Food Grinding Behavior: A Review of Causality and Influential Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
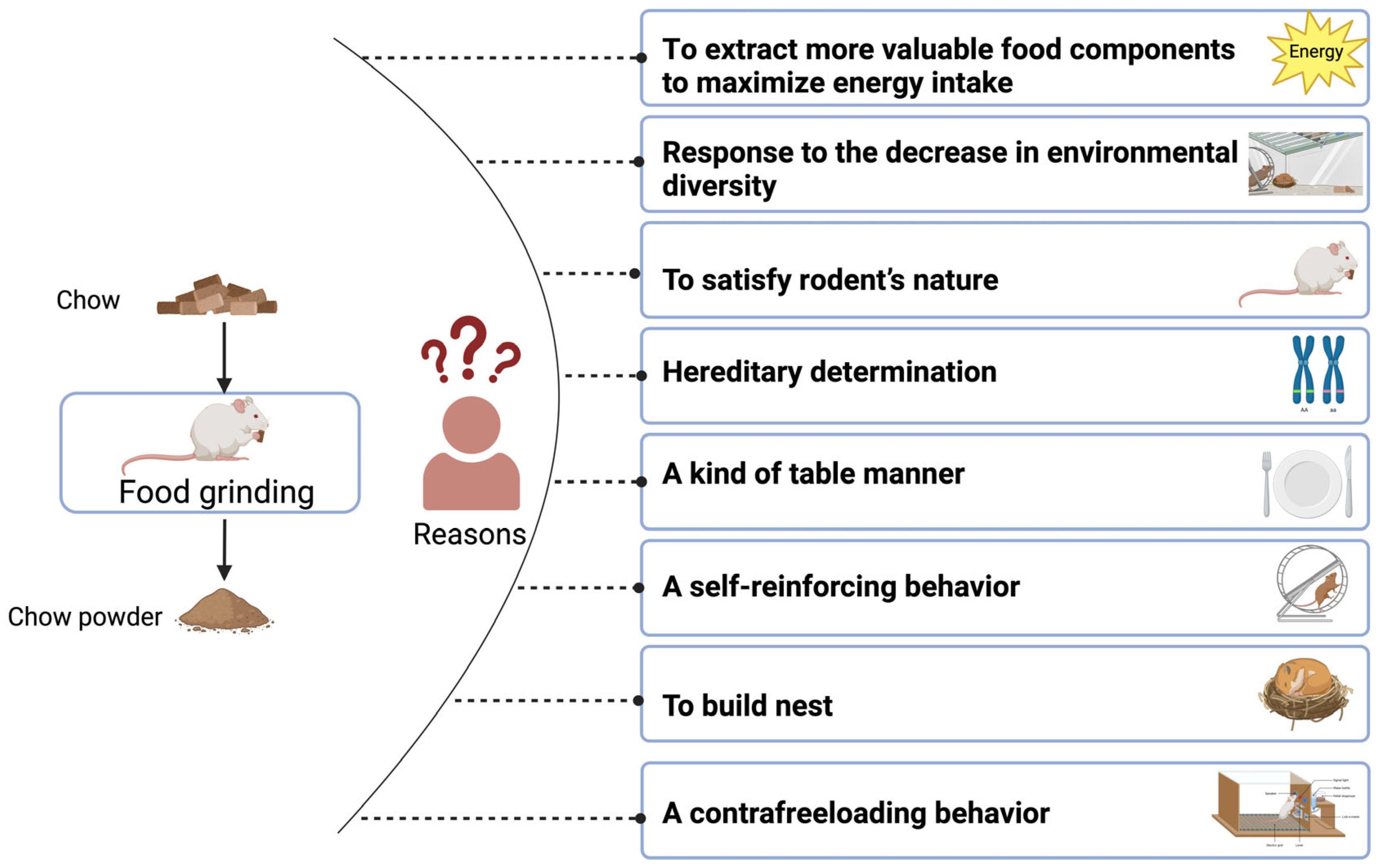
3. Reasons for the Rodent Food Grinding Phenomenon
3.1. Optimal Foraging Theory
3.2. Reduced Environmental Diversity
3.3. Rodent Nature
3.4. Hereditary Determination
3.5. Type of Table Manners
3.6. Self-Reinforcing Behavior
3.7. Nest Building
3.8. Contrafreeloading Behavior
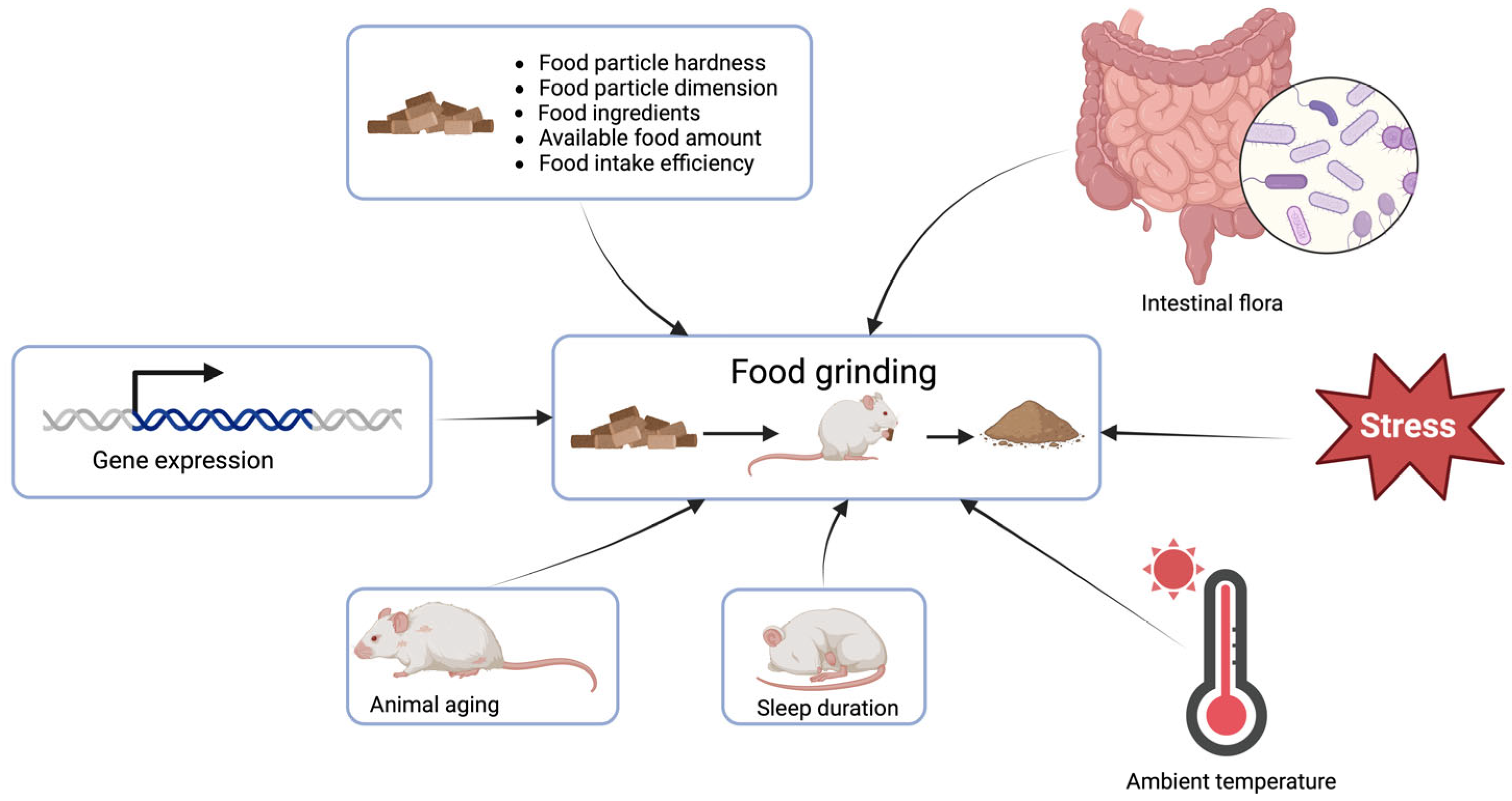
4. Factors Affecting Rodent Food Grinding
4.1. Hardness of Food Particles
4.2. Food Availability and Food Intake Efficiency
4.3. Ingredients in Food
4.4. Length of Sleep
4.5. Aging of Animals
4.6. Intestinal Flora of Animals
4.7. Stress in Animals
4.8. Gene Expression
4.9. Other Factors
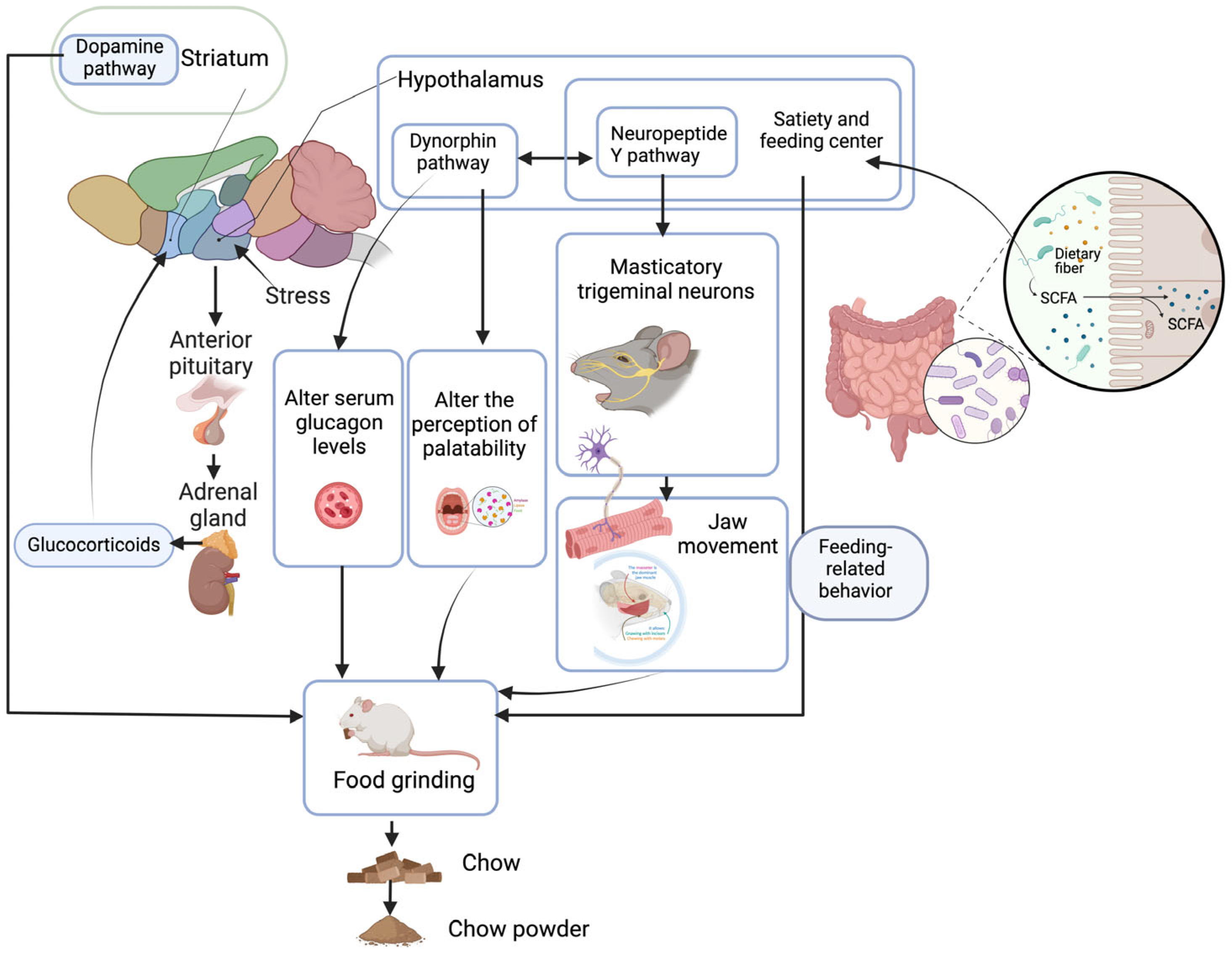
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: New York, NY, USA, 1994. [Google Scholar]
- Starr, M.E.; Saito, H. Age-related increase in food spilling by laboratory mice may lead to significant overestimation of actual food consumption: Implications for studies on dietary restriction, metabolism, and dose calculations. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Koteja, P.; Carter, P.A.; Swallow, J.G.; Garland, T., Jr. Food wasting by house mice: Variation among individuals, families, and genetic lines. Physiol. Behav. 2003, 80, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Wunder, B.A. Food Sorting by Collared Lemmings (Dicrostonyx groenlandicus) and Prairie Voles (Microtus ochrogaster): A Cautionary Note for Digestibility Studies. Comp. Biochem. Physiol. A Physiol. 1997, 116, 119–124. [Google Scholar] [CrossRef]
- Gesing, A.; Masternak, M.M.; Wang, F.; Joseph, A.M.; Leeuwenburgh, C.; Westbrook, R.; Lewinski, A.; Karbownik-Lewinska, M.; Bartke, A. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sadighi Akha, A.A.; Miller, R.A.; Harper, J.M. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Flurkey, K.; Astle, C.M.; Harrison, D.E. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1275–1284. [Google Scholar] [CrossRef]
- Barnes, R.H.; Neely, C.S.; Kwong, E.; Labadan, B.A.; Frankova, S. Postnatal nutritional deprivations as determinants of adult rat behavior toward food, its consumption and utilization. J. Nutr. 1968, 96, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hastings, I.M.; Moruppa, S.M.; Bunger, L.; Hill, W.G. Effects of selection on food intake in the adult mouse. J. Anim. Breed. Genet. 1997, 114, 419–434. [Google Scholar] [CrossRef]
- Owl, M.Y.; Batzli, G.O. The integrated processing response of voles to fibre content of natural diets. Funct. Ecol. 1998, 12, 4–13. [Google Scholar] [CrossRef]
- Kerley, G.I.; Erasmus, T. What do mice select for in seeds? Oecologia 1991, 86, 261–267. [Google Scholar] [CrossRef]
- Felicetti, L.A.; Shipley, L.A.; Witmer, G.W.; Robbins, C.T. Digestibility, nitrogen excretion, and mean retention time by North American porcupines (Erethizon dorsatum) consuming natural forages. Physiol. Biochem. Zool. 2000, 73, 772–780. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, Y.X.; Wen, Y.X. Rodents in China; Fudan University Press: Shanghai, China, 1995. [Google Scholar]
- Shen, Q.Y.; Shi, J.Y.; Gu, K.H.; Wei, W.H.; Yang, S.M.; Dai, X. Relationship between food grinding and gut microbiota in Brandt’s voles. Can. J. Zool. 2023, 101, 623–634. [Google Scholar] [CrossRef]
- Tertil, R. Impact of the Common Vole, Microtus arvalis (Pallas) on Winter Wheat and Alfalfa Crops. EPPO Bull. 1977, 7, 317–339. [Google Scholar] [CrossRef]
- Dai, X.; Han, Y.X.; Shen, Q.Y.; Tang, H.; Cheng, L.Z.; Yang, F.P.; Wei, W.H.; Yang, S.M. Effect of Food Restriction on Food Grinding in Brandt’s Voles. Animals 2023, 13, 3424. [Google Scholar] [CrossRef]
- Cameron, K.M.; Speakman, J.R. The extent and function of ‘food grinding’ in the laboratory mouse (Mus musculus). Lab. Anim. 2010, 44, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.H. Comments on relationships between native seed preferences of shrub-steppe granivores and seed nutritional characteristics. Oecologia 1988, 75, 481–482. [Google Scholar] [CrossRef]
- Pritchett-Corning, K.R.; Keefe, R.; Garner, J.P.; Gaskill, B.N. Can seeds help mice with the daily grind? Lab. Anim. 2013, 47, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Chi, Q.S.; Zhao, L.; Zhu, Q.X.; Cao, J.; Wang, D.H. Effect of food restriction on energy budget in warm-acclimated striped hamsters. Physiol. Behav. 2015, 147, 220–226. [Google Scholar] [CrossRef]
- Olsson, I.A.; Dahlborn, K. Improving housing conditions for laboratory mice: A review of “environmental enrichment”. Lab. Anim. 2002, 36, 243–270. [Google Scholar] [CrossRef]
- Martins, P.J.; Nobrega, J.N.; Tufik, S.; D’Almeida, V. Sleep deprivation-induced gnawing-relationship to changes in feeding behavior in rats. Physiol. Behav. 2008, 93, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Fiala, B.; Snow, F.M.; Greenough, W.T. “Impoverished” rats weigh more than “enriched” rats because they eat more. Dev. Psychobiol. 1977, 10, 537–541. [Google Scholar] [CrossRef]
- Wurbel, H.; Stauffacher, M. Prevention of stereotypy in laboratory mice: Effects on stress physiology and behaviour. Physiol. Behav. 1996, 59, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Koteja, P.; Swallow, J.G.; Carter, P.A.; Garland, T. Individual variation and repeatability of maximum cold-induced energy assimilation in house mice. Acta Theriol. 2000, 45, 455–470. [Google Scholar] [CrossRef]
- Hammond, K.A.; Diamond, J. Maximal sustained energy budgets in humans and animals. Nature 1997, 386, 457–462. [Google Scholar] [CrossRef]
- Sherwin, C.M. Voluntary wheel running: A review and novel interpretation. Anim. Behav. 1998, 56, 11–27. [Google Scholar] [CrossRef] [PubMed]
- van Zeeland, Y.R.A.; Schoemaker, N.J.; Lumeij, J.T. Contrafreeloading indicating the behavioural need to forage in healthy and feather damaging grey parrots. Animals 2023, 13, 2635. [Google Scholar] [CrossRef]
- Osborne, S.R. The free food (contrafreeloading) phenomenon: A review and analysis. Anim. Learn. Behav. 1977, 5, 221–235. [Google Scholar] [CrossRef]
- Höhne, A.; Petow, S.; Bessei, W.; Schrader, L. Contrafreeloading and foraging-related behavior in hens differing in laying performance and phylogenetic origin. Poult. Sci. 2023, 102, 102489. [Google Scholar] [CrossRef]
- Larson, L.D.; Tarte, R.D. The effects of training and effortfulness on rats’ choice behavior in a modified T-maze. Bull. Psychonom. Soc. 1976, 7, 506–508. [Google Scholar] [CrossRef]
- Forkman, B. The foraging behaviour of Mongolian gerbils: A behavioural need or a need to know? Behaviour 1996, 133, 129–143. [Google Scholar] [CrossRef]
- Reinhardt, V. Caged rhesus macaques voluntarily work for ordinary food. Primates 1994, 35, 95–98. [Google Scholar] [CrossRef]
- Inglis, I.R.; Forkman, B.; Lazarus, J. Free food or earned food? A review and fuzzy model of contrafreeloading. Anim. Behav. 1997, 53, 1171–1191. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.J. Stereotypies and suffering. Behav. Process. 1991, 25, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.J. Effect of autoclaving and physical structure of diets on their utilization by mice. Lab. Anim. 1977, 11, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.J. Influence of diet pellet hardness and particle size on food utilization by mice, rats and hamsters. Lab. Anim. 1977, 11, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, A.; Lin, S.; McNamara, K.; Slack, K.; Enriquez, R.; Lee, N.J.; Boey, D.; Smythe, G.A.; Schwarzer, C.; Baldock, P.; et al. Dynorphin knockout reduces fat mass and increases weight loss during fasting in mice. Mol. Endocrinol. 2007, 21, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, A.R.; Levitsky, D.A. Spillage behavior and thiamin deficiency in the rat. Physiol. Behav. 1982, 28, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.J.; D’Almeida, V.; Nobrega, J.N.; Tufik, S. A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation. Sleep 2006, 29, 1233–1238. [Google Scholar] [CrossRef]
- Nez, L.; Kubota, J.; Warren, M.; Henderson, R.; Phillips, M.; Schreiber, H.L., 3rd. REM sleep deprivation effect on apomorphine-induced gnawing is reversed by dexamethasone. Sleep 1985, 8, 283–287. [Google Scholar] [CrossRef]
- Nunes Junior, G.P.; Tufik, S.; Nobrega, J.N. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res. Bull. 1994, 34, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xie, T.; Xi, Y.; Li, L.; Mo, F.; Liu, X.; Liu, Z.; Gao, J.M.; Yuan, T. Sesamol Attenuates Amyloid Peptide Accumulation and Cognitive Deficits in APP/PS1 Mice: The Mediating Role of the Gut-Brain Axis. J. Agric. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef] [PubMed]
- Baldock, P.A.; Allison, S.J.; Lundberg, P.; Lee, N.J.; Slack, K.; Lin, E.J.; Enriquez, R.F.; McDonald, M.M.; Zhang, L.; During, M.J.; et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J. Biol. Chem. 2007, 282, 19092–19102. [Google Scholar] [CrossRef] [PubMed]
- Lyengar, S.; Li, D.L.; Simmons, R.M. Characterization of neuropeptide Y-induced feeding in mice: Do Y1-Y6 receptor subtypes mediate feeding? J. Pharmacol. Exp. Ther. 1999, 289, 1031–1040. [Google Scholar]
- Uguru-Okorie, D.C. Feeding behavior and sensorimotor functioning in rats with unilateral medial forebrain bundle damage. Psychobiology 2013, 12, 311–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Ge, W.-W.; Wei, W.-H.; Yang, S.-M.; Dai, X. Food Grinding Behavior: A Review of Causality and Influential Factors. Animals 2024, 14, 1865. https://doi.org/10.3390/ani14131865
Tang H, Ge W-W, Wei W-H, Yang S-M, Dai X. Food Grinding Behavior: A Review of Causality and Influential Factors. Animals. 2024; 14(13):1865. https://doi.org/10.3390/ani14131865
Chicago/Turabian StyleTang, Hao, Wei-Wei Ge, Wan-Hong Wei, Sheng-Mei Yang, and Xin Dai. 2024. "Food Grinding Behavior: A Review of Causality and Influential Factors" Animals 14, no. 13: 1865. https://doi.org/10.3390/ani14131865
APA StyleTang, H., Ge, W.-W., Wei, W.-H., Yang, S.-M., & Dai, X. (2024). Food Grinding Behavior: A Review of Causality and Influential Factors. Animals, 14(13), 1865. https://doi.org/10.3390/ani14131865




