0.452 | AA (127) | 0.380 | AA (33) | 0.452 |
| AG (138) | 0.413 | AG (36) | 0.493 | TC (136) | 0.407 | TC (36) | 0.493 | CT (137) | 0.410 | TC (36) | 0.493 | AG (137) | 0.410 | AG (36) | 0.493 | |
| GG (73) | 0.219 | GG (4) | 0.055 | CC (71) | 0.213 | CC (4) | 0.055 | TT (69) | 0.207 | CC (4) | 0.055 | GG (70) | 0.210 | GG (4) | 0.055 | |
| Allele frequency | A | 0.575 | A | 0.699 | T | 0.584 | T | 0.699 | C | 0.588 | C | 0.699 | A | 0.585 | A | 0.699 |
| G | 0.425 | G | 0.301 | C | 0.416 | C | 0.301 | T | 0.412 | T | 0.301 | G | 0.415 | G | 0.301 | |
| He | 0.413 | 0.493 | 0.407 | 0.493 | 0.410 | 0.493 | 0.410 | 0.493 | ||||||||
| PIC | 0.489 | 0.421 | 0.486 | 0.421 | 0.484 | 0.421 | 0.485 | 0.421 | ||||||||
| Ne | 1.704 | 1.972 | 1.686 | 1.972 | 1.695 | 1.972 | 1.695 | 1.972 | ||||||||
| Traits | g.21272246 A>G | g.21272306 T>C | g.21272422 C>T | g.21272529 A>G | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (n = 123) | AG (n = 123) | GG (n = 73) | TT (n = 123) | TC (n = 123) | CC (n = 71) | CC (n = 128) | CT (n = 123) | TT (n = 67) | AA (n = 127) | AG (n = 137) | GG (n = 70) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Carcass traits | LW1 (kg) | 487.73 ± 58.66 | 500.31 ± 58.54 | 487.52 ± 64.2 | 486.73 ± 57.83 b | 501.28 ± 57.29 a | 488.69 ± 67.38 a | 486.36 ± 57.65 | 501.78 ± 59.05 | 487.32 ± 64.67 | 486.24 ± 57.86 b | 502.41 ± 58.59 a | 486.30 ± 64.76 b |
| CW (kg) | 254.11 ± 35.34 b | 262.81 ± 36.77 a | 253.52 ± 41.81 b | 253.7 ± 35.03 b | 263.51 ± 36.22 a | 253.68 ± 43.05 b | 253.50 ± 34.95 b | 263.68 ± 37.05 a | 253.01 ± 41.89 b | 253.39 ± 35.07 b | 264.11 ± 36.75 a | 252.38 ± 41.92 b | |
| DP (%) | 52.02 ± 2.30 | 52.42 ± 2.15 | 51.82 ± 2.86 | 52.05 ± 2.31 | 52.46 ± 2.15 | 51.73 ± 2.85 | 52.04 ± 2.30 | 52.44 ± 2.15 | 51.74 ± 2.88 | 52.03 ± 2.30 | 52.47 ± 2.14 | 51.72 ± 2.86 | |
| CL (cm) | 140.52 ± 8.13 a | 139.42 ± 8.36 | 138.19 ± 7.90 b | 140.34 ± 8.15 a | 139.50 ± 8.23 | 138.23 ± 8.22 b | 140.44 ± 8.14 a | 139.57 ± 8.33 | 137.90 ± 7.90 b | 140.36 ± 8.12 a | 139.65 ± 8.38 | 137.91 ± 7.84 b | |
| CD (cm) | 64.78 ± 3.14 | 64.32 ± 3.48 | 64.14 ± 3.33 | 64.67 ± 3.09 | 64.38 ± 3.43 | 64.17 ± 3.56 | 64.73 ± 3.11 | 64.42 ± 3.49 | 63.99 ± 3.35 | 64.69 ± 3.07 | 64.45 ± 3.53 | 64.01 ± 3.34 | |
| CBD (cm) | 65.33 ± 3.95 | 65.45 ± 3.47 | 64.91 ± 3.62 | 65.21 ± 3.89 | 65.48 ± 3.38 | 65.03 ± 3.88 | 65.27 ± 3.90 | 65.53 ± 3.46 | 64.84 ± 3.69 | 65.23 ± 3.88 | 65.56 ± 3.49 | 64.87 ± 3.67 | |
| HLC (cm) | 48.72 ± 4.08 | 48.97 ± 3.45 | 49.06 ± 3.56 | 48.73 ± 4.03 | 48.99 ± 3.42 | 49.07 ± 3.68 | 48.72 ± 4.02 | 49.02 ± 3.45 | 48.97 ± 3.66 | 48.70 ± 4.03 | 49.03 ± 3.45 | 48.99 ± 3.63 | |
| HLW (cm) | 44.56 ± 2.92 | 44.85 ± 2.56 | 44.56 ± 2.71 | 44.49 ± 2.92 | 44.86 ± 2.51 | 44.73 ± 2.74 | 44.49 ± 2.94 | 44.87 ± 2.51 | 44.66 ± 2.72 | 44.46 ± 2.92 | 44.89 ± 2.53 | 44.66 ± 2.7 | |
| HLL (cm) | 80.22 ± 4.40 | 80.17 ± 4.01 | 80.01 ± 3.95 | 80.12 ± 4.47 | 80.12 ± 3.94 | 80.24 ± 3.95 | 80.19 ± 4.48 | 80.14 ± 3.92 | 80.11 ± 3.92 | 80.14 ± 4.46 | 80.16 ± 3.95 | 80.16 ± 3.92 | |
| TMT (cm) | 17.81 ± 1.81 | 17.88 ± 1.68 | 18.20 ± 1.58 | 17.79 ± 1.82 | 17.88 ± 1.69 | 18.29 ± 1.50 | 17.76 ± 1.81 | 17.91 ± 1.70 | 18.26 ± 1.48 | 17.77 ± 1.82 | 17.90 ± 1.70 | 18.25 ± 1.48 | |
| BFT (cm) | 0.93 ± 0.61 B | 1.10 ± 0.64 A | 0.93 ± 0.60 B | 0.96 ± 0.63 B | 1.10 ± 0.63 A | 0.87 ± 0.57 B | 0.94 ± 0.63 B | 1.10 ± 0.63 A | 0.89 ± 0.57 B | 0.95 ± 0.63 B | 1.10 ± 0.63 A | 0.88 ± 0.57 B | |
| WMT (cm) | 6.77 ± 0.92 b | 6.94 ± 0.85 a | 6.91 ± 0.93 ab | 6.78 ± 0.91 b | 6.97 ± 0.87 a | 6.89 ± 0.90 ab | 6.76 ± 0.91 b | 6.96 ± 0.87 a | 6.91 ± 0.90 ab | 6.77 ± 0.91 b | 6.97 ± 0.87 a | 6.89 ± 0.90 ab | |
| Meat quality traits | pH (0 h) | 6.33 ± 0.51 A | 6.19 ± 0.49 B | 6.16 ± 0.51 B | 6.31 ± 0.51 A | 6.18 ± 0.49 B | 6.18 ± 0.52 B | 6.32 ± 0.51 A | 6.19 ± 0.49 B | 6.17 ± 0.52 B | 6.32 ± 0.51 A | 6.19 ± 0.49 B | 6.17 ± 0.51 B |
| pH (24 h) | 5.60 ± 0.34 A | 5.55 ± 0.31 AB | 5.49 ± 0.37 B | 5.60 ± 0.34 A | 5.55 ± 0.31 AB | 5.49 ± 0.38 B | 5.60 ± 0.33 A | 5.55 ± 0.31 AB | 5.48 ± 0.38 B | 5.60 ± 0.33 A | 5.55 ± 0.31 AB | 5.49 ± 0.38 B | |
| MBS | 5.39 ± 0.72 ab | 5.26 ± 0.74 b | 5.48 ± 0.69 a | 5.40 ± 0.72 AB | 5.24 ± 0.75 B | 5.49 ± 0.67 A | 5.40 ± 0.71 ab | 5.26 ± 0.75 b | 5.48 ± 0.68 a | 5.40 ± 0.72 ab | 5.25 ± 0.75 b | 5.49 ± 0.68 a | |
| MCS | 5.72 ± 1.08 | 5.57 ± 1.08 | 5.64 ± 1.12 | 5.70 ± 1.08 | 5.62 ± 1.10 | 5.58 ± 1.10 | 5.70 ± 1.07 | 5.61 ± 1.09 | 5.59 ± 1.12 | 5.70 ± 1.07 | 5.61 ± 1.09 | 5.59 ± 1.11 | |
| REA (cm2) | 79.28 ± 13.44 ab | 81.30 ± 12.56 a | 77.23 ± 11.88 b | 79.21 ± 13.28 ab | 81.12 ± 12.69 a | 77.73 ± 12.11 b | 79.18 ± 13.17 ab | 81.07 ± 12.8 a | 77.78 ± 11.98 b | 79.19 ± 13.23 ab | 81.20 ± 12.68 a | 77.53 ± 12.08 b | |
| Traits | g.21272246 A>G | g.21272306 T>C | g.21272422 C>T | g.21272529 A>G | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (n = 123) | AG (n= 138) | GG (n = 73) | TT (n = 127) | TC (n = 136) | CC (n = 71) | CC (n = 128) | CT (n = 137) | TT (n = 69) | AA (n = 127) | AG (n = 137) | GG (n = 70) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Adipogenesis traits | MBS | 5.39 ± 0.72 ab | 5.26 ± 0.74 b | 5.48 ± 0.69 a | 5.40 ± 0.72 AB | 5.24 ± 0.75 B | 5.49 ± 0.67 A | 5.40 ± 0.71 ab | 5.26 ± 0.75 b | 5.48 ± 0.68 a | 5.40 ± 0.72 AB | 5.25 ± 0.75 B | 5.49 ± 0.68 A |
| FCS | 2.79 ± 1.03 a | 2.77 ± 0.87 a | 2.53 ± 1.04 b | 2.79 ± 1.02 a | 2.75 ± 0.85 | 2.55 ± 1.08 b | 2.80 ± 1.02 A | 2.77 ± 0.87 a | 2.49 ± 1.04 B,b | 2.80 ± 1.02 a | 2.76 ± 0.85 a | 2.53 ± 1.07 b | |
| BFT (cm) | 0.93 ± 0.61 B | 1.10 ± 0.64 A | 0.93 ± 0.60 B | 0.96 ± 0.63 B | 1.10 ± 0.63 A | 0.87 ± 0.57 B | 0.94 ± 0.63 B | 1.10 ± 0.63 A | 0.89 ± 0.57 B | 0.95 ± 0.63 B | 1.10 ± 0.63 A | 0.88 ± 0.57 B | |
| FCR% | 47.88 ± 21.66 b | 50.44 ± 20.91 a | 51.16 ± 19.93 a | 48.16 ± 21.5 B | 51.21 ± 20.99 A | 49.8 ± 19.78 AB | 47.7 ± 21.65 B | 51.09 ± 20.95 A | 50.43 ± 19.67 AB | 47.91 ± 21.60 b | 50.99 ± 21.07 a | 50.21 ± 19.6 ab | |
| RRAW (kg) | 7.50 ± 0.98 | 7.47 ± 0.92 | 7.55 ± 0.96 | 7.48 ± 0.98 | 7.45 ± 0.87 | 7.62 ± 1.04 | 7.49 ± 0.98 | 7.47 ± 0.91 | 7.57 ± 0.97 | 7.48 ± 0.98 | 7.48 ± 0.91 | 7.57 ± 0.96 | |
| HW1 (kg) | 23.53 ± 2.39 | 23.84 ± 2.34 | 23.15 ± 2.58 | 23.39 ± 2.33 | 23.86 ± 2.26 | 23.27 ± 2.76 | 23.44 ± 2.36 | 23.89 ± 2.34 | 23.18 ± 2.64 | 23.43 ± 2.37 | 23.92 ± 2.33 | 23.16 ± 2.62 | |
| FHW (kg) | 5.90 ± 0.62 b | 6.04 ± 0.71 a | 5.86 ± 0.71 b | 5.87 ± 0.62 b | 6.06 ± 0.69 a | 5.88 ± 0.74 b | 5.87 ± 0.62 b | 6.07 ± 0.70 a | 5.86 ± 0.71 b | 5.87 ± 0.62 b | 6.07 ± 0.7 a | 5.86 ± 0.71 b | |
| HHW (kg) | 3.50 ± 1.09 | 3.40 ± 0.95 | 3.38 ± 0.98 | 3.47 ± 1.08 | 3.39 ± 0.93 | 3.44 ± 1.03 | 3.49 ± 1.09 | 3.04 ± 0.94 | 3.39 ± 0.99 | 3.48 ± 1.09 | 3.41 ± 0.95 | 3.40 ± 0.99 | |
| OmW (kg) | 4.02 ± 0.76 | 3.93 ± 0.69 | 3.88 ± 0.66 | 3.99 ± 0.75 | 3.92 ± 0.66 | 3.94 ± 0.74 | 4.00 ± 0.75 | 3.94 ± 0.70 | 3.90 ± 0.67 | 4.00 ± 0.75 | 3.94 ± 0.70 | 3.90 ± 0.67 | |
| HW2 (kg) | 1.78 ± 0.31 B | 1.88 ± 0.35 A | 1.78 ± 0.33 B | 1.77 ± 0.31 B | 1.89 ± 0.35 A | 1.78 ± 0.33 B | 1.77 ± 0.30 B | 1.88 ± 0.35 A | 1.78 ± 0.33 B | 1.77 ± 0.31 B | 1.89 ± 0.35 A | 1.77 ± 0.33 B | |
| SW (kg) | 0.86 ± 0.17 | 0.87 ± 0.20 | 0.83 ± 0.20 | 0.86 ± 0.17 | 0.87 ± 0.19 | 0.83 ± 0.20 | 0.86 ± 0.17 b | 0.87 ± 0.20 a | 0.82 ± 0.20 b | 0.86 ± 0.170 b | 0.87 ± 0.20 a | 0.82 ± 0.20 b | |
| LTW (kg) | 3.09 ± 0.42 B | 3.22 ± 0.49 A | 3.14 ± 0.51 AB | 3.09 ± 0.42 B | 3.24 ± 0.49 A | 3.14 ± 0.51 AB | 3.09 ± 0.42 B | 3.23 ± 0.49 A | 3.14 ± 0.52 AB | 3.08 ± 0.42 B | 3.24 ± 0.49 A | 3.14 ± 0.51 AB | |
| KW (kg) | 1.15 ± 0.18 B | 1.20 ± 0.22 A | 1.16 ± 0.21 AB | 1.15 ± 0.18 B | 1.20 ± 0.22 A | 1.17 ± 0.21 AB | 1.15 ± 0.18 B | 1.20 ± 0.22 A | 1.17 ± 0.21 AB | 1.15 ± 0.18 B | 1.20 ± 0.22 A | 1.17 ± 0.21 AB | |
| RAW (kg) | 4.58 ± 2.70 | 4.98 ± 2.80 | 4.81 ± 2.87 | 4.57 ± 2.67 B | 5.11 ± 2.83 A | 4.63 ± 2.84 AB | 4.53 ± 2.67 B | 5.09 ± 2.82 A | 4.71 ± 2.84 AB | 4.55 ± 2.67 B | 5.09 ± 2.83 A | 4.67 ± 2.84 AB | |
| CPW (kg) | 0.45 ± 0.09 A | 0.44 ± 0.08 AB | 0.42 ± 0.08 B | 0.45 ± 0.09 A | 0.44 ± 0.08 AB | 0.42 ± 0.09 B | 0.45 ± 0.09 A | 0.44 ± 0.08 AB | 0.42 ± 0.09 B | 0.45 ± 0.09 A | 0.44 ± 0.08 AB | 0.42 ± 0.09 B | |
| TaW (kg) | 42.22 ± 6.64 | 42.47 ± 5.98 | 41.03 ± 5.90 | 42.12 ± 6.71 | 42.31 ± 5.70 | 41.41 ± 6.32 | 42.27 ± 6.8 | 42.34 ± 5.74 | 41.10 ± 6.01 | 42.16 ± 6.70 | 42.43 ± 5.87 | 41.15 ± 5.98 | |
| TeW (kg) | 0.63 ± 0.12 | 0.61 ± 0.13 | 0.57 ± 0.11 | 0.67 ± 0.14 | 0.68 ± 0.15 | 0.65 ± 0.15 | 0.67 ± 0.14 | 0.68 ± 0.15 | 0.66 ± 0.15 | 0.67 ± 0.14 | 0.68 ± 0.15 | 0.65 ± 0.15 | |
| GFW (kg) | 0.93 ± 0.38 | 0.89 ± 0.34 | 0.85 ± 0.34 | 0.91 ± 0.37 | 0.89 ± 0.34 | 0.86 ± 0.35 | 0.92 ± 0.37 | 0.89 ± 0.34 | 0.85 ± 0.34 | 0.92 ± 0.37 | 0.89 ± 0.34 | 0.85 ± 0.34 | |
| OxW (kg) | 1.34 ± 0.22 | 1.38 ± 0.26 | 1.35 ± 0.27 | 1.33 ± 0.22 b | 1.39 ± 0.26 a | 1.34 ± 0.26 ab | 1.33 ± 0.22 b | 1.39 ± 0.27 a | 1.34 ± 0.25 ab | 1.33 ± 0.22 b | 1.40 ± 0.26 a | 1.33 ± 0.26 b | |
| BW (kg) | 20.24 ± 3.03 | 20.23 ± 3.22 | 19.77 ± 2.98 | 20.11 ± 3.04 | 20.27 ± 3.16 | 19.92 ± 3.12 | 20.14 ± 3.04 | 20.28 ± 3.20 | 19.84 ± 3.00 | 20.11 ± 3.03 | 20.32 ± 3.21 | 19.81 ± 2.99 | |
| Fatty acid composition | Myristic acid | 0.020 ± 0.017 | 0.020 ± 0.017 | 0.025 ± 0.018 | 0.020 ± 0.017 | 0.019 ± 0.017 | 0.027 ± 0.020 | 0.02 ± 0.017 | 0.021 ± 0.018 | 0.025 ± 0.019 | 0.020 ± 0.017 | 0.020 ± 0.018 | 0.025 ± 0.019 |
| Myristoleic acid | 0.002 ± 0.004 | 0.002 ± 0.004 | 0.003 ± 0.004 | 0.002 ± 0.006 | 0.002 ± 0.004 | 0.003 ± 0.004 | 0.002 ± 0.006 | 0.002 ± 0.004 | 0.003 ± 0.004 | 0.002 ± 0.006 | 0.002 ± 0.004 | 0.003 ± 0.004 | |
| Palmitic acid | 0.265 ± 0.223 | 0.246 ± 0.179 | 0.309 ± 0.183 | 0.264 ± 0.221 | 0.237 ± 0.174 | 0.321 ± 0.188 | 0.261 ± 0.220 | 0.250 ± 0.180 | 0.313 ± 0.186 | 0.264 ± 0.221 | 0.247 ± 0.181 | 0.309 ± 0.183 | |
| Palmitoleic acid | 0.031 ± 0.044 | 0.024 ± 0.020 | 0.031 ± 0.018 | 0.031 ± 0.044 | 0.024 ± 0.020 | 0.031 ± 0.018 | 0.03 ± 0.043 | 0.024 ± 0.020 | 0.031 ± 0.019 | 0.031 ± 0.044 | 0.024 ± 0.020 | 0.031 ± 0.018 | |
| Margaric acid | 0.011 ± 0.007 | 0.011 ± 0.007 | 0.013 ± 0.008 | 0.011 ± 0.007 | 0.011 ± 0.007 | 0.014 ± 0.008 | 0.011 ± 0.007 | 0.012 ± 0.008 | 0.014 ± 0.008 | 0.011 ± 0.007 | 0.012 ± 0.008 | 0.013 ± 0.008 | |
| Heptadecenoic acid | 0.006 ± 0.009 | 0.004 ± 0.006 | 0.005 ± 0.005 | 0.006 ± 0.009 | 0.004 ± 0.006 | 0.006 ± 0.005 | 0.006 ± 0.009 | 0.004 ± 0.006 | 0.006 ± 0.005 | 0.006 ± 0.009 | 0.004 ± 0.006 | 0.005 ± 0.005 | |
| Stearic acid | 0.183 ± 0.109 | 0.186 ± 0.119 | 0.227 ± 0.126 | 0.182 ± 0.108 b | 0.178 ± 0.105 b | 0.241 ± 0.140 a | 0.181 ± 0.108 | 0.189 ± 0.120 | 0.231 ± 0.128 | 0.182 ± 0.108 | 0.188 ± 0.120 | 0.227 ± 0.126 | |
| Oleic acid | 0.401 ± 0.541 | 0.332 ± 0.233 | 0.388 ± 0.201 | 0.400 ± 0.535 | 0.321 ± 0.230 | 0.401 ± 0.207 | 0.395 ± 0.530 | 0.333 ± 0.234 | 0.393 ± 0.204 | 0.400 ± 0.535 | 0.331 ± 0.235 | 0.387 ± 0.201 | |
| Linoleic acid | 0.096 ± 0.025 B | 0.101 ± 0.026 B | 0.123 ± 0.043 A | 0.097 ± 0.025 B | 0.098 ± 0.023 B | 0.126 ± 0.045 A | 0.097 ± 0.024 B | 0.101 ± 0.027 B | 0.123 ± 0.044 A | 0.097 ± 0.025 B | 0.100 ± 0.027 B | 0.123 ± 0.043 A | |
| α-linolenic acid | 0.004 ± 0.005 B | 0.006 ± 0.005 AB | 0.009 ± 0.012 A | 0.005 ± 0.005 B | 0.006 ± 0.005 AB | 0.009 ± 0.011 A | 0.004 ± 0.005 B | 0.006 ± 0.005 AB | 0.010 ± 0.012 A | 0.005 ± 0.005 B | 0.006 ± 0.005 AB | 0.009 ± 0.012 A | |
| Traits | g.21272246 A>G | g.21272306 T>C | g.21272422 C>T | g.21272529 A>G | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (n = 33) | AG (n = 36) | GG (n = 4) | TT (n = 33) | TC (n = 36) | CC (n = 4) | CC (n = 33) | CT (n = 36) | TT (n = 4) | AA (n = 33) | AG (n = 36) | GG (n = 4) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Average milk yield (kg/day) | 29.38 ± 5.71 ab | 26.36 ± 6.44 b | 31.86 ± 7.54 a | 29.38 ± 5.71 ab | 26.36 ± 6.44 b | 31.86 ± 7.54 a | 29.38 ± 5.71 ab | 26.36 ± 6.44 b | 31.86 ± 7.54 a | 29.38 ± 5.71 | 26.36 ± 6.44 b | 31.86 ± 7.54 a |
| Milk fat content (%) | 4.59 ± 0.38 a | 4.53 ± 0.48 ab | 4.15 ± 0.47 b | 4.59 ± 0.38 a | 4.53 ± 0.48 ab | 4.15 ± 0.47 b | 4.59 ± 0.38 a | 4.53 ± 0.48 ab | 4.15 ± 0.47 b | 4.59 ± 0.38 a | 4.53 ± 0.48 ab | 4.15 ± 0.47 b |
| Milk protein content (%) | 3.31 ± 0.23 | 3.29 ± 0.34 | 3.22 ± 0.25 | 3.31 ± 0.23 | 3.29 ± 0.34 | 3.22 ± 0.25 | 3.31 ± 0.23 | 3.29 ± 0.34 | 3.22 ± 0.25 | 3.31 ± 0.23 | 3.29 ± 0.34 | 3.22 ± 0.25 |
| Milk lactose (%) | 4.62 ± 0.26 | 4.52 ± 0.34 | 4.42 ± 0.36 | 4.62 ± 0.26 | 4.52 ± 0.34 | 4.42 ± 0.36 | 4.62 ± 0.26 | 4.52 ± 0.34 | 4.42 ± 0.36 | 4.62 ± 0.26 | 4.52 ± 0.34 | 4.42 ± 0.36 |
| Dry matter intake (kg) | 13.42 ± 0.92 | 13.29 ± 1.12 | 12.94 ± 0.87 | 13.42 ± 0.92 | 13.29 ± 1.12 | 12.94 ± 0.87 | 13.42 ± 0.92 | 13.29 ± 1.12 | 12.94 ± 0.87 | 13.42 ± 0.92 | 13.29 ± 1.12 | 12.94 ± 0.87 |
| Milk urea-nitrogen (mg/dl) | 18.7 ± 2.10 | 18.37 ± 1.74 | 18.07 ± 3.20 | 18.7 ± 2.10 | 18.37 ± 1.74 | 18.07 ± 3.20 | 18.7 ± 2.10 | 18.37 ± 1.74 | 18.07 ± 3.20 | 18.7 ± 2.10 | 18.37 ± 1.74 | 18.07 ± 3.20 |
| Traits | Haplotypes Combination | ||||
|---|---|---|---|---|---|
| H1H1 (n = 121) | H1H2 (n = 131) | H1H3 (n = 5) | H2H2 (n = 68) | H2H3 (n = 2) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| LW1 (kg) | 486.81 ± 58.71 | 499.98 ± 57.88 | 477.70 ± 42.14 | 486.54 ± 64.82 | 543.00 ± 4.36 |
| CW (kg) | 253.68 ± 35.46 | 262.68 ± 36.55 | 248.84 ± 30.65 | 252.54 ± 42.01 | 294.83 ± 10.80 |
| DP (%) | 52.03 ± 2.31 | 52.43 ± 2.15 | 51.99 ± 2.70 | 51.72 ± 2.90 | 54.29 ± 1.64 |
| BW (kg) | 20.21 ± 3.03 | 20.21 ± 3.18 | 18.60 ± 2.30 | 19.84 ± 3.02 | 20.33 ± 0.58 |
| CL (cm) | 140.43 ± 8.13 | 139.17 ± 8.16 | 139.60 ± 9.32 | 137.87 ± 7.95 | 146.00 ± 5.29 |
| CD (cm) | 64.72 ± 3.10 | 64.21 ± 3.35 | 63.60 ± 2.88 | 63.98 ± 3.38 | 66.67 ± 1.53 |
| CBD (cm) | 65.28 ± 3.93 | 65.37 ± 3.36 | 64.40 ± 2.79 | 64.84 ± 3.72 | 66.50 ± 0.87 |
| HLW (cm) | 44.52 ± 2.92 | 44.82 ± 2.51 | 43.80 ± 2.80 | 44.64 ± 2.74 | 44.17 ± 1.26 |
| HLC (cm) | 48.71 ± 4.10 | 48.94 ± 3.47 | 48.10 ± 2.30 | 49.01 ± 3.67 | 49.67 ± 1.44 |
| HLL (cm) | 80.17 ± 4.36 | 80.05 ± 3.88 | 80.80 ± 6.41 | 80.14 ± 3.94 | 78.17 ± 2.02 |
| WMT (cm) | 6.77 ± 0.92 | 6.96 ± 0.86 | 6.70 ± 0.72 | 6.91 ± 0.90 | 7.67 ± 1.31 |
| TMT (cm) | 17.8 ± 1.83 | 17.88 ± 1.67 | 17.60 ± 1.47 | 18.26 ± 1.49 | 18.37 ± 3.01 |
| HW1 (kg) | 23.48 ± 2.38 | 23.82 ± 2.28 | 22.61 ± 1.97 | 23.14 ± 2.63 | 24.49 ± 1.48 |
| FHW (kg) | 5.89 ± 0.62 | 6.05 ± 0.70 | 5.46 ± 0.50 | 5.86 ± 0.72 | 6.21 ± 0.36 |
| HHW (kg) | 3.50 ± 1.09 | 3.37 ± 0.92 | 3.33 ± 1.08 | 3.40 ± 0.99 | 2.94 ± 0.29 |
| TaW (kg) | 42.16 ± 6.66 | 42.18 ± 5.63 | 43.74 ± 8.10 | 41.07 ± 6.05 | 40.94 ± 1.59 |
| BFT (cm) | 0.93 ± 0.61 | 1.10 ± 0.62 | 1.22 ± 0.99 | 0.89 ± 0.57 | 1.60 ± 0.78 |
| FCR% | 48.01 ± 21.66 b | 51.18 ± 20.67 b | 43.20 ± 23.50 b | 50.29 ± 19.78 b | 73.33 ± 16.07 a |
| GFW (kg) | 0.92 ± 0.37 | 0.88 ± 0.34 | 0.78 ± 0.33 | 0.85 ± 0.35 | 0.75 ± 0.41 |
| RRAW (kg) | 7.49 ± 0.98 | 7.44 ± 0.86 | 7.42 ± 1.21 | 7.58 ± 0.98 | 7.23 ± 0.89 |
| OmW (kg) | 4.01 ± 0.76 | 3.91 ± 0.67 | 3.75 ± 0.51 | 3.88 ± 0.67 | 3.85 ± 0.52 |
| HW2 (kg) | 1.77 ± 0.31 | 1.88 ± 0.35 | 1.72 ± 0.19 | 1.77 ± 0.33 | 2.10 ± 0.02 |
| LW2 (kg) | 5.87 ± 1.05 | 6.12 ± 1.10 | 5.54 ± 0.78 | 5.84 ± 1.06 | 6.37 ± 0.59 |
| SW (kg) | 0.86 ± 0.18 | 0.87 ± 0.20 | 0.80 ± 0.10 | 0.82 ± 0.20 | 0.95 ± 0.10 |
| LTW (kg) | 3.09 ± 0.42 | 3.23 ± 0.50 | 2.93 ± 0.34 | 3.14 ± 0.51 | 3.46 ± 0.42 |
| KW (kg) | 1.15 ± 0.18 | 1.21 ± 0.23 | 1.06 ± 0.19 | 1.17 ± 0.21 | 1.08 ± 0.13 |
| RAW (kg) | 4.58 ± 2.68 b | 5.10 ± 2.80 b | 3.28 ± 1.92 b | 4.66 ± 2.83 b | 8.23 ± 0.89 a |
| CPW (kg) | 0.45 ± 0.09 | 0.43 ± 0.08 | 0.46 ± 0.16 | 0.42 ± 0.09 | 0.44 ± 0.06 |
| TeW (kg) | 0.67 ± 0.14 | 0.68 ± 0.15 | 0.69 ± 0.10 | 0.65 ± 0.15 | 0.60 ± 0.30 |
| OxW (kg) | 1.34 ± 0.23 b | 1.39 ± 0.26 b | 1.25 ± 0.12 b | 1.34 ± 0.26 b | 1.75 ± 0.11 a |
| pH (0 h) | 6.33 ± 0.51 | 6.18 ± 0.50 | 6.14 ± 0.50 | 6.17 ± 0.52 | 5.86 ± 0.19 |
| pH (24 h) | 5.60 ± 0.34 | 5.55 ± 0.31 | 5.52 ± 0.22 | 5.49 ± 0.37 | 5.35 ± 0.23 |
| MBS | 5.39 ± 0.72 | 5.24 ± 0.75 | 5.60 ± 0.55 | 5.49 ± 0.68 | 5.00 ± 1.00 |
| MCS | 5.71 ± 1.08 b | 5.60 ± 1.09 b | 5.20 ± 0.84 b | 5.57 ± 1.11 b | 7.00 ± 0.00 a |
| FCS | 2.80 ± 1.02 | 2.75 ± 0.85 | 2.80 ± 1.10 | 2.51 ± 1.03 | 2.33 ± 0.58 |
| REA (cm2) | 79.36 ± 13.48 | 81.42 ± 12.79 | 76.6 ± 5.68 | 77.71 ± 12.05 | 74.33 ± 5.77 |
| Fatty Acid Composition (g/100 g) | Haplotypes | |||
|---|---|---|---|---|
| H1H1 (n = 43) | H1H2 (n = 35) | H1H3 (n = 3) | H2H2 (n = 21) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Myristic acid | 0.020 ± 0.018 | 0.019 ± 0.017 | 0.017 ± 0.009 | 0.025 ± 0.019 |
| Myristoleic acid | 0.002 ± 0.006 | 0.002 ± 0.004 | 0.003 ± 0.004 | 0.003 ± 0.004 |
| Palmitic acid | 0.267 ± 0.226 | 0.241 ± 0.175 | 0.199 ± 0.097 | 0.313 ± 0.186 |
| Palmitoleic acid | 0.031 ± 0.045 | 0.024 ± 0.020 | 0.023 ± 0.018 | 0.031 ± 0.019 |
| Margaric acid | 0.011 ± 0.007 | 0.011 ± 0.007 | 0.009 ± 0.001 | 0.014 ± 0.008 |
| Heptadecenoic acid | 0.006 ± 0.009 | 0.004 ± 0.006 | 0.003 ± 0.004 | 0.006 ± 0.005 |
| Stearic acid | 0.184 ± 0.110 | 0.179 ± 0.106 | 0.136 ± 0.037 | 0.231 ± 0.128 |
| Oleic acid | 0.405 ± 0.547 | 0.325 ± 0.233 | 0.297 ± 0.166 | 0.393 ± 0.204 |
| Linoleic acid | 0.096 ± 0.025 B | 0.098 ± 0.023 B | 0.099 ± 0.001 AB | 0.123 ± 0.044 A |
| α-linolenic acid | 0.004 ± 0.005 B | 0.006 ± 0.005 AB | 0.005 ± 0.007 AB | 0.010 ± 0.012 A |
| Arachic acid | 0.000 ± 0.001 | 0.001 ± 0.005 | 0.000 ± 0.000 | 0.001 ± 0.002 |
| Eicosanic acid | 0.001 ± 0.003 | 0.001 ± 0.002 | 0.000 ± 0.000 | 0.001 ± 0.001 |
| Dihomo-γ-linolenic acid | 0.010 ± 0.003 | 0.010 ± 0.003 | 0.009 ± 0.001 | 0.010 ± 0.003 |
| Arachidonic acid | 0.049 ± 0.013 | 0.048 ± 0.011 | 0.058 ± 0.012 | 0.054 ± 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Fang, X.; Lu, X.; Liu, Y.; Li, Y.; Bai, X.; Ding, X.; Yang, R. Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle. Animals 2024, 14, 1759. https://doi.org/10.3390/ani14121759
Liu R, Fang X, Lu X, Liu Y, Li Y, Bai X, Ding X, Yang R. Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle. Animals. 2024; 14(12):1759. https://doi.org/10.3390/ani14121759
Chicago/Turabian StyleLiu, Ruimin, Xibi Fang, Xin Lu, Yue Liu, Yue Li, Xue Bai, Xiangbin Ding, and Runjun Yang. 2024. "Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle" Animals 14, no. 12: 1759. https://doi.org/10.3390/ani14121759
APA StyleLiu, R., Fang, X., Lu, X., Liu, Y., Li, Y., Bai, X., Ding, X., & Yang, R. (2024). Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle. Animals, 14(12), 1759. https://doi.org/10.3390/ani14121759







