Comparison of Two Intravenous Propofol Doses after Jugular Administration for Short Non-Surgical Procedures in Red-Eared Sliders (Trachemys scripta elegans)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
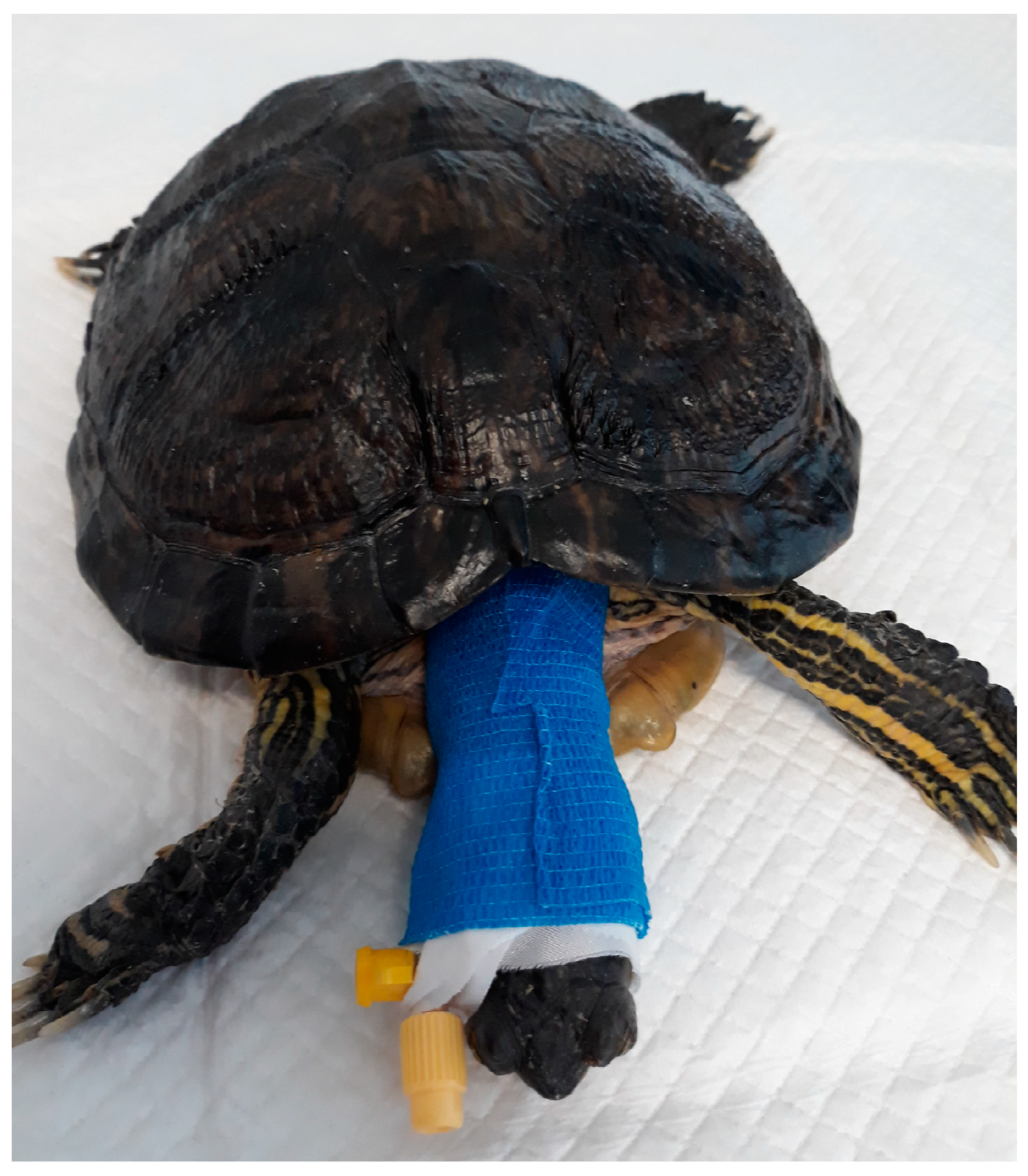
2.1. Turtles
2.2. Study Design
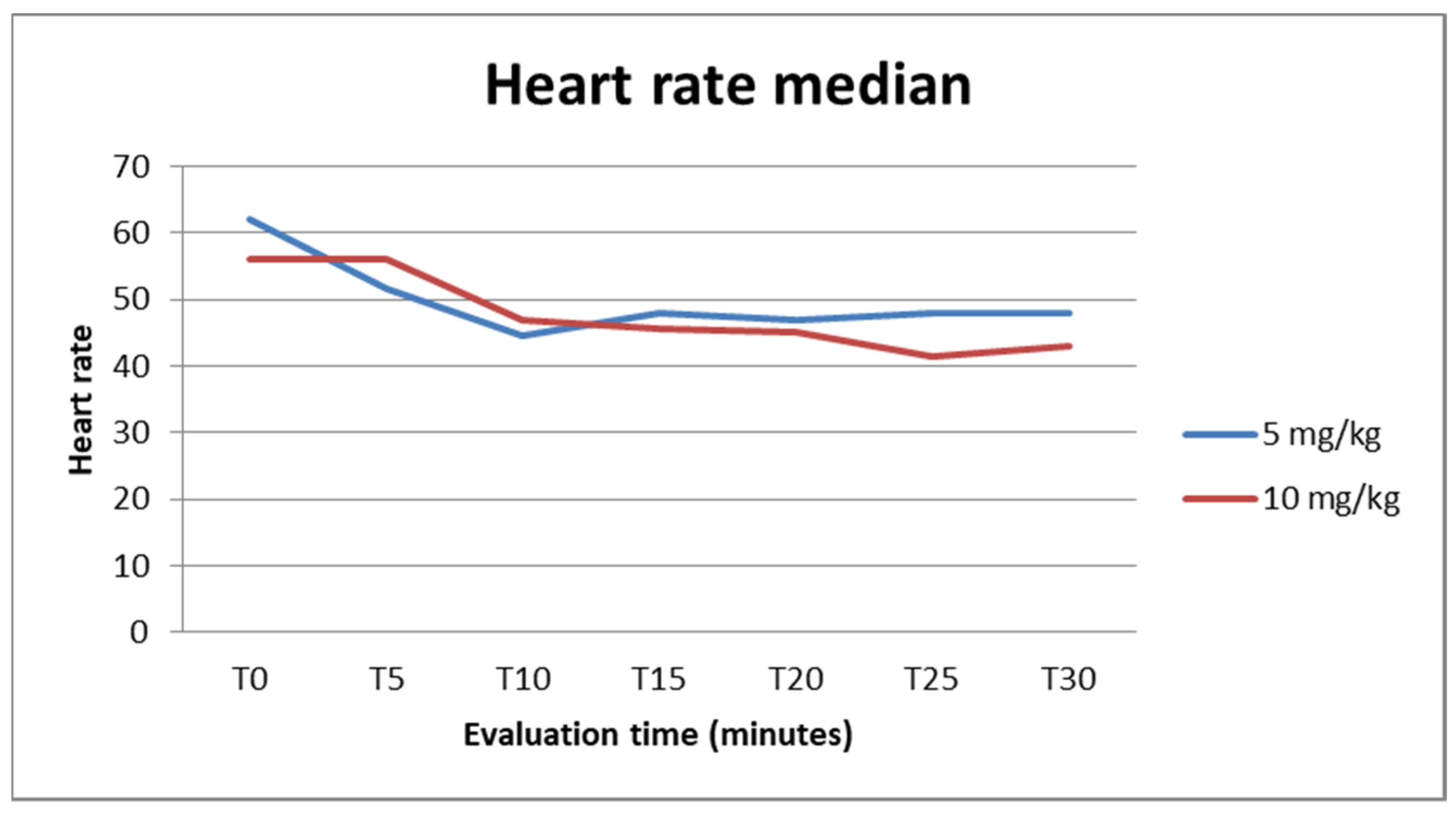
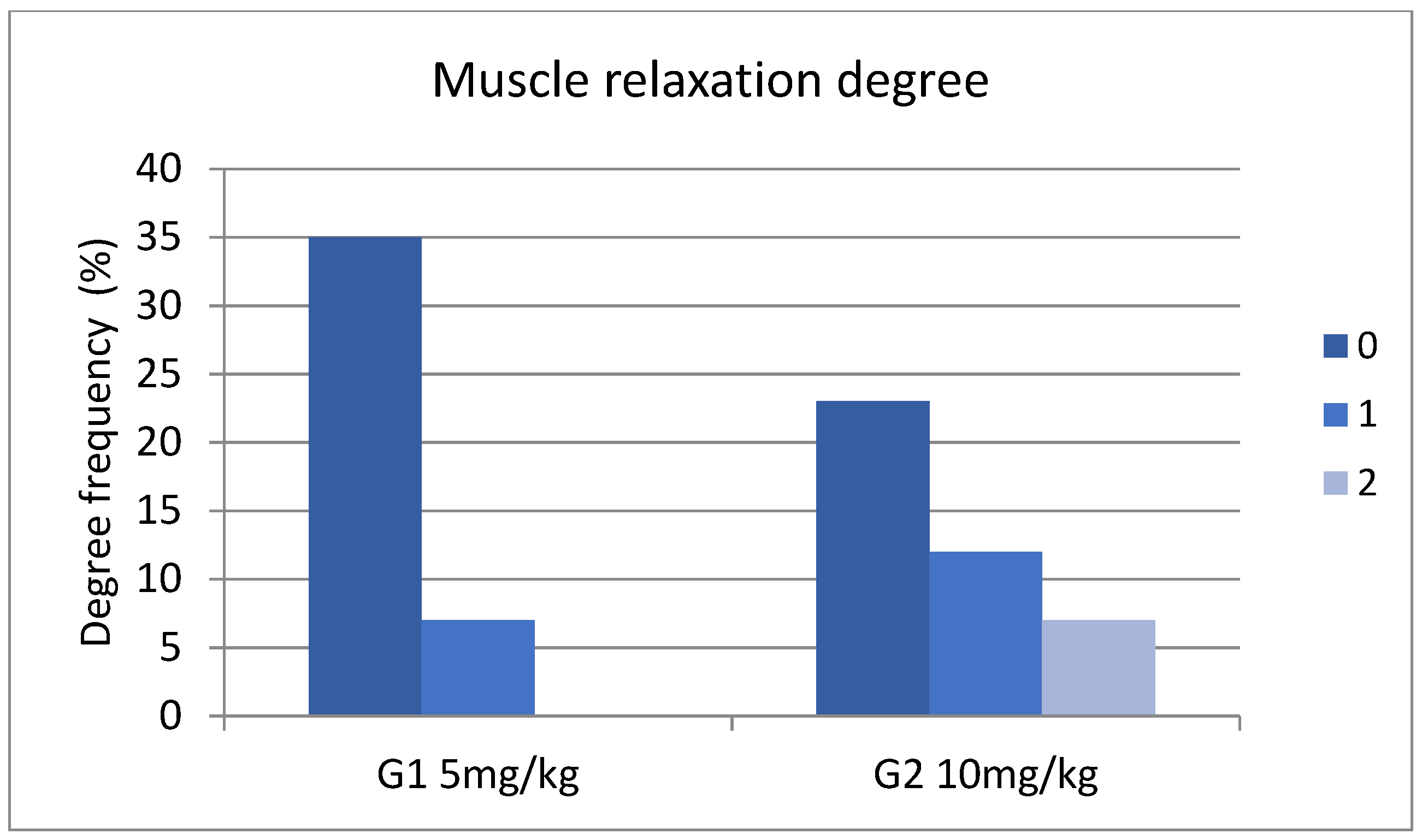
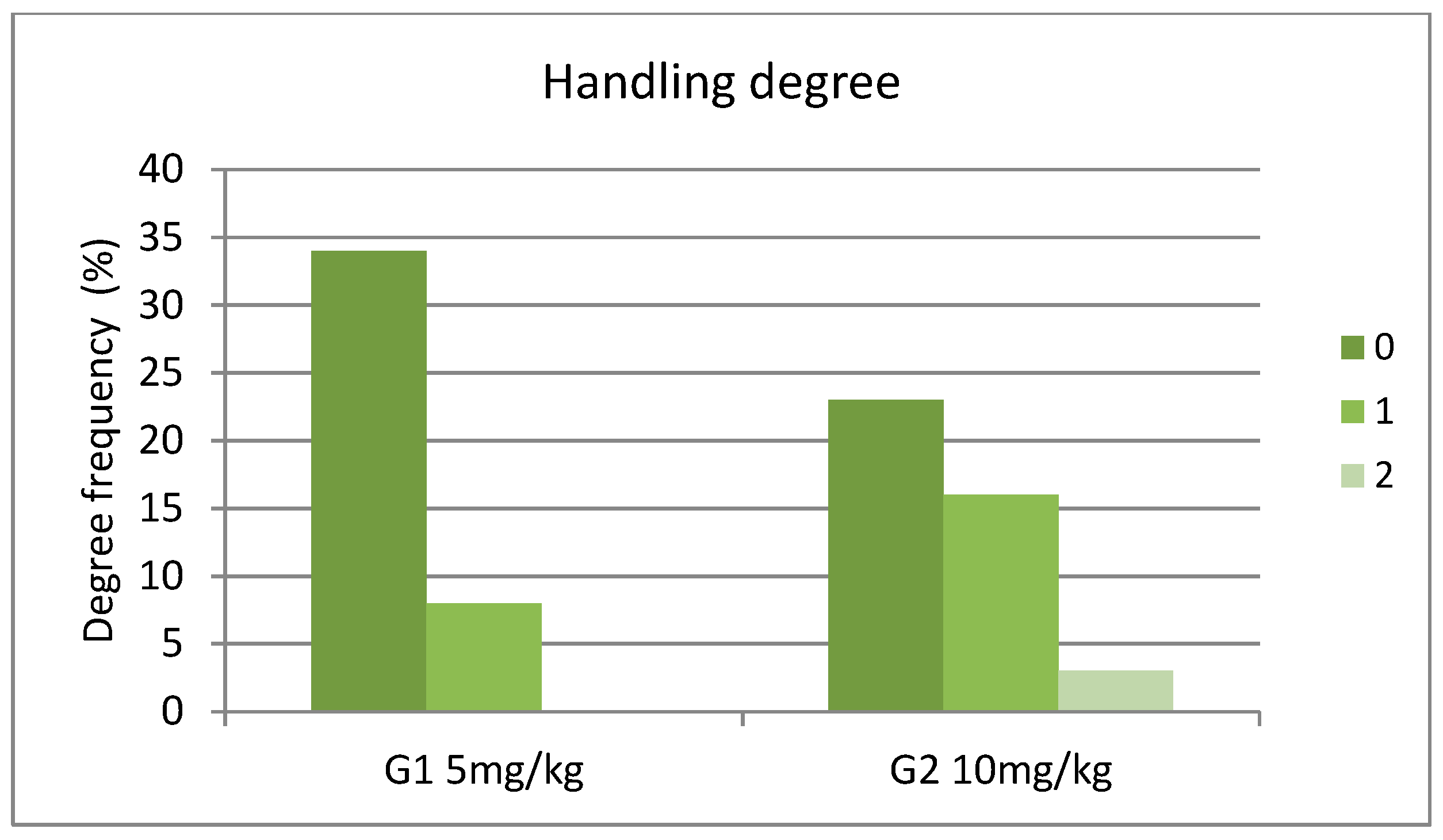
3. Results
4. Discussion
- Limitations of the Study
- Absence of Control Group: This study lacks a control group. This absence is justified by the lack of a consensus regarding a safe anesthetic protocol in turtles, making it difficult, if not impossible, to establish a positive control group.
- Small Sample Size: This study includes a small number of turtles due to the limited availability of individuals. As a result, the experiment was conducted as a small-scale crossover trial to limit the variability in the within-sliders comparisons.
- Generalization Limitation: Because of the small sample size and the absence of a control group, the results of this study cannot be generalized widely. However, they are considered an important advancement toward establishing a specific propofol dose for general use in turtle anesthesia.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hicks, J.W. Cardiac shunting in reptiles: Mechanisms, regulation and physiological functions. In Biology of the Reptilia; Morphology G: The Visceral Organs; Gans, C., Gaunt, A.S., Eds.; Society for the Study of Amphibians and Reptiles: Ohio, OH, USA, 1998; Volume 19, pp. 425–483. [Google Scholar]
- Eger, E.I.; Severinghaus, J.W. Effect of uneven pulmonary distribution of blood and gas on induction with inhalation anesthetics. Anesthesiology 1964, 25, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.E.; Angers, D.G.; Barash, P.G.; Mulla, A.; Miller, P.L.; Rothstein, P. Effect of left-to-right, mixed left-to-right, and right-to-left shunts on inhalational anesthetic induction in children: A computer model. Anesth. Analg. 1985, 64, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.A.; Malte, C.L.; Malte, H.; Bertelsen, M.F.; Wang, T. Ectothermy and cardiac shunts profoundly slow the equilibration of inhaled anesthetics in a multi-compartment model. Sci. Rep. 2020, 10, 17157. [Google Scholar] [CrossRef] [PubMed]
- Longley, L.A. Amphibian anesthesia. In Anesthesia of Exotic Pets; Saunders Elsevier: Edinburgh, UK, 2008; pp. 245–260. [Google Scholar]
- Bertelsen, M.F.; Buchanan, R.; Jensen, H.M.; Leite, C.A.; Abe, A.S.; Nielsen, S.S.; Wang, T. Assessing the influence of mechanical ventilation on blood gases and blood pressure in rattlesnakes. Vet. Anaesth. Analg. 2015, 42, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Digger, T.; Viira, D.J. Anaesthesia and surgical pain relief—The ideal general anesthetic agent. Hosp. Pharmacist. 2003, 10, 432–440. [Google Scholar]
- Bennet, R.A.; Schumacher, J.; Hedjazi Haring, K.; Newell, S.M. Cardiopulmonary and anesthetic effects of propofol administered intraosseously to green iguana. J. Am. Vet. Med. Ass. 1998, 212, 93–98. [Google Scholar] [CrossRef]
- Anderson, N.L.; Wack, R.F.; Calloway, L.; Hetherington, T. Cardiopulmonary effects and efficacy of propofol as an anesthetic agent in brown tree snakes (Boiga irregularis). Bull. Assoc. Reptil. Amphib. Vet. 1999, 9, 9–15. [Google Scholar] [CrossRef]
- MacLean, R.A.; Harms, C.A.; Braun-McNeill, J. Propofol anesthesia in loggerhead (Caretta caretta) sea turtles. J. Wildl. Dis. 2008, 44, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ziolo, M.S.; Bertelsen, F. Effects of propofol administered via the supravertebral sinus in red-eared sliders. J. Am. Vet. Med. Assoc. 2009, 234, 390–393. [Google Scholar] [CrossRef]
- Olsson, A.; Phalen, D.; Dart, C. Preliminary studies of alfaxalone for intravenous immobilization of juvenile captive estuarine crocodiles (Crocodylus porosus) and Australian freshwater crocodiles (Crocodylus johnstoni) at optimal and selected sub-optimal thermal zones. Vet. Anesth. Analg. 2013, 405, 494–502. [Google Scholar] [CrossRef]
- McKune, C.M.; Brosnan, R.J.; Dark, M.J.; Haldorson, G.J. Safety and efficacy of intramuscular propofol administration in rats. Vet. Anesth. Analg. 2008, 35, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.; Zardo, J.Q.; Hansen, S.M.; Bertelsen, M.F.; Alstrup, A.K.; Wang, T.; Williams, C.J. Effect of atropine and propofol on the minimum anaesthetic concentration of isoflurane in the freshwater turtle Trachemys scripta (yellow-bellied slider). Vet. Anesth. Analg. 2023, 50, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Yelen, T. Anesthesia and Analgesia. In Reptile Medicine and Surgery, 2nd ed.; Mader, D.R., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 442–452. [Google Scholar]
- Santos, A.L.; Bosso, A.C.; Alves Júnior, J.R.; Brito, F.M.M.; Pachally, J.R.; Ávila Junior, R.H. Pharmacological restraint of captivity giant amazonian turtle Podocnemis expansa (testudines, podocnemididae) with xylazine and propofol. Acta Cir. Bras. 2008, 23, 270–273. [Google Scholar] [CrossRef] [PubMed]
- McFadden, M.S.; Bennett, R.A.; Reavill, D.R.; Ragetly, G.R.; Clark-Price, S.C. Clinical and histologic effects of intracardiac administration of propofol for induction of anesthesia in ball pythons (Python regius). J. Am. Vet. Med. Assoc. 2011, 239, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Alves-Júnior, J.R.; Bosso, A.C.; Andrade, M.B.; Jayme, V.D.S.; Werther, K.; Santos, A.L.Q. Association of acepromazine with propofol in giant Amazon turtles Podocnemis expansa reared in captivity. Acta Cir. Bras. 2012, 27, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.A.; Johnson, R.A. The efficacy of intracoelomic fospropofol in red-eared sliders. J. Zoo. Wildl. Med. 2013, 44, 941. [Google Scholar] [CrossRef] [PubMed]
- Fonda, D. Intraosseous anesthesia with propofol in red-eared sliders (Trachemys scripta elegans). J. Vet. Anesth. 1999, 26, 46–47. [Google Scholar]
- Mans, C. Venipuncture techniques in chelonian species. Lab Anim. 2008, 37, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Bel, L.V.; Selleri, P. Use of a light source to help identify the jugular vein in chelonians. J. Small. Anim. Pract. 2018, 59, 197. [Google Scholar] [CrossRef]
- Murray, M.M. Cardiopulmonary anatomy and physiology. In Reptile Medicine and Surgery, 2nd ed.; Mader, D.R., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 124–128. [Google Scholar]
- O’Malley, B. (Ed.) Tortoises and Turtles. In Clinical Anatomy and Physiology of Exotic Species; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 41–56. [Google Scholar]
- Holz, P.; Barker, I.K.; Crawshaw, G.J.; Dobson, H. The anatomy and perfusion of the renal portal system in the red-eared slider (Trachemys scripta elegans). J. Zoo Wild. Med. 1997, 28, 378–385. [Google Scholar]
- Holz, P.; Raidal, S. Comparative renal anatomy of exotic species. Vet. Clin. N. Am. Exot. Anim. Pract. 2006, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lahner, L.; Mans, C.; Sladky, K.K. Comparison of anesthetic induction and recovery times after intramuscular, subcutaneous or intranasal dexmedetomidine-ketamine administration in red-eared slider turtles (Trachemys scripta elegans). In Proceedings of the AAZV Conference, Kansas City, MO, USA, 22–28 October 2011. [Google Scholar]
- Sladky, K.K.; Mans, C. Clinical anesthesia in reptiles. J. Exot. Pet. Med. 2012, 21, 17–31. [Google Scholar] [CrossRef]
- Holz, P.; Barker, I.K.; Burger, J.P.; Crawshaw, G.J.; Conlon, P.D. The effect of the renal portal system on pharmacokinetic parameters in the red-eared slider (Trachemys scripta elegans). J. Zoo Wildl. Med. 1997, 28, 386–393. [Google Scholar] [PubMed]
- Giorgi, M.; Salvadori, M.; De Vito, V.; Owen, H.; Demontis, M.P.; Varoni, M.V. Pharmacokinetic/ Pharmacodynamic assessments of 10 mg/kg tramadol intramuscular injection in yellow-bellied slider turtles (Trachemys scripta Scripta). J. Vet. Pharmacol. Ther. 2015, 38, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, M.D.; Faraci, L.; Luparello, M. Preliminary survey on the influence of renal portal system during propofol anesthesia in yellow-bellied turtle (Trachemys scripta scripta). Vet. Med. Allied Sci. 2018, 2, 1–3. [Google Scholar]
- Morici, M.; Lubian, E.; Costa, G.L.; Spadola, F. Difference between cranial and caudal intravenous alfaxalone administration in yellow-bellied sliders (Trachemys scripta scripta). Acta Vet. Eurasia 2021, 47, 88–92. [Google Scholar] [CrossRef]
- Shepard, M.K.; Divers, S.; Braun, C.; Hofmeister, E.H. Pharmacodynamics of alfaxalone after single-dose intramuscular administration in red-eared sliders (Trachemys scripta elegans): A comparison of two different doses at two different ambient temperatures. Vet. Anesth. Analg. 2013, 40, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Divers, S.M.; Hernandez-Divers, S.J.; Wyneken, J. Angiographic, anatomic and clinical technique descriptions of a subcarapacial venipuncture site for chelonians. J. Herpetol. Med. Surg. 2002, 12, 32–37. [Google Scholar] [CrossRef]
- Innis, C.; DeVoe, R.; Mylniczenko, N.A. Call for additional study of the safety of subcarapacial venipuncture in chelonians. In Proceedings of the ARAV, Seventh Annual Conference, South Padre Island, TX, USA, 23–39 October 2010; pp. 8–10. [Google Scholar]
- Quesada, R.J.; Aitken-Palmer, C.; Conley, K.; Heard, D.J. Accidental submeningeal injection of propofol in gopher tortoises (Gopherus polyphemus). Vet. Rec. 2010, 167, 494–495. [Google Scholar] [CrossRef]
- Rockwell, K.; Rademacher, N.; Osborn, M.L.; Nevarez, J.G. Extravasation of contrast media after subcarapacial vessel injection in three chelonian species. J. Zoo Wildl. Med. 2022, 53, 402–411. [Google Scholar] [CrossRef]
- Ciccarelli, S.; Valastro, C.; Di Bello, A.; Paci, S.; Caprio, F.; Corrente, M.L.; Trotta, A.; Franchini, D. Diagnosis and treatment of pulmonary disease in sea turtles (Caretta caretta). Animals 2020, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, M.F. Anaesthesia and analgesia. In BSAVA Manual of Reptiles; Girling, S.J., Raiti, P., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2019; pp. 200–209. [Google Scholar]
- Kischinovsky, M.; Duse, A.; Wang, T.; Bertelsen, M.F. Intramuscular administration of alfaxalone in red-eared sliders (Trachemys scripta elegans) effects of dose and body temperature. Vet. Anesth. Analg. 2013, 40, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Perpiñán, D. Reptile anesthesia and analgesia. Companion Anim. 2018, 23, 236–243. [Google Scholar] [CrossRef]
- Muir, W.W., 3rd; Gadawski, J.E. Respiratory depression and apnea induced by propofol in dogs. Am. J. Vet. Res. 1998, 59, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.A.; Dzikiti, B.T.; Stegmann, F.G.; Hartman, M. Comparison of alfaxalone and propofol administered as total intravenous anaesthesia for ovariohysterectomy in dogs. Vet. Anaesth. Analg. 2012, 3, 36–244. [Google Scholar] [CrossRef] [PubMed]
- Maney, J.K.; Shepard, M.K.; Braun, C.; Cremer, J.; Hofmeister, E.H. A comparison of cardiopulmonary and anesthetic effects of an induction dose of alfaxalone or propofol in dogs. Vet. Anaesth. Analg. 2013, 40, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cabanac, A.; Cabanac, M. Heart rate response to gentle handling of frog and lizard. Behav. Process. 2000, 52, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Bertelsen, M.F. Intravenous alfaxalone and propofol anesthesia in the bearded dragon (Pogona vitticeps). J. Herpetol. Med. Surg. 2017, 27, 123–126. [Google Scholar] [CrossRef]
- Fleming, G.J.; Heard, D.J.; Floyd, R.F.; Riggs, A. Evaluation of propofol and medetomidine-ketamine for short-term immobilization of Gulf of Mexico sturgeon (Acipenser oxyrinchus de Soti). J. Zoo Wild. Med. 2003, 34, 153–158. [Google Scholar] [CrossRef]
- Mitchell, M.A.; Riggs, S.M.; Singleton, C.B.; Diaz-Figueroa, O.; Hale, L.K. Evaluating the clinical and cardiopulmonary effects of clove oil and propofol in tiger salamanders (Ambystoma tigrinum). J. Exot. Pet. Med. 2008, 18, 50–56. [Google Scholar] [CrossRef]
- Wojick, K.B.; Langan, J.N.; Mitchell, M.A. Evaluation of MS-222 (Tricaine methanesulfonate) and propofol as anesthetic agents in Sonoran desert toads (Bufo alvarius). J. Herp. Med. Surg. 2010, 20, 79–83. [Google Scholar] [CrossRef]
- Machin, K.L.; Caulkett, N.A. Cardiopulmonary effects of propofol infusion in canvasback ducks (Aythya valisineria). J. Avian Med. Surg. 1999, 13, 167–172. [Google Scholar]
- Read, M.R. Evaluation of the use of anesthesia and analgesia in reptiles. J. Am. Vet. Med. Assoc. 2004, 224, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, M.F.; Buchanan, R.; Jensen, H.M.; Leite, C.A.; Abe, A.S.; Wang, T. Pharmacodynamics of propofol and alfaxalone in rattlesnakes (Crotalus durissus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 256, 110935. [Google Scholar] [CrossRef]
- Oppenheim, Y.C.; Moon, P.F. Sedative effects of midazolam in red-eared slider turtles (Trachemys scripta elegans). J. Zoo Wildl. Med. 1995, 26, 409–413. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel, L.V.; Selleri, P.; Turcu, C.M.; Cerbu, C.; Matei, I.A.; Masi, M.; Melega, I. Comparison of Two Intravenous Propofol Doses after Jugular Administration for Short Non-Surgical Procedures in Red-Eared Sliders (Trachemys scripta elegans). Animals 2024, 14, 1847. https://doi.org/10.3390/ani14131847
Bel LV, Selleri P, Turcu CM, Cerbu C, Matei IA, Masi M, Melega I. Comparison of Two Intravenous Propofol Doses after Jugular Administration for Short Non-Surgical Procedures in Red-Eared Sliders (Trachemys scripta elegans). Animals. 2024; 14(13):1847. https://doi.org/10.3390/ani14131847
Chicago/Turabian StyleBel, Lucia Victoria, Paolo Selleri, Carmen Maria Turcu, Constantin Cerbu, Ioana Adriana Matei, Marco Masi, and Iulia Melega. 2024. "Comparison of Two Intravenous Propofol Doses after Jugular Administration for Short Non-Surgical Procedures in Red-Eared Sliders (Trachemys scripta elegans)" Animals 14, no. 13: 1847. https://doi.org/10.3390/ani14131847
APA StyleBel, L. V., Selleri, P., Turcu, C. M., Cerbu, C., Matei, I. A., Masi, M., & Melega, I. (2024). Comparison of Two Intravenous Propofol Doses after Jugular Administration for Short Non-Surgical Procedures in Red-Eared Sliders (Trachemys scripta elegans). Animals, 14(13), 1847. https://doi.org/10.3390/ani14131847




