Effect of Rumen-Protected L-Tryptophan or L-Ascorbic Acid on Plasma Metabolites and Milk Production Characteristics of Lactating Holstein Cows during Summer Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
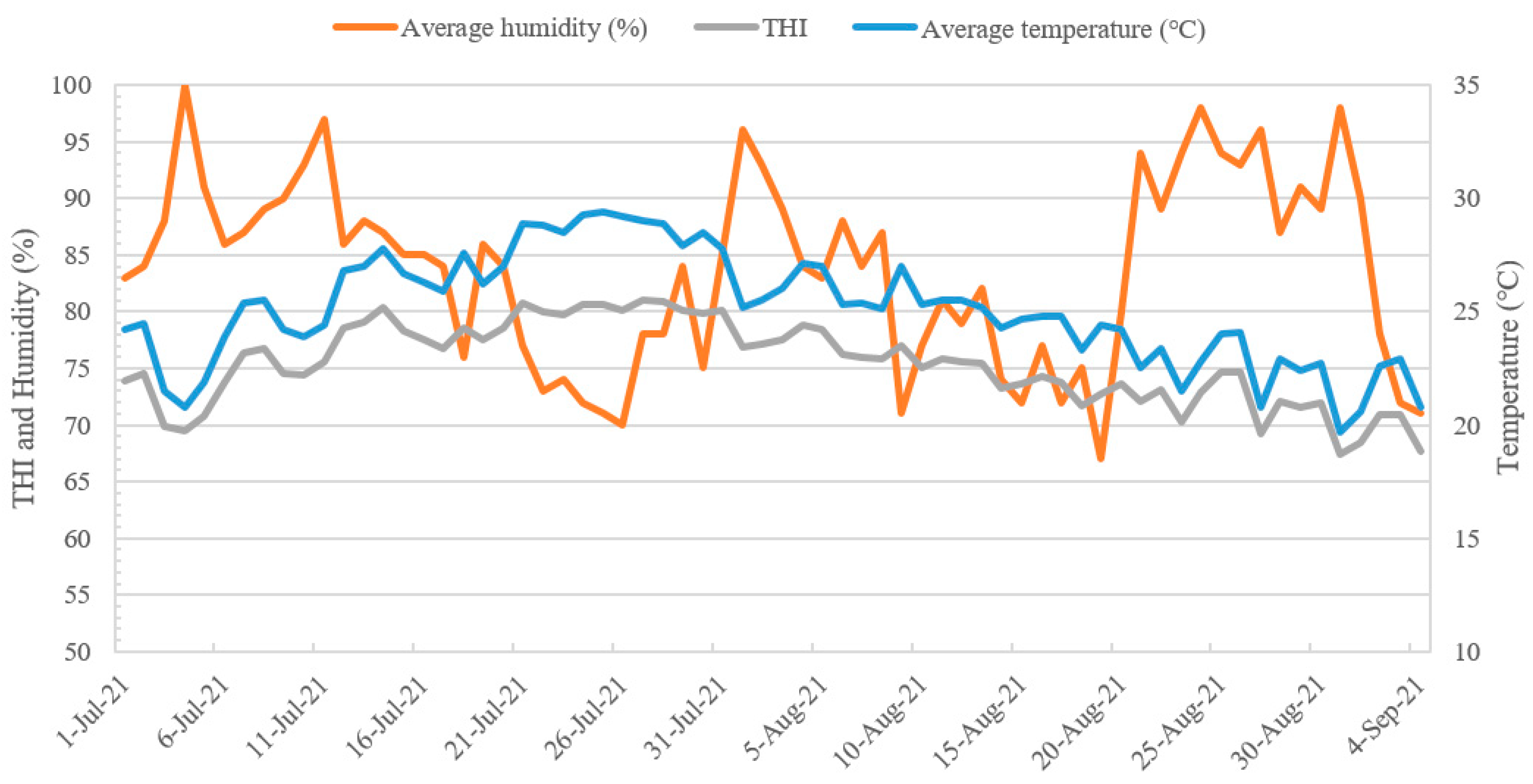
2.1. Period
2.2. Animals, Treatments, and Management
2.3. Milk Yield, Feed Intake, and Feed Efficiency
2.4. Milk Components, Fatty Acids, and Melatonin
2.5. Plasma Metabolites and Cortisol
2.6. Statistical Analysis
3. Results
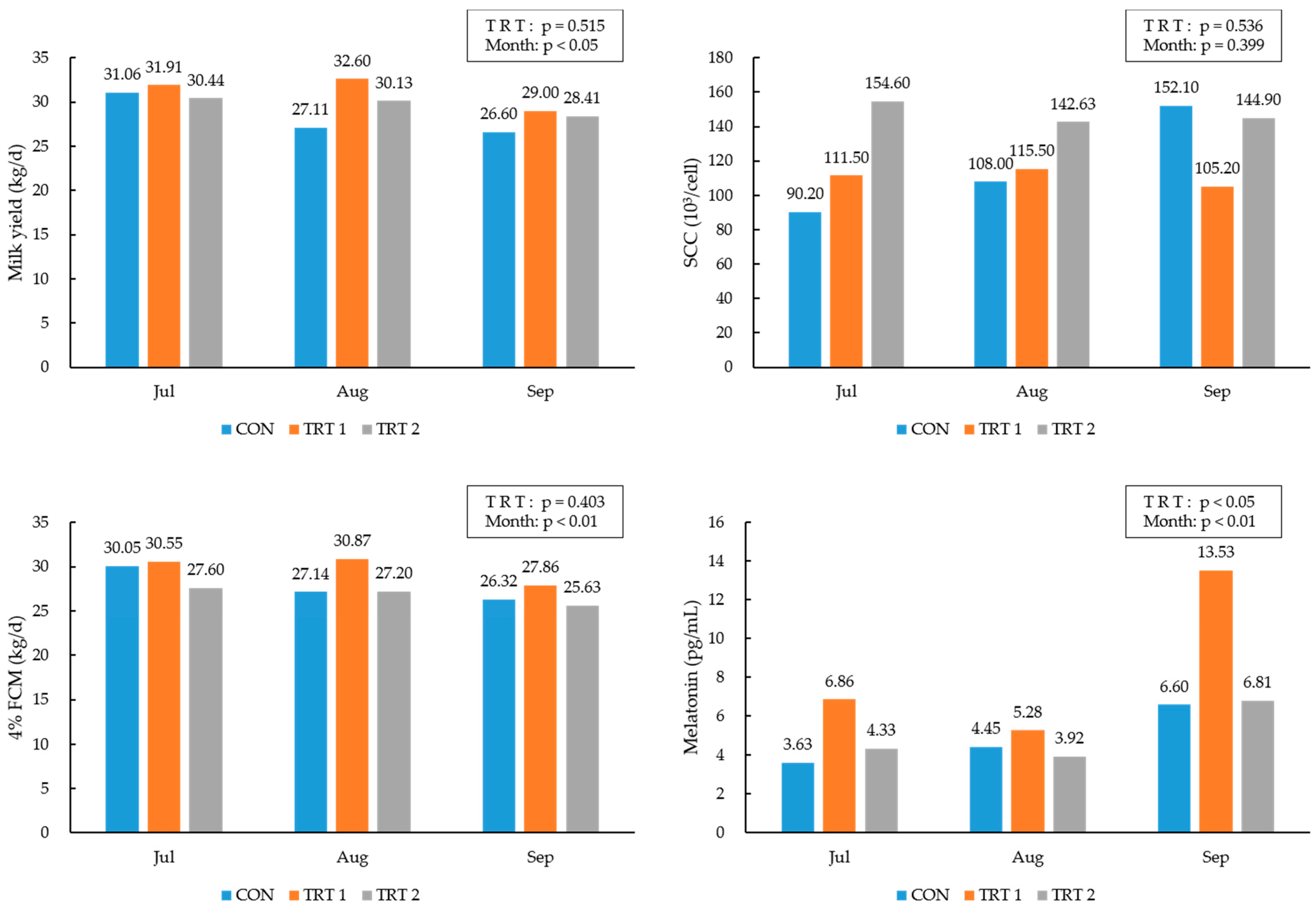
3.1. Milk Yield, Feed Intake, and Feed Efficiency
3.2. Plasma Metabolite and Cortisol
3.3. Milk Yield, Components and Melatonin
3.4. Fatty Acid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sejian, V.; Bahadur, S.; Indu, S.; Bagath, M.; Malik, P.; Soren, N.; Kumar, D.; Maurya, V.P.; Shinde, A.K.; Sahoo, A.; et al. Environmental Stress Impact on Small Ruminants Production. In SHEEP AND GOAT Meat Production and Processing; Satish Serial Publishing House: Delhi, India, 2016. [Google Scholar]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia risoria as a model. Int. J. Tryptophan Res. 2009, 2, 23–36. [Google Scholar] [CrossRef]
- Minton, J.E. Function of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. J. Anim. Sci. 1994, 72, 1891–1898. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, K.W.; Wang, T.; Lee, J.S.; Jung, U.S.; Nejad, J.G.; Oh, Y.K.; Baek, Y.C.; Kim, K.H.; Lee, H.G. Administration of encapsulated L-tryptophan improves duodenal starch digestion and increases gastrointestinal hormones secretions in beef cattle. Asian-Australas. J. Anim. Sci. 2020, 33, 91. [Google Scholar] [CrossRef]
- Choi, W.T.; Nejad, J.G.; Moon, J.O.; Lee, H.G. Dietary supplementation of acetate-conjugated tryptophan alters feed intake, milk yield and composition, blood profile, physiological variables, and heat shock protein gene expression in heat-stressed dairy cows. J. Therm. Biol. 2021, 98, 102949. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological significance of ascorbic acid (vitamin C) in human health—A review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar]
- Koopmans, S.J.; Ruis, M.; Dekker, R.; van Diepen, H.; Korte, M.; Mroz, Z. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Physiol. Behav. 2005, 85, 469–478. [Google Scholar] [CrossRef]
- Jo, J.H.; Jalil, G.N.; Kim, W.S.; Moon, J.O.; Lee, S.D.; Kwon, C.H.; Lee, H.G. Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions. Int. J. Mol. Sci. 2024, 25, 1217. [Google Scholar] [CrossRef]
- Min, L.; Li, D.; Tong, X.; Nan, X.; Ding, D.; Xu, B.; Wang, G. Nutritional strategies for alleviating the detrimental effects of heat stress in dairy cows: A review. Int. J. Biometeorol. 2019, 63, 1283–1302. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle, 8th ed.; Animal Nutrition Series; National Academy Press: Washington, DC, USA, 2021. [Google Scholar]
- Weiss, W.P.; Hogan, J.S. Effects of dietary vitamin C on neutrophil function and responses to intramammary infusion of lipopolysaccharide in periparturient dairy cows. J. Dairy Sci. 2007, 90, 731–739. [Google Scholar] [CrossRef]
- Naresh, R.; Dwivedi, S.K.; Swarup, D.; Patra, R.C. Evaluation of ascorbic acid treatment in clinical and subclinical mastitis of Indian dairy cows. Asian-Australas. J. Anim. Sci. 2002, 15, 905–911. [Google Scholar] [CrossRef]
- Kim, J.H.; Mamuad, L.L.; Yang, C.J.; Kim, S.H.; Ha, J.K.; Lee, W.S.; Cho, K.K.; Lee, S.S. Hemato-biochemical and cortisol profile of Holstein growing-calves supplemented with vitamin C during summer season. Asian-Australas. J. Anim. Sci. 2012, 25, 361. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Sutoh, M.; Kasuya, E.; Yayou, K.I. Effects of intravenous tryptophan infusion on thermoregulation in steers exposed to acute heat stress. Anim. Sci. J. 2018, 89, 777–783. [Google Scholar] [CrossRef]
- Bell, C.; Abrams, J.; Nutt, D. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry 2001, 178, 399–405. [Google Scholar] [CrossRef]
- Kollmann, M.T.; Locher, M.; Hirche, F.; Eder, K.; Meyer, H.H.D.; Bruckmaier, R.M. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Domest. Anim. Endocrinol. 2008, 34, 14–24. [Google Scholar] [CrossRef]
- Otto, F.; Vilela, F.; Harun, M.; Taylor, G.; Baggasse, P.; Bogin, E. Biochemical blood profile of Angoni cattle in Mozambique. Isr. J. Vet. Med. 2000, 55, 95–102. [Google Scholar]
- Melendez, P.; Donovan, A.; Hernandez, J. Milk urea nitrogen and infertility in Florida dairy cows. In Proceedings of the Thirty-Second Annual Conference, American Association of Bovine Practitioners, Nashville, TN, USA, 23–26 September 1999; p. 198. [Google Scholar]
- Tshuma, T.; Fosgate, G.; Webb, E.; Swanepoel, C.; Holm, D. Effect of temperature and humidity on milk urea nitrogen concentration. Animals 2023, 13, 295. [Google Scholar] [CrossRef]
- Farombi, E.O.; Onyema, O.O. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: Modulatory role of vitamin C, vitamin E and quercetin. Hum. Exp. Toxicol. 2006, 25, 251–259. [Google Scholar] [CrossRef]
- Akinmoladun, O.F. Stress amelioration potential of vitamin C in ruminants: A review. Trop. Anim. Health Prod. 2022, 54, 24. [Google Scholar] [CrossRef]
- Waage, S.; Sviland, S.; Ødegaard, S.A. Identification of risk factors for clinical mastitis in dairy heifers. J. Dairy Sci. 1998, 81, 1275–1284. [Google Scholar] [CrossRef]
- Nousiainen, J.; Shingfield, K.J.; Huhtanen, P. Evaluation of milk urea nitrogen as a diagnostic of protein feeding. J. Dairy Sci. 2004, 87, 386–398. [Google Scholar] [CrossRef]
- Manal Said, T.; Nawal, A.B. Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamins C and E. Food Nutr. Sci. 2012, 3, 651–659. [Google Scholar]
- Sımonov, M.; Vlızlo, V. Some Blood Markers of the Functional State of Liver in Dairy Cows with Clinical Ketosis. Bulgarian J. Vet. Med. 2015, 18, 74–82. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Wheelock, J.B.; Shwartz, G.; O’brien, M.; VanBaale, M.J.; Collier, R.J.; Rhoads, M.L.; Rhoads, R.P. Effects of heat stress on nutritional requirements of lactating dairy cattle. In Proceedings of the 5th Annual Arizona Dairy Production Conference, Tempe, Arizona, 10 October 2006; pp. 8–17. [Google Scholar]
- Tanaka, M.; Kamiya, Y.; Suzuki, T.; Kamiya, M.; Nakai, Y. Relationship between milk production and plasma concentrations of oxidative stress markers during hot season in primiparous cows. Anim. Sci. J. 2008, 79, 481–486. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.V. Growth rate, feed intake, physiological responses and hormonal profile of Murrah buffaloes implanted melatonin during summer season. Indian J. Anim. Sci. 2021, 91, 386–390. [Google Scholar] [CrossRef]
- Stoop, W.M.; Bovenhuis, H.; Heck, J.M.L.; Van Arendonk, J.A.M. Effect of lactation stage and energy status on milk fat composition of Holstein-Friesian cows. J. Dairy Sci. 2009, 92, 1469–1478. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.A.; Bruckmaier, R.M.; Schwarz, F.J. Milk fatty acid profile related to energy balance in dairy cows. J. Dairy Res. 2011, 78, 479–488. [Google Scholar] [CrossRef]
- Bohlouli, M.; Yin, T.; Hammami, H.; Gengler, N.; König, S. Climate sensitivity of milk production traits and milk fatty acids in genotyped Holstein dairy cows. J. Dairy Sci. 2021, 104, 6847–6860. [Google Scholar] [CrossRef]
| Items | CON | TRT 1 1 | TRT 2 2 |
|---|---|---|---|
| Yield | 31.06 ± 5.70 | 31.91 ± 8.08 | 30.44 ± 6.84 |
| Days in milk | 226.40 ± 96.83 | 217.78 ± 83.56 | 212.80 ± 84.98 |
| Months old | 60.50 ± 34.26 | 54.30 ± 27.48 | 56.11 ± 23.54 |
| Parity | 2.60 ± 2.46 | 2.40 ± 1.35 | 2.90 ± 2.23 |
| Items | Concentrate | TMR 1 | Tall Fescue Straw |
|---|---|---|---|
| Dry matter (%) | 93.69 ± 0.26 | 65.74 ± 0.34 | 84.60 ± 0.24 |
| Crude protein (%) | 21.01 ± 0.22 | 24.28 ± 0.10 | 4.87 ± 0.04 |
| Ether extract (%) | 6.80 ± 0.31 | 3.21 ± 0.54 | 0.99 ± 0.10 |
| Crude ash (%) | 7.57 ± 0.30 | 11.50 ± 0.03 | 10.44 ± 0.78 |
| Crude fiber (%) | 12.78 ± 0.39 | 28.81 ± 0.75 | 50.01 ± 0.54 |
| Neutral detergent fiber (%) | 48.61 ± 0.45 | 65.56 ± 0.56 | 93.64 ± 0.42 |
| Acid detergent fiber (%) | 25.39 ± 0.29 | 41.65 ± 0.86 | 65.35 ± 0.08 |
| Items | July | August | September | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | TRT 1 | TRT 2 | CON | TRT 1 | TRT 2 | CON | TRT 1 | TRT 2 | Month | TRT | TRT × Month | |
| Feed intake (DM kg/d) | ||||||||||||
| Concentrate | 3.85 ± 1.09 | 4.08 ± 1.41 | 3.77 ± 1.32 | 3.21 ± 1.23 | 4.06 ± 1.44 | 3.63 ± 1.16 | 2.98 ± 1.00 | 3.68 ± 0.92 | 3.44 ± 0.97 | 0.030 | 0.408 | 0.596 |
| Total mixed ration | 19.72 | 19.72 | 19.72 | 19.72 | 19.72 | 19.72 | 19.72 | 19.72 | 19.72 | - | - | - |
| Tall fescue straw | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | - | - | - |
| DMI 1 | 24.42 ± 1.09 | 24.57 ± 1.42 | 24.34 ± 1.32 | 23.69 ± 1.31 | 24.55 ± 1.48 | 24.11 ± 1.25 | 23.46 ± 1.13 | 24.16 ± 0.92 | 23.92 ± 1.13 | 0.024 | 0.475 | 0.587 |
| Feed efficiency (milk yield/DMI, kg/kg) | 1.27 ± 0.18 | 1.29 ± 0.27 | 1.24 ± 0.21 | 1.13 ± 0.21 | 1.31 ± 0.29 | 1.24 ± 0.22 | 1.12 ± 0.22 | 1.20 ± 0.18 | 1.18 ± 0.17 | 0.018 | 0.553 | 0.693 |
| Items | July | August | September | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | TRT 1 | TRT 2 | CON | TRT 1 | TRT 2 | CON | TRT 1 | TRT 2 | Month | TRT | TRT × Month | |
| GLU 1 (mg/dL) | 63.50 ± 2.95 | 62.60 ± 3.17 | 59.20 ± 12.93 | 60.40 ± 7.38 | 62.00 ± 4.24 | 59.44 ± 6.19 | 58.30 ± 3.20 | 61.20 ± 5.35 | 59.56 ± 3.81 | 0.241 | 0.325 | 0.329 |
| NEFA 2 (uEq/L) | 112.63 ± 11.34 | 126.67 ± 25.75 | 128.13 ± 22.43 | 54.00 ± 28.46 | 66.56 ± 27.67 | 74.63 ± 24.14 | 39.25 ± 15.27 | 47.00 ± 21.70 | 46.25 ± 11.46 | <0.001 | 0.232 | 0.632 |
| BUN 3 (mg/dL) | 19.74 ± 2.34 | 18.80 ± 2.92 | 19.15 ± 3.47 | 22.85 ± 2.73 | 24.81 ± 2.86 | 24.89 ± 2.60 | 21.50 ± 1.63 | 22.46 ± 3.26 | 21.26 ± 1.79 | <0.001 | 0.735 | 0.471 |
| TP 4 (g/dL) | 8.43 ± 0.36 | 8.57 ± 0.48 | 8.35 ± 0.25 | 7.34 ± 0.55 | 8.02 ± 0.58 | 7.93 ± 1.06 | 6.46 ± 0.37 | 7.03 ± 0.58 | 6.84 ± 0.50 | <0.001 | 0.033 | 0.191 |
| ALB 5 (g/dL) | 4.35 ± 0.18 | 4.29 ± 0.12 | 4.30 ± 0.26 | 3.74 ± 0.30 | 4.00 ± 0.24 | 3.97 ± 0.35 | 3.34 ± 0.17 | 3.57 ± 0.35 | 3.46 ± 0.21 | <0.001 | 0.097 | 0.144 |
| Ca 6 (mg/dL) | 9.65 ± 0.41 | 9.51 ± 0.34 | 9.45 ± 0.47 | 8.51 ± 0.59 | 8.79 ± 0.46 | 8.71 ± 1.11 | 7.56 ± 0.37 | 7.79 ± 0.67 | 7.58 ± 0.48 | <0.001 | 0.736 | 0.267 |
| IP 7 (mg/dL) | 6.74 ± 0.81 | 6.64 ± 1.19 | 6.93 ± 1.25 | 5.23 ± 1.00 | 6.17 ± 0.99 | 6.13 ± 1.05 | 4.54 ± 0.51 | 5.22 ± 0.89 | 4.83 ± 0.88 | <0.001 | 0.280 | 0.313 |
| Mg 8 (mg/dL) | 2.44 ± 0.16 | 2.43 ± 0.19 | 2.44 ± 0.21 | 2.49 ± 0.28 | 2.67 ± 0.29 | 2.73 ± 0.35 | 2.16 ± 0.18 | 2.30 ± 0.22 | 2.22 ± 0.16 | <0.001 | 0.295 | 0.348 |
| CHOL 9 (mg/dL) | 246.40 ± 56.56 | 258.40 ± 61.91 | 265.20 ± 64.71 | 241.60 ± 47.57 | 272.10 ± 55.56 | 267.00 ± 44.29 | 216.70 ± 35.93 | 254.00 ± 74.15 | 235.89 ± 47.90 | 0.066 | 0.465 | 0.564 |
| γ-GTP 10 (U/L) | 20.40 ± 11.51 | 18.20 ± 4.66 | 24.00 ± 11.76 | 37.00 ± 13.98 | 33.60 ± 7.21 | 45.67 ± 27.60 | 30.70 ± 6.80 | 32.00 ± 7.92 | 33.11 ± 13.12 | <0.001 | 0.484 | 0.292 |
| AST 11 (U/L) | 79.70 ± 21.44 | 76.00 ± 8.23 | 81.90 ± 21.87 | 87.10 ± 27.80 | 95.60 ± 21.91 | 101.33 ± 22.98 | 74.60 ± 15.26 | 79.80 ± 15.39 | 76.00 ± 13.11 | 0.287 | 0.699 | 0.227 |
| Cortisol (ng/mL) | 35.73 ± 0.59 | 35.06 ± 1.62 | 34.81 ± 2.57 | 36.33 ± 0.74 | 35.22 ± 1.61 | 34.80 ± 2.54 | 36.17 ± 0.52 | 32.82 ± 3.91 | 33.28 ± 3.99 | 0.012 | 0.192 | 0.043 |
| Items | July | August | September | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | TRT 1 | TRT 2 | CON | TRT 1 | TRT 2 | CON | TRT 1 | TRT 2 | Month | TRT | TRT × Month | |
| SFA 1 | 70.38 ± 3.54 | 67.99 ± 3.89 | 66.46 ± 5.06 | 67.99 ± 2.87 | 67.38 ± 3.38 | 67.74 ± 2.84 | 68.08 ± 2.24 | 66.70 ± 1.40 | 65.96 ± 1.55 | 0.595 | 0.398 | 0.248 |
| MUFA 2 | 27.30 ± 2.95 | 29.44 ± 3.84 | 30.99 ± 4.72 | 29.10 ± 2.65 | 30.01 ± 3.19 | 29.40 ± 2.67 | 28.94 ± 1.45 | 30.74 ± 1.54 | 31.34 ± 1.34 | 0.632 | 0.408 | 0.267 |
| PUFA 3 | 2.32 ± 0.86 | 2.56 ± 0.32 | 2.55 ± 0.56 | 2.91 ± 0.29 | 2.61 ± 0.26 | 2.86 ± 0.51 | 2.98 ± 1.19 | 2.57 ± 0.17 | 2.69 ± 0.28 | 0.445 | 0.628 | 0.088 |
| UFA 4 | 29.62 ± 3.54 | 32.01 ± 3.89 | 33.54 ± 5.06 | 32.01 ± 2.87 | 32.62 ± 3.38 | 32.26 ± 2.84 | 31.92 ± 2.24 | 33.30 ± 1.40 | 34.04 ± 1.55 | 0.595 | 0.398 | 0.248 |
| n-6/n-3 | 7.84 ± 1.06 | 7.03 ± 1.77 | 7.77 ± 2.57 | 7.39 ± 1.22 | 7.35 ± 0.99 | 7.50 ± 3.04 | 8.04 ± 0.92 | 7.91 ± 1.96 | 6.88 ± 1.63 | 0.895 | 0.985 | 0.196 |
| UFA/SFA | 0.42 ± 0.07 | 0.48 ± 0.09 | 0.51 ± 0.13 | 0.47 ± 0.06 | 0.49 ± 0.07 | 0.48 ± 0.06 | 0.47 ± 0.05 | 0.50 ± 0.03 | 0.52 ± 0.04 | 0.825 | 0.371 | 0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-L.; Lee, S.-H.; Son, G.-H.; Shin, J.-S.; Kim, M.-J.; Park, B.-K. Effect of Rumen-Protected L-Tryptophan or L-Ascorbic Acid on Plasma Metabolites and Milk Production Characteristics of Lactating Holstein Cows during Summer Conditions. Animals 2024, 14, 1820. https://doi.org/10.3390/ani14121820
Kim Y-L, Lee S-H, Son G-H, Shin J-S, Kim M-J, Park B-K. Effect of Rumen-Protected L-Tryptophan or L-Ascorbic Acid on Plasma Metabolites and Milk Production Characteristics of Lactating Holstein Cows during Summer Conditions. Animals. 2024; 14(12):1820. https://doi.org/10.3390/ani14121820
Chicago/Turabian StyleKim, Young-Lae, So-Hee Lee, Gi-Hwal Son, Jong-Suh Shin, Min-Ji Kim, and Byung-Ki Park. 2024. "Effect of Rumen-Protected L-Tryptophan or L-Ascorbic Acid on Plasma Metabolites and Milk Production Characteristics of Lactating Holstein Cows during Summer Conditions" Animals 14, no. 12: 1820. https://doi.org/10.3390/ani14121820





