The Impact of Avian Haemosporidian Infection on Feather Quality and Feather Growth Rate of Migratory Passerines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Species
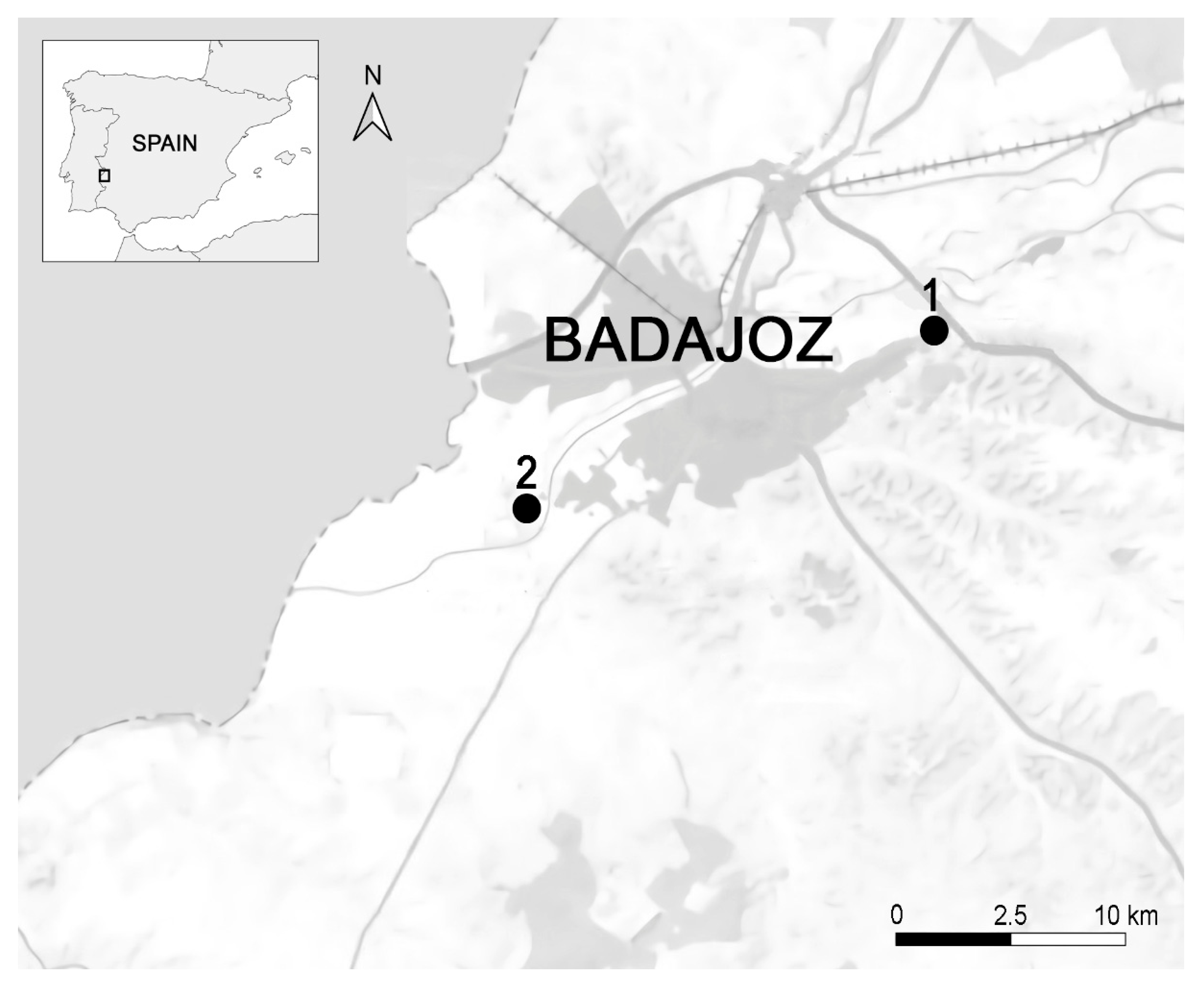
2.2. Study Area and Bird Sampling
2.3. Measurement of Feather Growth Rate and Determination of Feather Quality Index
2.4. Uropygial Gland Volume
2.5. Molecular Detection of Haemosporidian Infection
2.6. Statistical Analysis
3. Results
3.1. Haemosporidian Prevalence
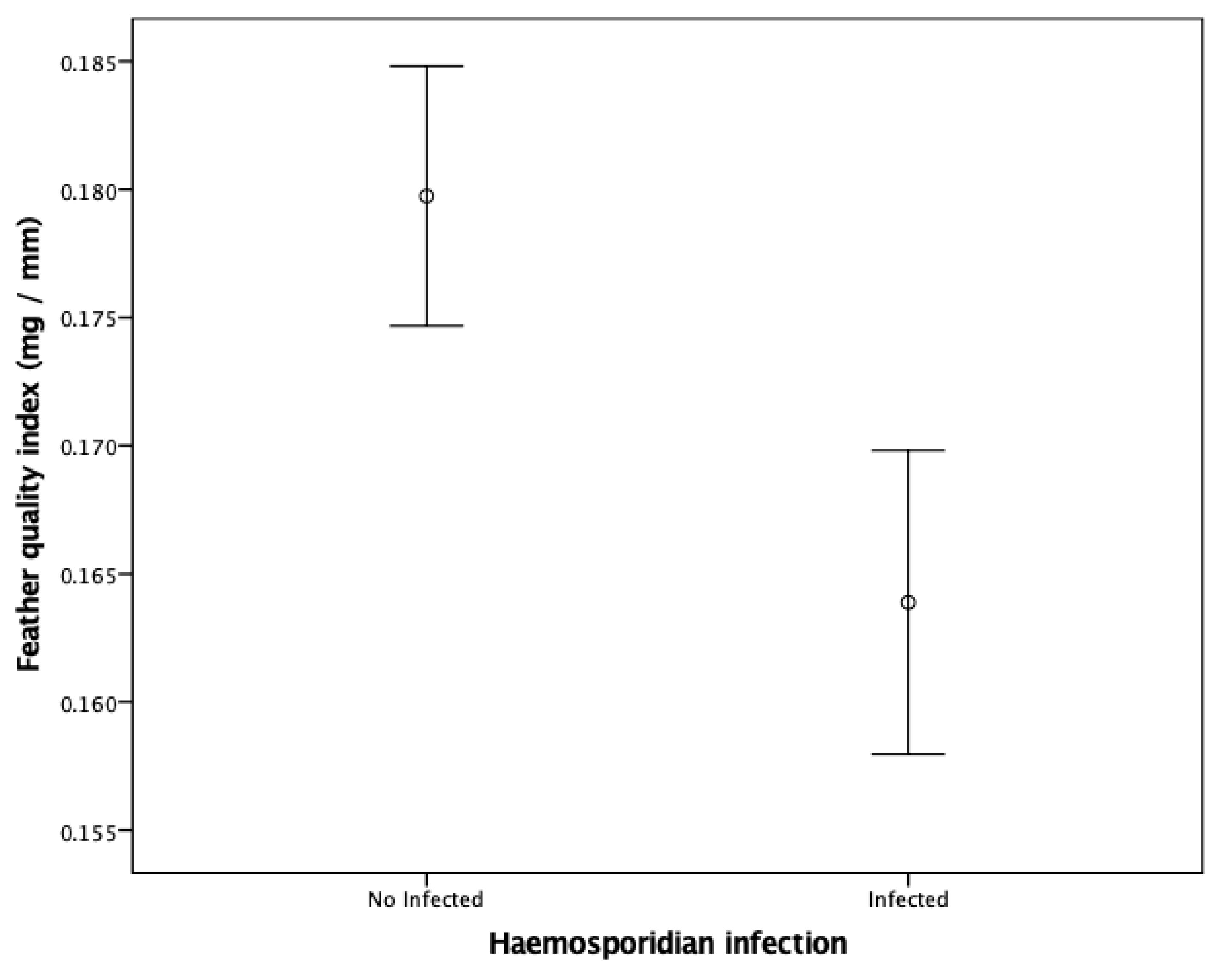
3.2. Factors Explaining Variation in Feather Quality Index
3.3. Factors Explaining Variation in Feather Growth Rate
4. Discussion
4.1. Differences in Haemosporidian Prevalence between Bird Species
4.2. Factors Influencing Feather Quality Index
4.3. Factors Influencing Feather Growth Rate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenni, L.; Winkler, R. Moult and Ageing of European Passerines, 2nd ed.; Bloomsbury Publishing: London, UK, 2020. [Google Scholar]
- Terrill, R.S.; Shultz, A.J. Feather function and the evolution of birds. Biol. Rev. 2023, 98, 540–566. [Google Scholar] [CrossRef]
- Hill, G.E.; McGraw, K.J. Bird Coloration: Function and Evolution; Harvard University Press: Cambridge, UK, 2006; Volume 2. [Google Scholar]
- Rijke, A.M.; Jesser, W.A.; Evans, S.W.; Bouwman, H. Water repellency and feather structure of the blue swallow Hirundo atrocaerulea. Ostrich 2000, 71, 143–145. [Google Scholar] [CrossRef]
- Sato, K.; Naito, Y.; Kato, A.; Niizuma, Y.; Watanuki, Y.; Charrassin, J.B.; Bost, C.A.; Handrich, Y.; Le Maho, Y. Buoyancy and maximal diving depth in penguins: Do they control inhaling air volume? J. Exp. Biol. 2002, 205, 1189–1197. [Google Scholar] [CrossRef]
- Hausmann, L.; von Campenhausen, M.; Endler, F.; Singheiser, M.; Wagner, H. Improvements of sound localization abilities by the facial ruff of the barn owl (Tyto alba) as demonstrated by virtual ruff removal. PLoS ONE 2009, 4, e7721. [Google Scholar] [CrossRef] [PubMed]
- Jenni, L.; Winkler, R. The Biology of Moult in Birds; Bloomsbury Publishing: London, UK, 2020. [Google Scholar]
- Holmgren, N.; Hedenström, A. The scheduling of molt in migratory birds. Evol. Ecol. 1995, 9, 354–368. [Google Scholar] [CrossRef]
- Bridge, E.S. Mind the gaps: What’s missing in our understanding of feather molt. Condor 2011, 113, 1–4. [Google Scholar] [CrossRef]
- Lind, J. Escape Flight in Moulting Tree Sparrows (Passer montanus). Funct. Ecol. 2001, 15, 29–35. [Google Scholar]
- Buttemer, W.A.; Bauer, S.; Emmenegger, T.; Dimitrov, D.; Peev, S.; Hahn, S. Moult-related reduction of aerobic scope in passerine birds. J. Comp. Physiol. B 2019, 189, 463–470. [Google Scholar] [CrossRef] [PubMed]
- de La Hera, I.; Schaper, S.V.; Díaz, J.A.; Pérez-Tris, J.; Bensch, S.; Tellería, J.L. How Much Variation in the Molt Duration of Passerines Can Be Explained by the Growth Rate of Tail Feathers? Auk 2011, 128, 321–329. [Google Scholar] [CrossRef]
- Bonser, R.H.C. Melanin and the Abrasion Resistance of Feathers. Condor 1995, 97, 590–591. [Google Scholar]
- Pennycuick, C.J. Mechanics of flight. In Avian Biology; Farner, D.S., King, J.R., Eds.; Academic Press: New York, NY, USA, 1975; Volume 5. [Google Scholar]
- Norberg, U.M. Vertebrate Flight: Mechanics, Physiology, Morphology, Ecology and Evolution; Springer—Verlag: Berlin, Germany, 1990. [Google Scholar]
- Vágási, C.I.; Pap, P.L.; Tökölyi, J.; Székely, E.; Barta, Z. Correlates of variation in flight feather quality in the great tit Parus major. Ardea 2011, 99, 53–60. [Google Scholar] [CrossRef]
- Rohwer, V.G.; Rohwer, S. How do birds adjust the time required to replace their flight feathers? Auk 2013, 130, 699–707. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Helfenstein, F.; Vallat, A.; Glauser, G.; Jenni, L. Corticosterone: Effects on feather quality and deposition into feathers. Methods Ecol. Evol. 2015, 6, 237–246. [Google Scholar] [CrossRef]
- Sarasola, J.H.; Jovani, R. Risk of feather damage explains fault bar occurrence in a migrant hawk, the Swainson’s hawk Buteo swainsoni. J. Avian Biol. 2006, 37, 29–35. [Google Scholar] [CrossRef]
- Møller, A.P.; Erritzøe, J.; Nielsen, J.T. Frequency of fault bars in feathers of birds and susceptibility to predation. Biol. J. Linn. Soc. 2009, 97, 334–345. [Google Scholar] [CrossRef]
- Matyjasiak, P.; Boniecki, P.; Fuszara, M.; Okołowski, M.; Olejniczak, I. Feather holes and flight performance in the barn swallow Hirundo rustica. Anim. Cells Syst. 2018, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Vágási, C.I. The origin of feather holes: A word of caution. J. Avian Biol. 2014, 45, 431–436. [Google Scholar] [CrossRef]
- Valkiunas, G. Avian Malaria Parasites and other Haemosporidia, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Santiago-Alarcon, S.; Marzal, A. Avian Malaria and Related Parasites in the Tropics; Springer Nature Switzerland: Cham, Swizterland, 2020. [Google Scholar]
- Valkiūnas, G.; Atkinson, C.T. Introduction to life cycles, taxonomy, distribution, and basic research techniques. In Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2020; pp. 45–80. [Google Scholar]
- Rivero, A.; Gandon, S. Evolutionary Ecology of Avian Malaria: Past to Present. Trends Parasitol. 2018, 34, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Martínez-de la Puente, J.; Merino, S.; Tomás, G.; Moreno, J.; Morales, J.; Lobato, E.; García-Fraile, S.; Belda, E.J. The blood parasite Haemoproteus reduces survival in a wild bird: A medication experiment. Biol. Lett. 2010, 6, 663–665. [Google Scholar] [CrossRef]
- Palinauskas, V.; Žiegytė, R.; Ilgūnas, M.; Iezhova, T.A.; Bernotienė, R.; Bolshakov, C.; Valkiūnas, G. Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int. J. Parasitol. 2015, 45, 51–62. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.A.; Dinhopl, N.; Nedorost, N.; Weissenbacher-Lang, C.; Weissenböck, H.; Valkiūnas, G. Mortality and pathology in birds due to Plasmodium (Giovannolaia) homocircumflexum infection, with emphasis on the exoerythrocytic development of avian malaria parasites. Malar. J. 2016, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Balbontín, J.; Reviriego, M.; García-Longoria, L.; Relinque, C.; Hermosell, I.G.; Magallanes, S.; López-Calderón, C.; de Lope, F.; Møller, A.P. A longitudinal study of age-related changes in Haemoproteus infection in a passerine bird. Oikos 2016, 125, 1092–1099. [Google Scholar] [CrossRef]
- Marzal, A.; Bensch, S.; Reviriego, M.; Balbontin, J.; de Lope, F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008, 21, 979–987. [Google Scholar] [CrossRef]
- Carlson, J.S.; Giannitti, F.; Valkiūnas, G.; Tell, L.A.; Snipes, J.; Wright, S.A.; Cornel, A.J. A method to preserve low parasitaemia Plasmodium-infected avian blood for host and vector infectivity assays. Malar. J. 2016, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Moreno, J.; Sanz, J.J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; de Lope, F.; Navarro, C.; Møller, A.P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Langston, N.; Hillgarth, N. Moult Varies with Parasites in Laysan Albatrosses. Proc. R. Soc. Lond. B 1995, 261, 239–243. [Google Scholar]
- Pérez-Tris, J.; Carbonell, R.; Tellería, J.L. Parasites and the blackcap’s tail: Implications for the evolution of feather ornaments. Biol. J. Linn. Soc. 2002, 76, 481–492. [Google Scholar] [CrossRef]
- Pap, P.L.; Vágási, C.I.; Bărbos, L.; Marton, A. Chronic coccidian infestation compromises flight feather quality in house sparrows Passer domesticus. Biol. J. Linn. Soc. 2013, 108, 414–428. [Google Scholar] [CrossRef]
- Fithian, R. Prothonotary Warbler (Protonotaria citrea) Plumage as an Indicator for Infection: The Relationship between Haemosporidia Infection and Breast Feather Reflectance in a Neotropical Migrant Passerine. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2009. [Google Scholar]
- Marzal, A.; Reviriego, M.; Hermosell, I.G.; Balbontín, J.; Bensch, S.; Relinque, C.; Rodríguez, L.; Garcia-Longoria, L.; de Lope, F. Malaria infection and feather growth rate predict reproductive success in house martins. Oecologia 2013, 171, 853–861. [Google Scholar] [CrossRef]
- Marzal, A.; Asghar, M.; Rodríguez, L.; Reviriego, M.; Hermosell, I.G.; Balbontín, J.; Garcia-Longoria, L.; de Lope, F.; Bensch, S. Co-infections by malaria parasites decrease feather growth but not feather quality in house martin. J. Avian Biol. 2013, 44, 001–008. [Google Scholar] [CrossRef]
- Coon, C.A.C.; Garcia-Longoria, L.; Martin, L.B.; Magallanes, S.; de Lope, F.; Marzal, A. Malaria infection negatively affects feather growth rate in the house sparrow Passer domesticus. J. Avian Biol. 2016, 47, 779–787. [Google Scholar] [CrossRef]
- Clark, G.A., Jr. Form and function: The external bird. In Handbook of Bird Biology, 3rd ed.; Podulka, S., Rohrbaugh, R.W., Jr., Bonney, R., Eds.; Cornell Lab of Ornithology and Princeton University Press: New York, NY, USA, 2004; Volume 3, pp. 3:1–3:70. [Google Scholar]
- Soini, H.A.; Whittaker, D.J.; Wiesler, D.; Ketterson, E.D.; Novotny, M.V. Chemosignaling diversity in songbirds: Chromatographic profiling of preen oil volatiles in different species. J. Chromatogr. A 2013, 1317, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rueda, G. Preen oil and bird fitness: A critical review of the evidence. Biol. Rev. Camb. Philos. Soc. 2017, 92, 2131–2143. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rueda, G. Uropygial gland size correlates with feather holes, body condition and wingbar size in the house sparrow Passer domesticus. J. Avian Biol. 2010, 41, 229–236. [Google Scholar] [CrossRef]
- Fülöp, A.; Czirják, G.Á.; Pap, P.L.; Vágási, C.I. Feather-degrading bacteria, uropygial gland size and feather quality in house sparrows Passer domesticus. Ibis 2016, 158, 362–370. [Google Scholar] [CrossRef]
- Pap, P.L.; Tökölyi, J.; Szép, T. Frequency and consequences of feather holes in Barn Swallows Hirundo rustica. Ibis 2005, 147, 169–175. [Google Scholar] [CrossRef]
- Martín-Vivaldi, M.; Pena, A.; Peralta-Sanchez, J.M.; Sanchez, L.; Ananou, S.; Ruiz-Rodriguez, M.; Soler, J.J. Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc. R. Soc. Lond. B 2010, 277, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, M.; Tomás, G.; Martín-Gálvez, D.; Ruiz-Castellano, C.; Soler, J.J. Bacteria and the evolution of honest signals. The case of ornamental throat feathers in spotless starlings. Funct. Ecol. 2015, 29, 701–709. [Google Scholar] [CrossRef]
- Bodawatta, K.H.; Schierbech, S.K.; Petersen, N.R.; Sam, K.; Bos, N.; Jønsson, K.A.; Poulsen, M. Great Tit (Parus major) Uropygial Gland Microbiomes and Their Potential Defensive Roles. Front. Microbiol. 2020, 11, 1735. [Google Scholar] [CrossRef]
- Leclaire, S.; Pierret, P.; Chatelain, M.; Gasparini, J. Feather bacterial load affects plumage condition, iridescent color, and investment in preening in pigeons. Behav. Ecol. 2014, 25, 1192–1198. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Uropygial gland size, feather holes and moult performance in the House Sparrow Passer domesticus. Int. J. Avian Sci. 2014, 156, 457–460. [Google Scholar]
- del Hoyo, J.; Collar, N.J. Passerines. In HBW and BirdLife International Illustrated Checklist of the Birds of the World; Lynx Edicions: Barcelona, Spain, 2016; Volume 2. [Google Scholar]
- Marzal, A. Avión común occidental Delichon urbicum. In III Atlas de las aves en época de reproducción en España, 1st ed.; Molina, B., Nebreda, A., Muñoz, A.R., Seoane, J., Real, R., Bustamante, J., del Moral, J.C., Eds.; SEO/BirdLife: Madrid, Spain, 2022; Available online: https://atlasaves.seo.org/ave/avion-comun-occidental/ (accessed on 25 November 2023).
- Fernández-García, J.M.; Ruiz De Azua Pérez De Luco, N. Avión zapador Riparia riparia. In III Atlas de las aves en época de reproducción en España, 1st ed.; Molina, B., Nebreda, A., Muñoz, A.R., Seoane, J., Real, R., Bustamante, J., del Moral, J.C., Eds.; SEO/BirdLife: Madrid, Spain, 2022; Available online: https://atlasaves.seo.org/ave/avion-zapador/ (accessed on 25 November 2023).
- Svensson, L.; Mullarney, K.K.; Zetterström, D. Collins Bird Guide, 2nd ed.; Harper Collins: London, UK, 2009. [Google Scholar]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Riddle, O. The genesis of fault-bars in feathers and the cause of alternation of light and dark fundamental bars. Biol. Bull. 1908, 14, 328–370. [Google Scholar] [CrossRef]
- Michener, H.; Michener, J.R. Bars in flight feathers. Condor 1938, 40, 149–160. [Google Scholar] [CrossRef]
- Brodin, A. Radio-ptilochronology tracing radioactively labelled food in feathers. Ornis. Scand. 1993, 24, 167–173. [Google Scholar] [CrossRef]
- Grubb, T.C., Jr. Ptilochronology: Feather Time and the Biology of Birds; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Shawkey, M.D.; Beck, M.L.; Hill, G.E. Use of a gel documentation system to measure feather growth bars. J. Field Ornithol. 2003, 74, 125–128. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics. Int. 2004, 11, 36–42. [Google Scholar]
- Dawson, A.; Hinsley, S.A.; Ferns, P.N.; Bonser, R.H.C.; Eccleston, L. Rate of moult affects feather quality: A mechanism linking current reproductive effort to future survival. Proc. R. Soc. Lond. B 2000, 267, 2093–2098. [Google Scholar] [CrossRef]
- Galván, I.; Sanz, J.J. Feather mite abundance increases with uropygial gland size and plumage yellowness in Great Tits Parus major. Ibis 2006, 148, 687–697. [Google Scholar] [CrossRef]
- Møller, A.P.; Czirjak, G.Á.; Heeb, P. Feather micro-organisms and uropygial antimicrobial defences in a colonial passerine bird. Funct. Ecol. 2009, 23, 1097–1102. [Google Scholar] [CrossRef]
- Pap, P.L.; Vágási, C.I.; Osváth, G.; Mureşan, C.; Barta, Z. Seasonality in the uropygial gland size and feather mite abundance in house sparrows Passer domesticus: Natural covariation and an experiment. J. Avian Biol. 2010, 41, 653–661. [Google Scholar] [CrossRef]
- Martín-Vivaldi, M.; Ruiz-Rodríguez, M.; Soler, J.J.; Peralta-Sánchez, J.M.; Méndez, M.; Valdivia, E.; Martín-Platero, A.M.; Martínez-Bueno, M. Seasonal, sexual and developmental differences in hoopoe (Upupa epops) preen gland morphology and secretions: Evidence for a role of bacteria. J. Avian Biol. 2009, 40, 191–205. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Body-mass-dependent trade-off between immune response and uropygial gland size in house sparrows Passer domesticus. J. Avian Biol. 2015, 46, 40–45. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Inumaru, M.; Odaya, Y.; Sato, Y.; Marzal, A. First records of prevalence and diversity of avian haemosporidia in snipe species (genus Gallinago) of Japan. Int. J. Parasitol. Parasites Wildl. 2021, 16, 5–17. [Google Scholar] [CrossRef]
- Dubiec, A.; Podmokła, E.; Zagalska-Neubauer, M.; Drobniak, S.M.; Arct, A.; Gustafsson, L.; Cichoń, M. Differential prevalence and diversity of haemosporidian parasites in two sympatric closely related non-migratory passerines. Parasitology 2016, 143, 1320–1329. [Google Scholar] [CrossRef]
- Ellis, V.A.; Huang, X.; Westerdahl, H.; Jönsson, J.; Hasselquist, D.; Neto, J.M.; Nilsson, J.; Nilsson, A.; Hegemann, A.; Hellgren, O.; et al. Explaining prevalence, diversity and host specificity in a community of avian haemosporidian parasites. Oikos 2020, 129, 1314–1329. [Google Scholar] [CrossRef]
- Bukauskaitė, D.; Dementavičius, D.; Rumbutis, S.; Treinys, R. Differences in haemosporidian parasite prevalence and diversity in migratory and resident birds of prey species revealed by a non-invasive sampling method. Ecol. Evol. 2024, 14, e11038. [Google Scholar] [CrossRef]
- van Rooyen, J.; Lalubin, F.; Glaizot, O.; Christe, P. Altitudinal variation in haemosporidian parasite distribution in great tit populations. Parasit. Vectors 2013, 6, 139. [Google Scholar] [CrossRef]
- Reinoso-Pérez, M.T.; Canales-Delgadillo, J.C.; Chapa-Vargas, L.; Riego-Ruiz, L. Haemosporidian parasite prevalence, parasitemia, and diversity in three resident bird species at a shrubland dominated landscape of the Mexican highland plateau. Parasit. Vectors 2016, 9, 307. [Google Scholar] [CrossRef]
- Schumm, Y.R.; Bakaloudis, D.; Barboutis, C.; Cecere, J.G.; Eraud, C.; Fischer, D.; Hering, J.; Hillerich, K.; Lormée, H.; Mader, V.; et al. Prevalence and genetic diversity of avian haemosporidian parasites in wild bird species of the order Columbiformes. Parasitol. Res. 2021, 120, 1405–1420. [Google Scholar] [CrossRef]
- Rodriguez, M.D.; Doherty, P.F.; Piaggio, A.J.; Huyvaert, K.P. Sex and nest type influence avian blood parasite prevalence in a high-elevation bird community. Parasit. Vectors 2021, 14, 145. [Google Scholar] [CrossRef]
- Fecchio, A.; Dias, R.I.; Ferreira, T.V.; Reyes, A.O.; Dispoto, J.H.; Weckstein, J.D.; Bell, J.A.; Tkach, V.V.; Pinho, J.B. Host foraging behavior and nest type influence prevalence of avian haemosporidian parasites in the Pantanal. Parasitol. Res. 2022, 121, 1407–1417. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Regulation and Stability of Host-Parasite Population Interactions: I. Regulatory Processes. J. Anim. Ecol. 1978, 47, 219–247. [Google Scholar] [CrossRef]
- Ortego, J.; Cordero, P.J. Factors associated with the geographic distribution of Leucocytozoa parasitizing nestling eagle owls (Bubo bubo): A local spatial-scale analysis. Conserv. Genet. 2010, 11, 1479–1487. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; MacGregor-Fors, I.; Kühnert, K.; Segelbacher, G.; Schaefer, H.M. Avian haemosporidian parasites in an urban forest and their relationship to bird size and abundance. Urban Ecosyst. 2016, 19, 331–346. [Google Scholar] [CrossRef]
- Turner, A.K.; Rose, C. A Handbook to the Swallows and Martins of the World; Christopher Helm: London, UK, 1989. [Google Scholar]
- Marzal, A.; Magallanes, S.; Garcia-Longoria, L. Stimuli Followed by Avian Malaria Vectors in Host-Seeking Behaviour. Biology 2022, 11, 726. [Google Scholar] [CrossRef]
- Ferraguti, M.; Magallanes, S.; Mora-Rubio, C.; Bravo-Barriga, D.; Marzal, A.; Hernandez-Caballero, I.; Aguilera-Sepúlveda, P.; Llorente, F.; Pérez-Ramírez, E.; Guerrero-Carvajal, F.; et al. Implications of migratory and exotic birds and the mosquito community on West Nile virus transmission. Infect. Dis. 2024, 56, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, L.; Bijlsma, R.G.; van der Kamp, J.; Wymenga, E. Common Sand Martin Riparia riparia. In Living on the Edge: Wetlands and Birds in a Changing Sahel, 2nd ed.; KNNV: Zeist, The Netherlands, 2009; pp. 396–405. [Google Scholar]
- López-Calderón, C.; Hobson, K.A.; Marzal, A.; Balbontín, J.; Reviriego, M.; Magallanes, S.; García-Longoria, L.; de Lope, F.; Møller, A.P. Environmental conditions during winter predict age- and sex-specific differences in reproductive success of a trans-Saharan migratory bird. Sci. Rep. 2017, 7, 18082. [Google Scholar] [CrossRef]
- Sehgal, R.N.M. Manifold habitat effects on the prevalence and diversity of avian blood parasites. Int. J. Parasitol. Parasites Wildl. 2015, 4, 421–430. [Google Scholar] [CrossRef]
- López-Calderón, C.; Hobson, K.A.; Balbontín, J.; Reviriego, M.I.; Magallanes, S.; García-Longoria, L.; Relinque, C.; De Lope, F.; Møller, A.P.; Marzal, A. Rainfall at African wintering grounds predicts age-specific probability of haemosporidian infection in a migratory passerine bird. Ibis 2019, 161, 759–769. [Google Scholar] [CrossRef]
- Garcia-Longoria, L.; Marzal, A.; de Lope, F.; Garamszegi, L. Host-parasite interaction explains variation in the prevalence of avian haemosporidians at the community level. PLoS ONE 2019, 14, e0205624. [Google Scholar] [CrossRef]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How Malaria Parasites Acquire Nutrients from Their Host. Front. Cell Dev. Biol. 2021, 9, 649184. [Google Scholar] [CrossRef]
- Robel, E.J. A feather abnormality in chicks fed diets deficient in certain amino acids. Poult. Sci. 1977, 56, 1968–1971. [Google Scholar] [CrossRef]
- Zeng, Q.F.; Zhang, Q.; Chen, X.; Doster, A.; Murdoch, R.; Makagon, M.; Gardner, A.; Applegate, T.J. Effect of dietary methionine content on growth performance, carcass traits, and feather growth of Pekin duck from 15 to 35 days of age. Poult. Sci. 2015, 94, 1592–1599. [Google Scholar] [CrossRef]
- Costantini, D. A meta-analysis of impacts of immune response and infection on oxidative status in vertebrates. Conserv. Physiol. 2022, 10, coac018. [Google Scholar] [CrossRef]
- Ben-Hamo, M.; Downs, C.J.; Burns, D.J.; Pinshow, B. House sparrows offset the physiological trade-off between immune response and feather growth by adjusting foraging behavior. J. Avian Biol. 2017, 48, 837–845. [Google Scholar] [CrossRef]
- Palinauskas, V.; Martínez-de la Puente, J.; Hernández-Soto, S.R.; Marzal, A. Experimental Parasitology and Ecoimmunology: Concepts and Opportunities in Avian Haemosporidian Studies. In Avian Malaria and Related Parasites in the Tropics, 1st ed.; Santiago-Alarcon, D., Marzal, A., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2020. [Google Scholar]
- Ágh, N.; Csörgő, T.; Szöllősi, E. Delay in arrival: Lineage-specific influence of haemosporidians on autumn migration of European robins. Parasitol. Res. 2022, 121, 2831–2840. [Google Scholar] [CrossRef]
- Møller, A.P.; Nielsen, J.T. The trade-off between rapid feather growth and impaired feather quality increases risk of predation. J. Ornithol. 2018, 159, 165–171. [Google Scholar] [CrossRef]
- de la Hera, I.; Pérez-Tris, J.; Tellería, J.L. Migratory behaviour affects the trade-off between feather growth rate and feather quality in a passerine bird. Biol. J. Linn. Soc. 2009, 97, 98–105. [Google Scholar] [CrossRef]
- Vágási, C.I.; Pap, P.L.; Vincze, O.; Benkő, Z.; Marton, A.; Barta, Z. Haste Makes Waste but Condition Matters: Molt Rate–Feather Quality Trade-Off in a Sedentary Songbird. PLoS ONE 2012, 7, e40651. [Google Scholar] [CrossRef]
- Saino, N.; Romano, M.; Caprioli, M.; Lardelli, R.; Micheloni, P.; Scandolara, C.; Rubolini, D.; Fasola, M. Molt, feather growth rate and body condition of male and female Barn Swallows. J. Ornithol. 2013, 154, 537–547. [Google Scholar] [CrossRef]
- Szép, T.; Dobránszky, J.; Møller, A.P.; Dyke, G.; Lendvai, Á.Z. Older birds have better feathers: A longitudinal study on the long-distance migratory sand martin, Riparia riparia. PLoS ONE 2019, 14, e0209737. [Google Scholar] [CrossRef]
- Pap, P.L.; Vágási, C.I.; Czirják, G.Á.; Barta, Z. Diet quality affects postnuptial molting and feather quality of the House Sparrow (Passer domesticus): Interaction with humoral immune function? Can. J. Zool. 2008, 86, 834–842. [Google Scholar] [CrossRef]
- de la Hera, I.; Reichert, M.S.; Davidson, G.L.; Quinn, J.L. A longitudinal analysis of the growth rate and mass of tail feathers in a great tit population: Ontogeny, genetic effects and relationship between both traits. J. Avian Biol. 2022, e02894. [Google Scholar] [CrossRef]
- Horák, K.; Bobek, L.; Adámková, M.; Kauzál, O.; Kauzálová, T.; Manialeu, J.P.; Nguelefack, T.B.; Nana, E.D.; Jønsson, K.A.; Munclinger, P.; et al. Feather growth and quality across passerines is explained by breeding rather than moulting latitude. Proc. R. Soc. B 2022, 289, 20212404. [Google Scholar] [CrossRef]
- Jacob, J.; Ziswiler, V. The uropygial gland. In Avian Biology; Farner, D.S., King, J.R., Parkes, K.C., Eds.; Academic Press: New York, NY, USA, 1982; Volume VI, pp. 199–324. [Google Scholar]
- Soini, H.A.; Schrock, S.E.; Bruce, K.E.; Wiesler, D.; Ketterson, E.D.; Novotny, M.V. Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J. Chem. Ecol. 2007, 33, 183–198. [Google Scholar] [CrossRef]
- Dunn, J.C.; Goodman, S.J.; Benton, T.G.; Hammer, K.C. Avian blood parasite infection during the non-breeding season: An overlooked issue in declining populations? BMC Ecol. 2013, 13, 30. [Google Scholar] [CrossRef]
- Romano, A.; Nodari, R.; Bandi, C.; Caprioli, M.; Costanzo, A.; Ambrosini, R.; Rubolini, D.; Parolini, M.; Epis, S.; Saino, N. Haemosporidian parasites depress breeding success and plumage coloration in female barn swallows (Hirundo rustica). J. Avian Biol. 2019, 50, 325. [Google Scholar] [CrossRef]
- Henschen, A.E.; Whittingham, L.A.; Dunn, P.O. The relationship between blood parasites and ornamentation depends on the level of analysis in the common yellowthroat. J. Avian Biol. 2017, 48, 1263–1272. [Google Scholar] [CrossRef]
- Woodworth, B.L.; Atkinson, C.T.; LaPointe, D.A.; Hart, P.J.; Spiegel, C.S.; Tweed, E.J.; Henneman, C.; LeBrun, J.; Denette, T.; DeMots, R.; et al. Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc. Natl. Acad. Sci. USA 2005, 102, 1531–1536. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Bensch, S.; Iezhova, T.A.; Krizanauskienė, A.; Hellgren, O.; Bolshakov, C.V. Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: Microscopy is still essential. J. Parasitol. 2006, 92, 418–422. [Google Scholar] [CrossRef]
- Bensch, S.; Waldenström, J.; Jonzén, N.; Westerdahl, H.; Hansson, B.; Sejberg, D.; Hasselquist, D. Temporal dynamics and diversity of avian malaria parasites in a single host species. J. Anim. Ecol. 2007, 76, 112–122. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Uropygial gland and bib colouration in the house sparrow. PeerJ 2016, 4, e2102. [Google Scholar] [CrossRef]
- Magallanes, S.; García-Longoria, L.; Muriel, J.; de Lope, F.; Marzal, A. El volumen de la glándula uropigial y la infección por malaria varía entre hábitats urbano–rural en el gorrión común. Ecosistemas 2020, 29, 1977. [Google Scholar]
- Møller, A.P.; Laursen, K. Function of the uropygial gland in eiders (Somateria mollissima). Avian Res. 2019, 10, 24. [Google Scholar] [CrossRef]

| Independent Variables | Estimate | Std. Error | t | p |
|---|---|---|---|---|
| Haemosporidian infection (infected) | −0.043 | 0.014 | −3.060 | 0.003 |
| Sex | 0.006 | 0.011 | 0.495 | 0.622 |
| Scaled body mass index | −0.269 | 0.197 | −1.363 | 0.176 |
| Uropygial gland volume | <0.001 | <0.001 | 0.137 | 0.891 |
| Feather growth rate | 0.262 | 0.087 | 3.004 | 0.004 |
| Independent Variables | Estimate | Std. Error | t | p |
|---|---|---|---|---|
| Haemosporidian infection (infected) | 0.004 | 0.018 | 0.219 | 0.827 |
| Sex | −0.014 | 0.013 | −1.011 | 0.315 |
| Scaled body mass index | 0.359 | 0.234 | 1.535 | 0.129 |
| Uropygial gland volume | −0.001 | 0.001 | −1.627 | 0.107 |
| Feather quality | 0.370 | 0.123 | 3.004 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Rubio, C.; Garcia-Longoria, L.; Ferraguti, M.; Magallanes, S.; Cruz, J.T.; de Lope, F.; Marzal, A. The Impact of Avian Haemosporidian Infection on Feather Quality and Feather Growth Rate of Migratory Passerines. Animals 2024, 14, 1772. https://doi.org/10.3390/ani14121772
Mora-Rubio C, Garcia-Longoria L, Ferraguti M, Magallanes S, Cruz JT, de Lope F, Marzal A. The Impact of Avian Haemosporidian Infection on Feather Quality and Feather Growth Rate of Migratory Passerines. Animals. 2024; 14(12):1772. https://doi.org/10.3390/ani14121772
Chicago/Turabian StyleMora-Rubio, Carlos, Luz Garcia-Longoria, Martina Ferraguti, Sergio Magallanes, João T. Cruz, Florentino de Lope, and Alfonso Marzal. 2024. "The Impact of Avian Haemosporidian Infection on Feather Quality and Feather Growth Rate of Migratory Passerines" Animals 14, no. 12: 1772. https://doi.org/10.3390/ani14121772





