Studying Chondrichthyans Using Baited Remote Underwater Video Systems: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Literature Survey and Data Selection
| Source | Survey Location | Type of BRUVS | Camera Configurations | Bait Types | Topics |
|---|---|---|---|---|---|
| [28] | South Africa | benthic | Stereo, mono | Sardinops sargax | P, A |
| [29] | Caribbean | benthic | Stereo | Tuna | P, A, B |
| [30] | Colombian Pacific | mid-water | Mono | Tuna | P, A |
| [31] | North Western Australia | benthic | Stereo | - | P, DN, A |
| [27] | Australia | benthic | Stereo | Pilchards | P, A, HU |
| [32] | South Africa | benthic | Mono | Sardinops sargax | P, A |
| [19] | North Western Australia | benthic | Stereo | - | P, DN, A, S, HS |
| [33] | The north coast of Brittany | benthic | Stereo | Scomber scombrus | P, A, DY |
| [34] | Belize | benthic | Mono | Crushed baitfish | P, DN, A |
| [35] | Western Australia | drifting deep-water | Stereo | Sardinops sagax | P, HS |
| [36] | South-western Pacific | benthic | Stereo | Sardinops sagax | P, DN, DY |
| [37] | Central Pacific | benthic | Stereo | Mackerel | P, DN, A |
| [11] | Bahamas | benthic | Mono | Bonito tuna | P, DN, A, DY, S |
| [38] | Turks and Caicos Islands | benthic | Mono | Barracuda sphyraena | P, DN, A, DY |
| [39] | Caribbean coast of Colombia | benthic | Mono | Opisthonema oglinum | P, A |
| [40] | Eastern Tropical Pacific, Costa Rica, Galapagos Islands | drifting-pelagic | Mono | Thunnus albacares | P, DN, A |
| [18] | North-east Australia | benthic | Mono | Sardinops neopilchardus | P, DN, A, DY |
| [41] | Central Mediterranean Sea | mid-water | Mono | Sardinella aurita | P, S |
| [42] | Panama | benthic | Stereo | Euthynnus alletteratus | P, A, DY |
| [43] | Eastern Tropical Pacific | benthic | Stereo | Mugil cephalus, Katsuwonus pelamis | P |
| [44] | Western Indian Ocean | benthic | Mono | Mackerel | P, DN, A |
| [45] | Southeast Australia | benthic | Mono | - | P, A, DE |
| [26] | Pacific Ocean | benthic | Mono | Clupeidae, Scombridae | P, D |
| [46] | South Africa | benthic | Mono | Sardinops sagax | P, DN, A, DY |
| [47] | South Africa | benthic | Stereo | Sardinops sagax | P, DN, A, DY |
| [24] | North-east Australia | benthic | Mono | Sardinops, Sardinella spp. | P, DN, A, TH |
| [48] | Eastern Tropical Pacific | benthic & mid-water | Mono | Scomber japonicus | P, DN, A |
| [49] | Belize | benthic | Mono | Sardines | P, A |
| [50] | Western Atlantic | benthic | Mono | Sardines | P |
| [51] | Fiji | benthic | Stereo | Sardinops sp. | P, A, B |
| [52] | Southern California | benthic | Mono | Loligo sp. | P, A |
| [53] | Seychelles | benthic | Mono | Scomber scombrus, Euthynnus affinis | P, DN, A, M |
| [54] | New South Wales, Australia | benthic | Stereo | Sardinops neopilchardus | P, S |
| [55] | New South Wales, Australia | benthic | Stereo | Sardinops neopilchardus | P, A, G, S |
| [56] | Western Australian | benthic | Stereo | Sardinops sagax | P, A, S |
| [57] | Western Australia | benthic | Stereo | Sardinops sagax | P, A |
| [58] | Eastern Australia | mid-water | Mono | Minced pilchards, bread, tuna oil | P, DN, A, TH |
| [59] | Southern New Zealand | downward-facing benthic | Mono | Thyrsites atun, Sardinella sirm | P, A |
| [60] | Antilles | drumline camera | Mono | Sphyraena barracuda | P, BR |
| [61] | Southeast Australia | benthic | Stereo | Pilchards | P, DN, DY, TH |
| [62] | Arabian Gulf | benthic | Mono | Sardinella longiceps | P, DN, A, TH |
| [63] | South-Western Pacific | benthic | Stereo | Sardinops sagax | P, DN, A, DY |
| [64] | New South Wales, Australia | benthic | Mono | Sardinops sagax | P, A |
| [65] | French Polynesia | benthic | Mono | Sardines | P, A |
| [66] | New South Wales, Australia | benthic | Stereo, Mono | Sardinops sagax | P |
| [67] | Western Atlantic | benthic | Mono | Scomber scombrus | P, A |
| [13] | North-west Australia | benthic | Stereo | Sardinops spp. | P, A, BR |
| [68] | Western Australia | mid-water | Stereo | Sardinops sagax, squid | P, DN, DY, S |
| [69] | New Zealand | downward-facing benthic | Stereo | - | P, S |
| [20] | Worldwide | benthic | Mono | Clupeidae, Scombridae | P, DN, A |
| [70] | Western Australia | mid-water | Stereo | Tuna, Sardinops sp. | P, A, BR |
| [71] | Philippines | benthic | Mono | Sphyraena barracuda, Caranx ignobilis, Serranidae spp., Acanthocybium solandri, Katsuwonus pelamis, Sardinus spp. | P, A, DY |
| [23] | Worldwide | mid-water | Stereo | Sardinops sagax | P, DN, A, S |
| [72] | South-West Australia | benthic | Mono | Sepioteuthis australis, Seriola lalandi, Sardinops sagax | P, DN |
| [73] | Northeastern Brazil | benthic | Mono | Sardinella brasiliensis, Sphyraena barracuda | P |
| [74] | South Africa | benthic | Mono | Sardinops sagax | P, A, DY |
| [75] | Sweden | benthic | Stereo | Sardines | P, A, S |
| [76] | Central Indian Ocean | drifting-pelagic | Mono | - | P |
| [77] | Antilles | benthic | Mono | Sarda spp. | P, BR |
| [78] | Western Mediterranean | drifting-pelagic | Mono | Tuna | P |
| [79] | North-east Atlantic | benthic | Mono | Scomber scombrus | P |
| [80] | Brazil | benthic | Stereo | Sardinella brasiliensis, Harengula sp. | P, A, S |
| [81] | North-west Australia | benthic | Mono | Sardinops sagax | P, A, BR |
| [16] | Central Pacific Ocean | benthic | Mono | - | P, A, BR |
| [82] | Western Australia | mid-water | Stereo | Mugil cephalus | P, A, DY, S |
| [25] | Mid-west coast of Western Australia | mid-water | Stereo | Sardinops sagax | P, A |
| [83] | Western Australia | mid-water | Stereo | Sardinops sagax | P |
| [15] | Massachusetts | benthic | Mono | Mackerel | P, BR |
| [84] | Malaysian Borneo | benthic | Mono | Sardinella spp, Scomber australiasicus | P, A |
| [85] | Indonesia | benthic | Mono | Clupeidae, Scombridae | P, A |
| [14] | Worldwide | benthic | Mono | - | P, DN, A, DY |
| [86] | Red Sea, Sudan | benthic | Mono | Sarda orientalis, Caranx spp., Lutjanus spp. | P, DN, A |
| [87] | Spain, France | benthic | Mono | Flour, dried chopped fish, sardine oil, sunflower oil, sodium bicarbonate, citric acid | P, A |
| [88] | Sydney metropolitan area | benthic | Mono | Pilchards, falafel, tuna oil | P, A |
| [22] | Worldwide | mid-water | Stereo | - | P, A, DY, S, B |
| [89] | Western Mediterranean Sea | mid-water | Mono | Fish scraps, cephalopods, cetacean flesh and oil | P |
| [90] | Wales | benthic | Stereo | Meal and fish oils | P, A |
| [21] | South-western Australia | benthic | Stereo | Sardinops sagax | P, A, DE |
| [91] | North-east Australia | benthic | Mono | Sardinops spp., Sardinella spp. | P, DN |
| [92] | New South Wales, Australia | benthic | Mono | Pilchard, abalone, urchin | P, A |
| [93] | North-eastern New Zealand | benthic | Stereo | Sardinops sagax | P, DN, A, DE |
3. Results
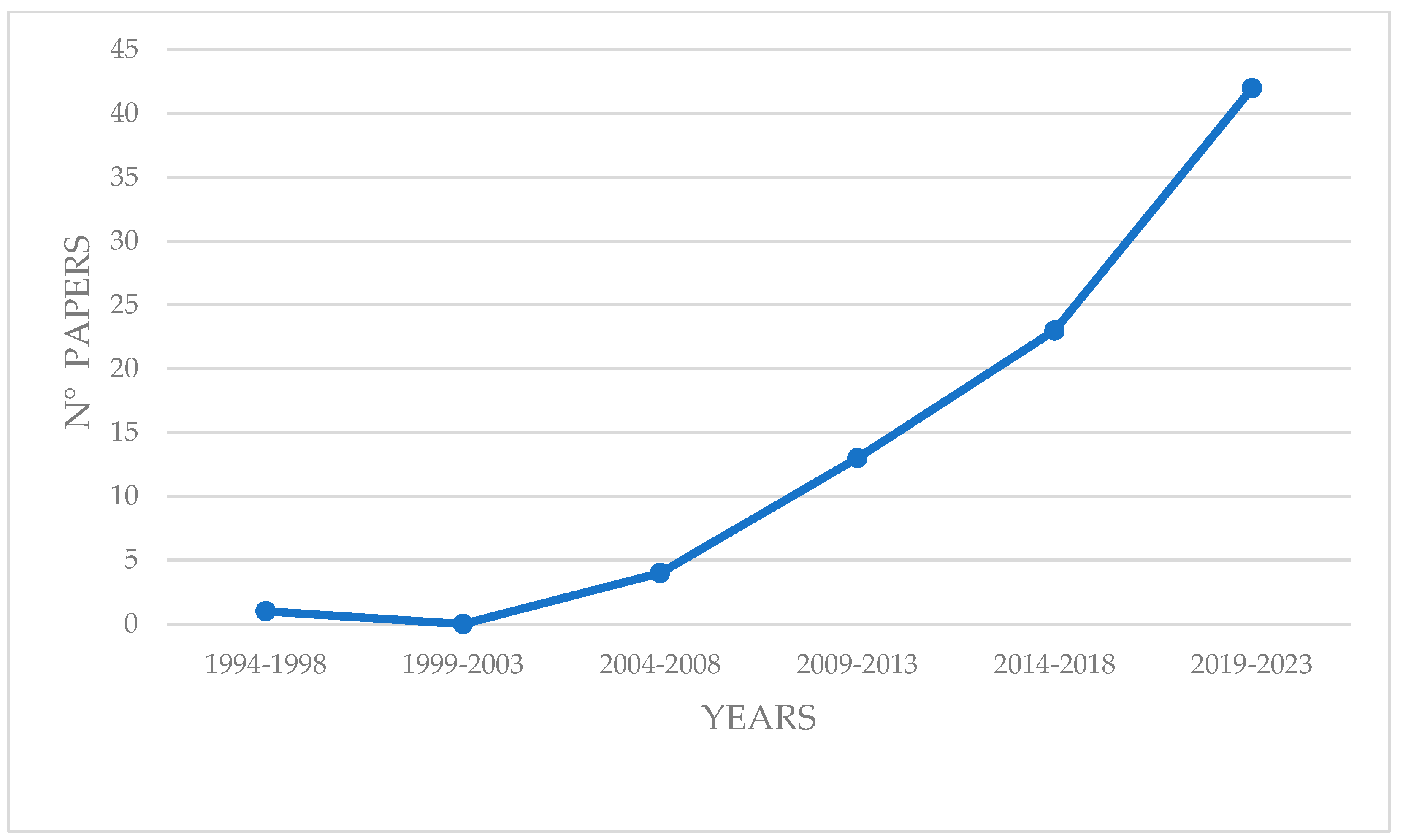
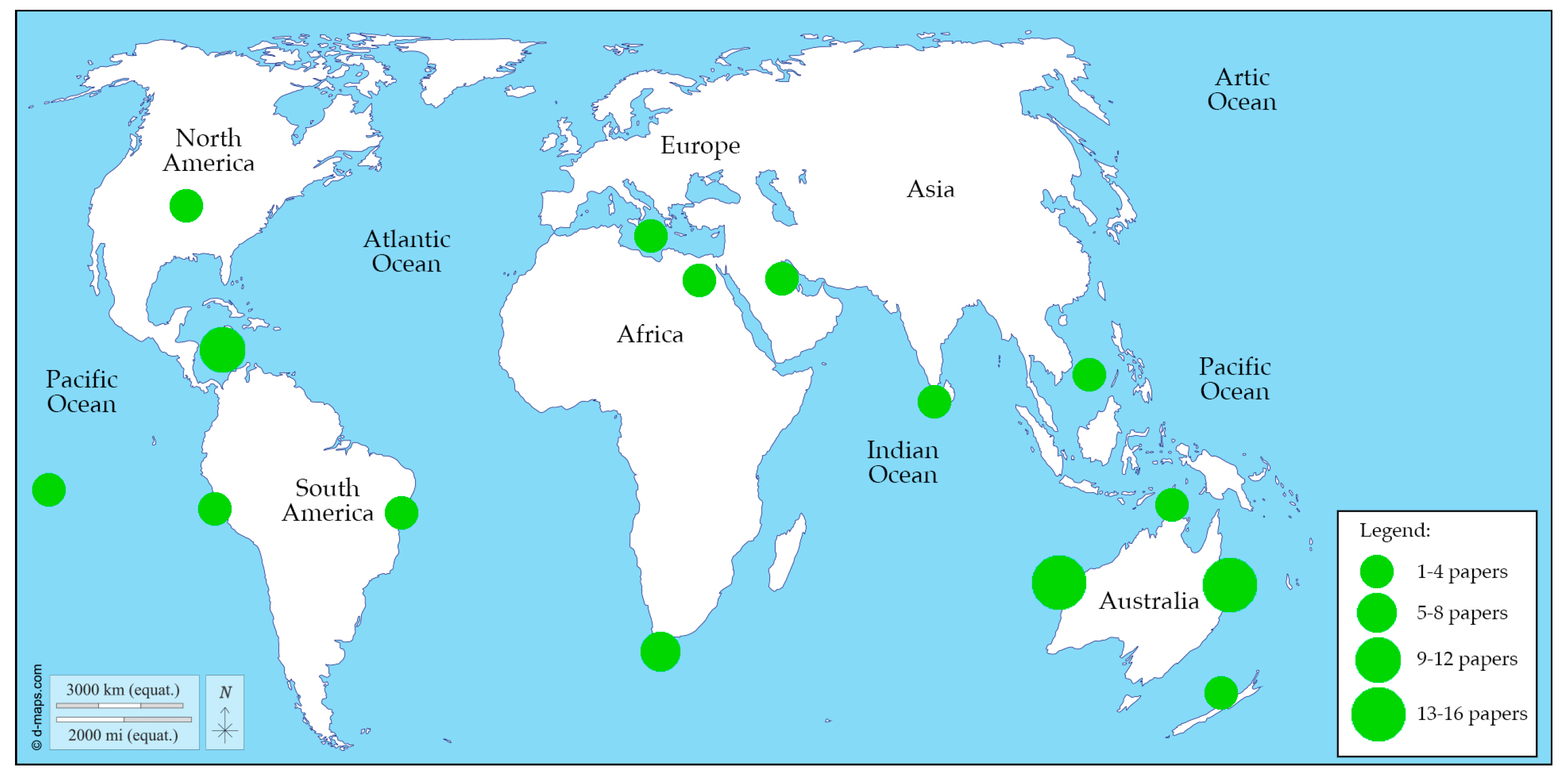
3.1. Temporal and Spatial Trends in the Use of BRUVS for Studying Cartilaginous Fish
3.2. Chondrichthyans Studied through BRUVS
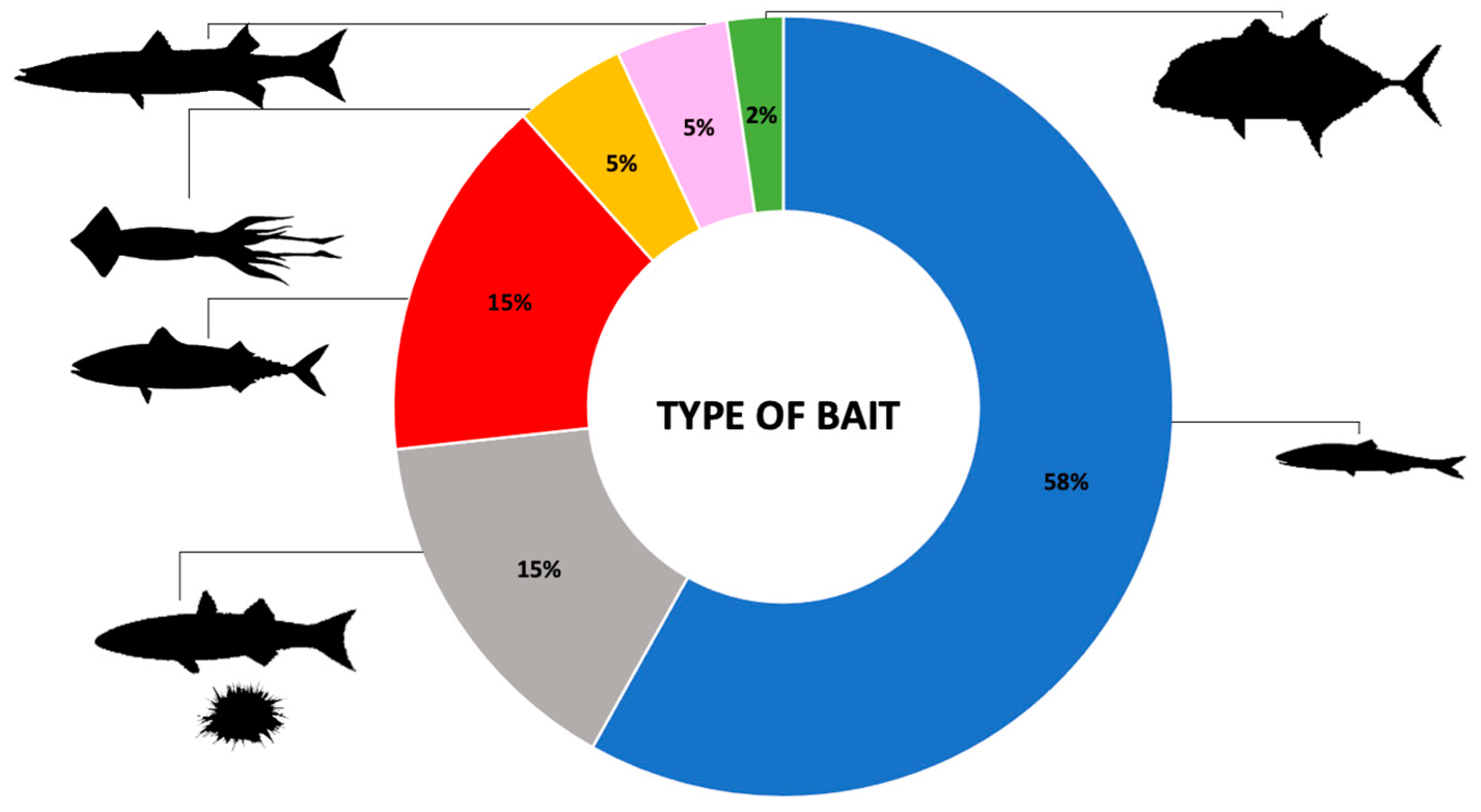
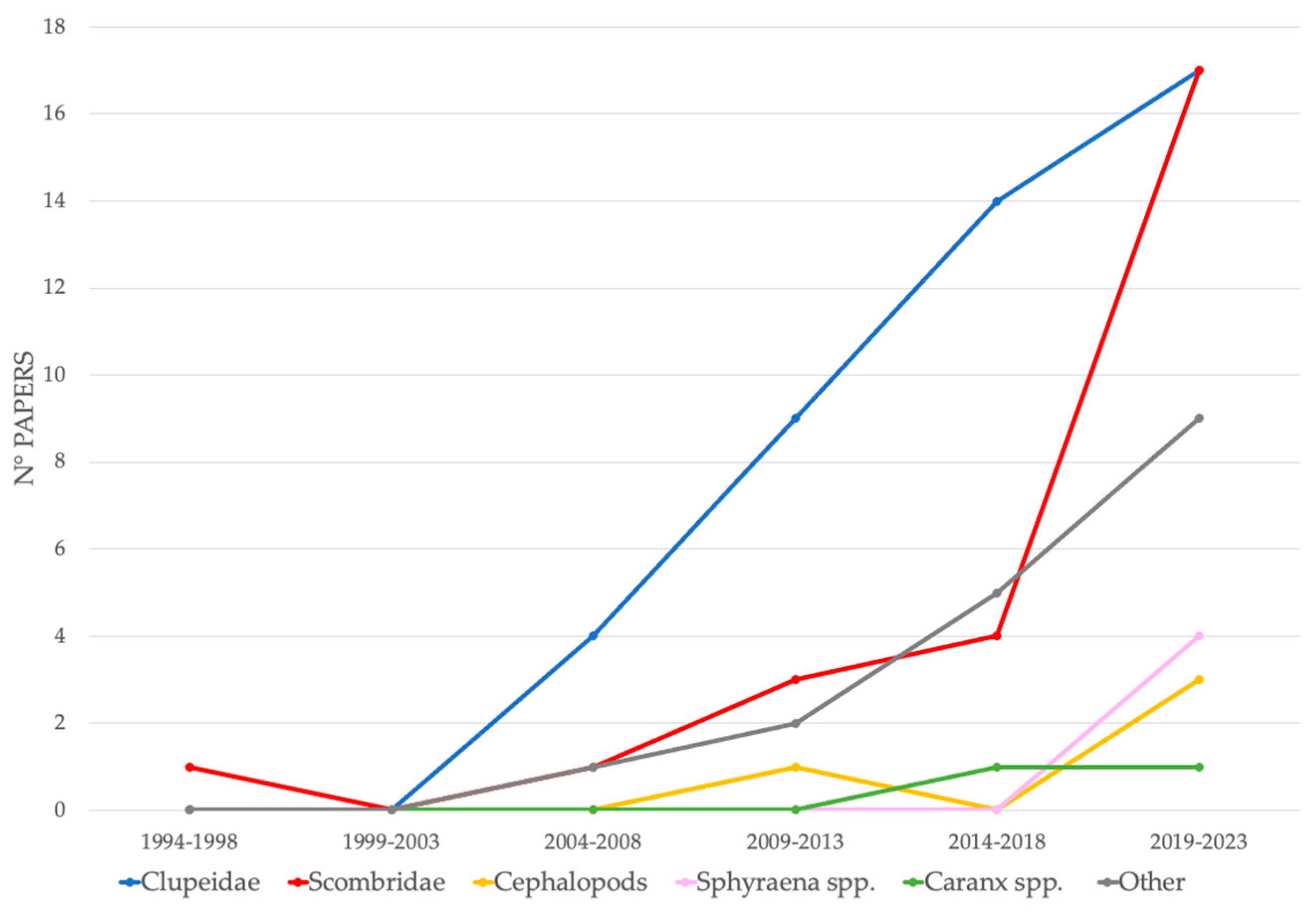
3.3. Baits Used to Attract Chondrichthyans
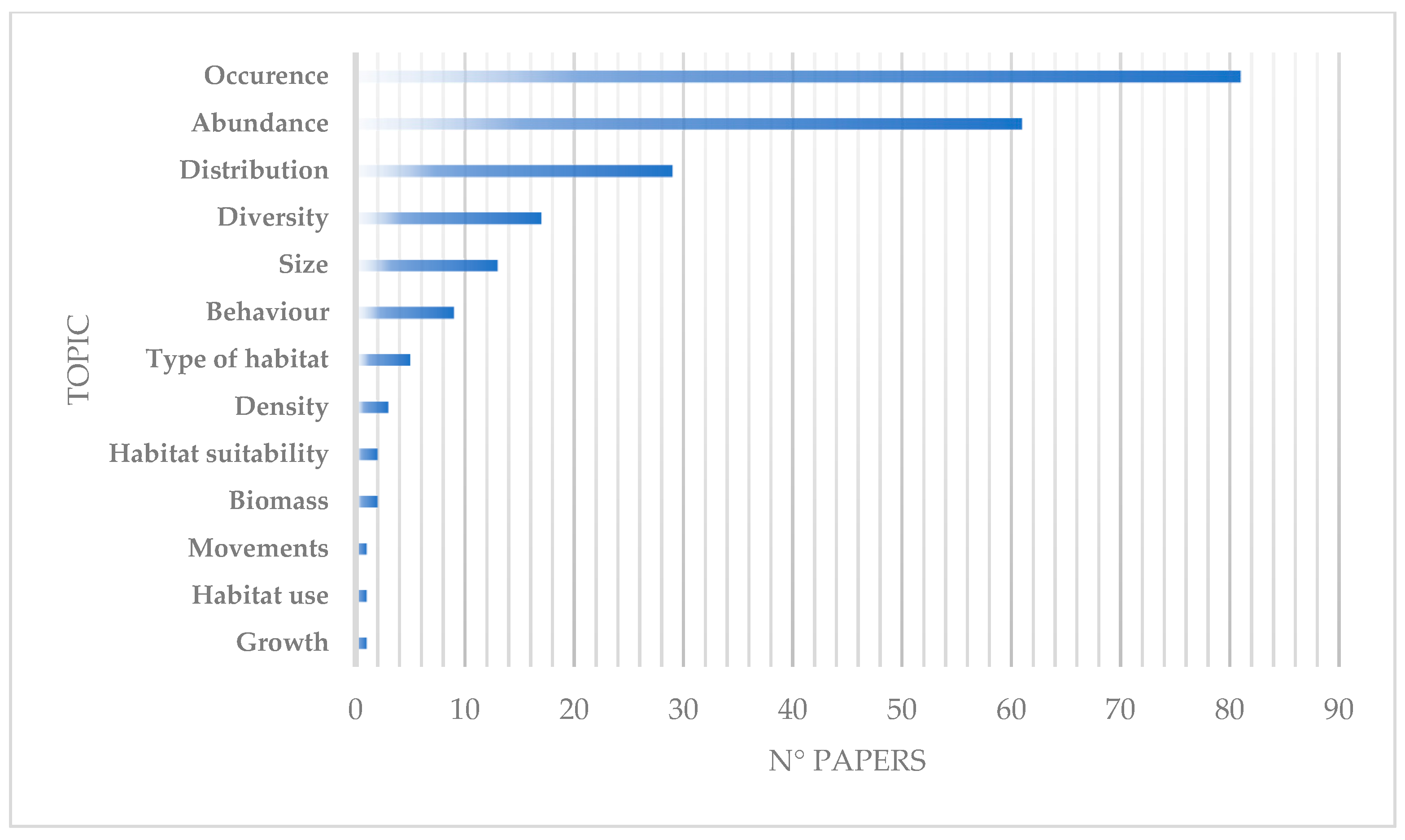
3.4. Topics of Investigation
4. Discussion
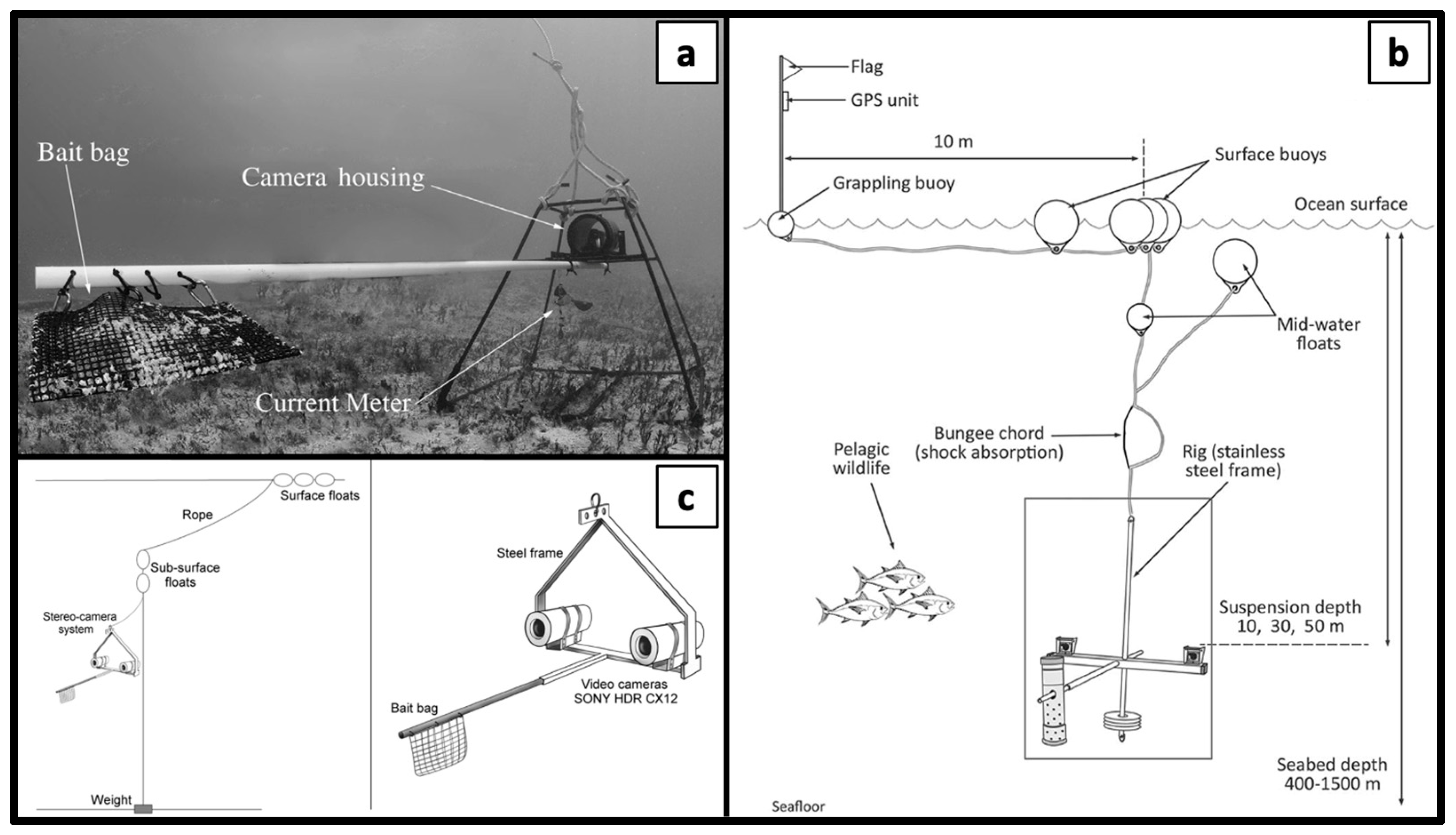
4.1. Implementing BRUVS
4.2. Informing Conservation Initiatives
4.3. Challenges and Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, J.K.; Myers, R.A. Shifting Baselines and the Decline of Pelagic Sharks in the Gulf of Mexico. Ecol. Lett. 2004, 7, 135–145. [Google Scholar] [CrossRef]
- Myers, R.; Baum, J.; Shepherd, T.; Powers, S.; Peterson, C. Cascading Effects of the Loss of Apex Predatory Sharks from a Coastal Ocean. Science 2007, 315, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Field, I.C.; Meekan, M.G.; Buckworth, R.C.; Bradshaw, C.J.A. Chapter 4 Susceptibility of Sharks, Rays and Chimaeras to Global Extinction. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2009; Volume 56, pp. 275–363. [Google Scholar]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a Century of Global Decline in Oceanic Sharks and Rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Dulvy, N.; Allen, D.; Ralph, G.; Walls, R. The Conservation Status of Sharks, Rays, and Chimaeras in the Mediterranean Sea [Brochure]; IUCN: Malaga, Spain, 2016. [Google Scholar]
- Leonetti, F.L.; Giglio, G.; Leone, A.; Coppola, F.; Romano, C.; Bottaro, M.; Reinero, F.R.; Milazzo, C.; Micarelli, P.; Tripepi, S.; et al. An Updated Checklist of Chondrichthyans of Calabria. Mediterr. Mar. Sci. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Cavanagh, R.; Gibson, C. Overview of the Conservation Status of Cartilaginous Fishes (Chondrichthyans) in the Mediterranean Sea; IUCN: Gland, Switzerland, 2007. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World: A Complete Guide; Illustrated edizione; Princeton University Press: Princeton, NJ, USA, 2021; ISBN 978-0-691-20599-1. [Google Scholar]
- IUCN The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 22 December 2023).
- Gallo, S.; Nania, G.; Caruso, V.; Zicarelli, G.; Leonetti, F.L.; Giglio, G.; Fedele, G.; Romano, C.; Bottaro, M.; Mangoni, O.; et al. Bioaccumulation of Trace Elements in the Muscle of the Blackmouth Catshark Galeus Melastomus from Mediterranean Waters. Biology 2023, 12, 951. [Google Scholar] [CrossRef]
- Brooks, E.; Sloman, K.; Sims, D.; Danylchuk, A. Validating the Use of Baited Remote Underwater Video Surveys for Assessing the Diversity, Distribution and Abundance of Sharks in the Bahamas. Endanger. Species Res. 2011, 13, 231–243. [Google Scholar] [CrossRef]
- Skomal, G. Evaluating the Physiological and Physical Consequences of Capture on Post-release Survivorship in Large Pelagic Fishes. Fish. Manag. Ecol. 2007, 14, 81–89. [Google Scholar] [CrossRef]
- Lester, E.; Langlois, T.; Lindgren, I.; Birt, M.; Bond, T.; McLean, D.; Vaughan, B.; Holmes, T.H.; Meekan, M. Drivers of Variation in Occurrence, Abundance, and Behaviour of Sharks on Coral Reefs. Sci. Rep. 2022, 12, 728. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A.; Heithaus, M.R.; Heupel, M.R.; Macneil, M.A.; Meekan, M.; Harvey, E.; Sherman, C.S.; Currey-Randall, L.M.; Goetze, J.S.; Kiszka, J.J.; et al. Widespread Diversity Deficits of Coral Reef Sharks and Rays. Science 2023, 380, 1155–1160. [Google Scholar] [CrossRef]
- Shea, B.D.; Benson, C.W.; De Silva, C.; Donovan, D.; Romeiro, J.; Bond, M.E.; Creel, S.; Gallagher, A.J. Effects of Exposure to Large Sharks on the Abundance and Behavior of Mobile Prey Fishes along a Temperate Coastal Gradient. PLoS ONE 2020, 15, e0230308. [Google Scholar] [CrossRef]
- Sabando, M.A.; Rieucau, G.; Bradley, D.; Caselle, J.E.; Papastamatiou, Y.P. Habitat-Specific Inter and Intraspecific Behavioral Interactions among Reef Sharks. Oecologia 2020, 193, 371–376. [Google Scholar] [CrossRef]
- Cappo, M.; Harvey, E.; Shortis, M. Counting and Measuring Fish with Baited Video Techniques—An Overview. Aust. Soc. Fish Biol. 2007, 1, 101–114. [Google Scholar]
- Cappo, M.; Speare, P.; De’ath, G. Comparison of Baited Remote Underwater Video Stations (BRUVS) and Prawn (Shrimp) Trawls for Assessments of Fish Biodiversity in Inter-Reefal Areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol. 2004, 302, 123–152. [Google Scholar] [CrossRef]
- Oh, B.Z.L.; Sequeira, A.M.M.; Meekan, M.G.; Ruppert, J.L.W.; Meeuwig, J.J. Predicting Occurrence of Juvenile Shark Habitat to Improve Conservation Planning. Conserv. Biol. 2017, 31, 635–645. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, M.A.; Chapman, D.D.; Heupel, M.; Simpfendorfer, C.A.; Heithaus, M.; Meekan, M.; Harvey, E.; Goetze, J.; Kiszka, J.; Bond, M.E.; et al. Global Status and Conservation Potential of Reef Sharks. Nature 2020, 583, 801–806. [Google Scholar] [CrossRef]
- Watson, D.L.; Harvey, E.S.; Anderson, M.J.; Kendrick, G.A. A Comparison of Temperate Reef Fish Assemblages Recorded by Three Underwater Stereo-Video Techniques. Mar. Biol. 2005, 148, 415–425. [Google Scholar] [CrossRef]
- Thompson, C.D.H.; Meeuwig, J.J. Sharks Are the Preferred Scraping Surface for Large Pelagic Fishes: Possible Implications for Parasite Removal and Fitness in a Changing Ocean. PLoS ONE 2022, 17, e0275458. [Google Scholar] [CrossRef]
- Murray, S.; Meeuwig, J.J.; Thompson, C.D.H.; Mouillot, D. Identifying the Drivers of Silky Shark Distribution and an Evaluation of Protection Measures. Environ. Biol. Fishes 2023, 106, 1693–1713. [Google Scholar] [CrossRef]
- Espinoza, M.; Cappo, M.; Heupel, M.R.; Tobin, A.J.; Simpfendorfer, C.A. Quantifying Shark Distribution Patterns and Species-Habitat Associations: Implications of Marine Park Zoning. PLoS ONE 2014, 9, e106885. [Google Scholar] [CrossRef]
- Santana-Garcon, J.; Newman, S.J.; Langlois, T.J.; Harvey, E.S. Effects of a Spatial Closure on Highly Mobile Fish Species: An Assessment Using Pelagic Stereo-BRUVs. J. Exp. Mar. Biol. Ecol. 2014, 460, 153–161. [Google Scholar] [CrossRef]
- Currey-Randall, L.M.; Cappo, M.; Simpfendorfer, C.A.; Farabaugh, N.F.; Heupel, M.R. Optimal Soak Times for Baited Remote Underwater Video Station Surveys of Reef-Associated Elasmobranchs. PLoS ONE 2020, 15, e0231688. [Google Scholar] [CrossRef]
- Barnett, A.; Fitzpatrick, R.; Bradley, M.; Miller, I.; Sheaves, M.; Chin, A.; Smith, B.; Diedrich, A.; Yick, J.L.; Lubitz, N.; et al. Scientific Response to a Cluster of Shark Bites. People Nat. 2022, 4, 963–982. [Google Scholar] [CrossRef]
- Albano, P.S.; Fallows, C.; Fallows, M.; Schuitema, O.; Bernard, A.T.F.; Sedgwick, O.; Hammerschlag, N. Successful Parks for Sharks: No-Take Marine Reserve Provides Conservation Benefits to Endemic and Threatened Sharks off South Africa. Biol. Conserv. 2021, 261, 109302. [Google Scholar] [CrossRef]
- Andradi-Brown, D.A.; Macaya-Solis, C.; Exton, D.A.; Gress, E.; Wright, G.; Rogers, A.D. Assessing Caribbean Shallow and Mesophotic Reef Fish Communities Using Baited-Remote Underwater Video (BRUV) and Diver-Operated Video (DOV) Survey Techniques. PLoS ONE 2016, 11, e0168235. [Google Scholar] [CrossRef] [PubMed]
- Archila, F.; Cardeñosa, D.; Bessudo, S.; Cuellar, A.; Muriel, F.; Carvajal, J.; Amariles, D.; Duarte, A. Monitoreo de Fauna Pelágica de Los Montes Submarinos Del Pacífico Colombiano Usando BRUVS. Biota Colomb. 2022, 24, e1103. [Google Scholar] [CrossRef]
- Barley, S.; Meekan, M.; Meeuwig, J. Species Diversity, Abundance, Biomass, Size and Trophic Structure of Fish on Coral Reefs in Relation to Shark Abundance. Mar. Ecol. Prog. Ser. 2017, 565, 163–179. [Google Scholar] [CrossRef]
- Bernard, A.; Götz, A. Bait Increases the Precision in Count Data from Remote Underwater Video for Most Subtidal Reef Fish in the Warm-Temperate Agulhas Bioregion. Mar. Ecol. Prog. Ser. 2012, 471, 235–252. [Google Scholar] [CrossRef]
- Blampied, S.R.; Rees, S.E.; Attrill, M.J.; Binney, F.C.T.; Sheehan, E.V. Removal of Bottom-Towed Fishing from Whole-Site Marine Protected Areas Promotes Mobile Species Biodiversity. Estuar. Coast. Shelf Sci. 2022, 276, 108033. [Google Scholar] [CrossRef]
- Bond, M.E.; Babcock, E.A.; Pikitch, E.K.; Abercrombie, D.L.; Lamb, N.F.; Chapman, D.D. Reef Sharks Exhibit Site-Fidelity and Higher Relative Abundance in Marine Reserves on the Mesoamerican Barrier Reef. PLoS ONE 2012, 7, e32983. [Google Scholar] [CrossRef]
- Bouchet, P.J.; Meeuwig, J.J. Drifting Baited Stereo-videography: A Novel Sampling Tool for Surveying Pelagic Wildlife in Offshore Marine Reserves. Ecosphere 2015, 6, 1–29. [Google Scholar] [CrossRef]
- Boussarie, G.; Bakker, J.; Wangensteen, O.S.; Mariani, S.; Bonnin, L.; Juhel, J.-B.; Kiszka, J.J.; Kulbicki, M.; Manel, S.; Robbins, W.D.; et al. Environmental DNA Illuminates the Dark Diversity of Sharks. Sci. Adv. 2018, 4, eaap9661. [Google Scholar] [CrossRef]
- Bradley, D.; Papastamatiou, Y.; Caselle, J. No Persistent Behavioural Effects of SCUBA Diving on Reef Sharks. Mar. Ecol. Prog. Ser. 2017, 567, 173–184. [Google Scholar] [CrossRef]
- Bruns, S.; Henderson, A.C. A Baited Remote Underwater Video System (BRUVS) Assessment of Elasmobranch Diversity and Abundance on the Eastern Caicos Bank (Turks and Caicos Islands); an Environment in Transition. Environ. Biol. Fishes 2020, 103, 1001–1012. [Google Scholar] [CrossRef]
- Cáceres, C.; Kiszka, J.J.; Luna-Acosta, A.; Herrera, H.; Zarza, E.; Heithaus, M.R. Predatory Fish Exploitation and Relative Abundance in a Data-poor Region from the Caribbean Coast of Colombia, Inferred from Artisanal Fishery Interview Surveys and Baited Remote Underwater Video Systems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1401–1415. [Google Scholar] [CrossRef]
- Cambra, M.; Lara-Lizardi, F.; Peñaherrera-Palma, C.; Hearn, A.; Ketchum, J.T.; Zarate, P.; Chacón, C.; Suárez-Moncada, J.; Herrera, E.; Espinoza, M. A First Assessment of the Distribution and Abundance of Large Pelagic Species at Cocos Ridge Seamounts (Eastern Tropical Pacific) Using Drifting Pelagic Baited Remote Cameras. PLoS ONE 2021, 16, e0244343. [Google Scholar] [CrossRef] [PubMed]
- Cattano, C.; Gambardella, C.; Grancagnolo, D.; Principato, E.; Aglieri, G.; Turco, G.; Quattrocchi, F.; Milazzo, M. Multiple Interannual Records of Young-of-the-Year Identify an Important Area for the Protection of the Shortfin Mako, Isurus Oxyrinchus. Mar. Environ. Res. 2023, 192, 106217. [Google Scholar] [CrossRef]
- Chevis, M.G.; Graham, R.T. Insights into Elasmobranch Composition, Abundance, and Distribution in the Bocas Del Toro Archipelago, Panama Using Fisheries-Independent Monitoring. Lat. Am. J. Aquat. Res. 2022, 50, 492–506. [Google Scholar] [CrossRef]
- Chiriboga-Paredes, Y.; Palomino, Á.; Goodman, L.; Córdova, F.; Páez, V.; Yépez, M.; Jorgensen, S.; Armijos, D.; Pazmiño, D.; Hearn, A. Discovery of a Putative Scalloped Hammerhead Shark Sphyrna Lewini (Carcharhiniformes: Sphyrnidae) Nursery Site at the Galapagos Islands, Eastern Tropical Pacific. Environ. Biol. Fishes 2022, 105, 181–192. [Google Scholar] [CrossRef]
- Clarke, C.; Lea, J.; Ormond, R. Comparative Abundance of Reef Sharks in the Western Indian Ocean. In Proceedings of the 12th International Coral Reef Symposium, Cairns, QLD, Australia, 9–13 July 2012; pp. 9–13. [Google Scholar]
- Colton, M.; Swearer, S. A Comparison of Two Survey Methods: Differences between Underwater Visual Census and Baited Remote Underwater Video. Mar. Ecol. Prog. Ser. 2010, 400, 19–36. [Google Scholar] [CrossRef]
- De Vos, L.; Götz, A.; Winker, H.; Attwood, C. Optimal BRUVs (Baited Remote Underwater Video System) Survey Design for Reef Fish Monitoring in the Stilbaai Marine Protected Area. Afr. J. Mar. Sci. 2014, 36, 1–10. [Google Scholar] [CrossRef]
- De Vos, L.; Watson, R.; Götz, A.; Attwood, C. Baited Remote Underwater Video System (BRUVs) Survey of Chondrichthyan Diversity in False Bay, South Africa. Afr. J. Mar. Sci. 2015, 37, 209–218. [Google Scholar] [CrossRef]
- Espinoza, M.; Araya-Arce, T.; Chaves-Zamora, I.; Chinchilla, I.; Cambra, M. Monitoring Elasmobranch Assemblages in a Data-Poor Country from the Eastern Tropical Pacific Using Baited Remote Underwater Video Stations. Sci. Rep. 2020, 10, 17175. [Google Scholar] [CrossRef]
- Flowers, K.; Babcock, E.; Papastamatiou, Y.; Bond, M.; Lamb, N.; Miranda, A.; Nuñez, R.; Valentin-Albanese, J.; Clementi, G.; Kelley, M.; et al. Varying Reef Shark Abundance Trends inside a Marine Reserve: Evidence of a Caribbean Reef Shark Decline. Mar. Ecol. Prog. Ser. 2022, 683, 97–107. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Shipley, O.N.; De Silva, C.; Kohler, J.K.; Fernandes, T.F.; Austin, T.; Ormond, R.F.; Gore, M.A. First Records of the Blurred Lantern Shark Etmopterus Bigelowi from the Cayman Islands, Western Atlantic. Front. Mar. Sci. 2023, 10, 1165207. [Google Scholar] [CrossRef]
- Goetze, J.S.; Fullwood, L.A.F. Fiji’s Largest Marine Reserve Benefits Reef Sharks. Coral Reefs 2013, 32, 121–125. [Google Scholar] [CrossRef]
- Gold, Z.; Koch, M.Q.; Schooler, N.K.; Emery, K.A.; Dugan, J.E.; Miller, R.J.; Page, H.M.; Schroeder, D.M.; Hubbard, D.M.; Madden, J.R.; et al. A Comparison of Biomonitoring Methodologies for Surf Zone Fish Communities. PLoS ONE 2023, 18, e0260903. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Ormond, R.; Clarke, C.; Kohler, J.; Millar, C.; Brooks, E. Application of Photo-Identification and Lengthened Deployment Periods to Baited Remote Underwater Video Stations (BRUVS) Abundance Estimates of Coral Reef Sharks. Oceans 2020, 1, 274–299. [Google Scholar] [CrossRef]
- Harasti, D.; Lee, K.A.; Laird, R.; Bradford, R.; Bruce, B. Use of Stereo Baited Remote Underwater Video Systems to Estimate the Presence and Size of White Sharks (Carcharodon Carcharias). Mar. Freshw. Res. 2017, 68, 1391. [Google Scholar] [CrossRef]
- Harasti, D.; Davis, T.; Williams, J.; Bradford, R. Estimating Growth in Juvenile White Sharks Using Stereo Baited Remote Underwater Video Systems (Stereo-BRUVs). Rep. Natl. Environ. Sci. Program Mar. Biodivers. Hub 2019, 1–23. [Google Scholar]
- Hardinge, J.; Harvey, E.S.; Saunders, B.J.; Newman, S.J. A Little Bait Goes a Long Way: The Influence of Bait Quantity on a Temperate Fish Assemblage Sampled Using Stereo-BRUVs. J. Exp. Mar. Biol. Ecol. 2013, 449, 250–260. [Google Scholar] [CrossRef]
- Harvey, E.S.; Newman, S.J.; McLean, D.L.; Cappo, M.; Meeuwig, J.J.; Skepper, C.L. Comparison of the Relative Efficiencies of Stereo-BRUVs and Traps for Sampling Tropical Continental Shelf Demersal Fishes. Fish. Res. 2012, 125–126, 108–120. [Google Scholar] [CrossRef]
- Heagney, E.; Lynch, T.; Babcock, R.; Suthers, I. Pelagic Fish Assemblages Assessed Using Mid-Water Baited Video: Standardising Fish Counts Using Bait Plume Size. Mar. Ecol. Prog. Ser. 2007, 350, 255–266. [Google Scholar] [CrossRef]
- Heldsinger, M.; Hepburn, C.; Jowett, T.; Rayment, W. Small Marine Reserves Provide Conservation Benefits for Coastal Sharks in Southern New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 144–159. [Google Scholar] [CrossRef]
- Henderson, A.C.; Smith, C.; Bruns, S. Fussy Eaters, Bait Loss and Escapees: How Reliable Are Baited-Hook Assessments of Shark Abundance in Shallow, Coastal Waters? J. Exp. Mar. Biol. Ecol. 2022, 556, 151794. [Google Scholar] [CrossRef]
- Hill, N.A.; Barrett, N.; Lawrence, E.; Hulls, J.; Dambacher, J.M.; Nichol, S.; Williams, A.; Hayes, K.R. Quantifying Fish Assemblages in Large, Offshore Marine Protected Areas: An Australian Case Study. PLoS ONE 2014, 9, e110831. [Google Scholar] [CrossRef] [PubMed]
- Jabado, R.W.; Al Hameli, S.M.; Grandcourt, E.M.; Al Dhaheri, S.S. Low Abundance of Sharks and Rays in Baited Remote Underwater Video Surveys in the Arabian Gulf. Sci. Rep. 2018, 8, 15597. [Google Scholar] [CrossRef] [PubMed]
- Juhel, J.; Vigliola, L.; Mouillot, D.; Kulbicki, M.; Letessier, T.B.; Meeuwig, J.J.; Wantiez, L. Reef Accessibility Impairs the Protection of Sharks. J. Appl. Ecol. 2018, 55, 673–683. [Google Scholar] [CrossRef]
- Kiggins, R.S.; Knott, N.A.; Davis, A.R. Miniature Baited Remote Underwater Video (Mini-BRUV) Reveals the Response of Cryptic Fishes to Seagrass Cover. Environ. Biol. Fishes 2018, 101, 1717–1722. [Google Scholar] [CrossRef]
- Kilfoil, J.; Wirsing, A.; Campbell, M.; Kiszka, J.; Gastrich, K.; Heithaus, M.; Zhang, Y.; Bond, M. Baited Remote Underwater Video Surveys Undercount Sharks at High Densities: Insights from Full-Spherical Camera Technologies. Mar. Ecol. Prog. Ser. 2017, 585, 113–121. [Google Scholar] [CrossRef]
- Klages, J.; Broad, A.; Kelaher, B.P.; Davis, A.R. The Influence of Gummy Sharks, Mustelus Antarcticus, on Observed Fish Assemblage Structure. Environ. Biol. Fishes 2014, 97, 215–222. [Google Scholar] [CrossRef]
- Kohler, J.; Gore, M.; Ormond, R.; Austin, T. First Estimates of Population Size and Home Range of Caribbean Reef and Nurse Sharks Using Photo-Identification and BRUVS. Front. Mar. Sci. 2023, 10, 1230896. [Google Scholar] [CrossRef]
- Letessier, T.B.; Meeuwig, J.J.; Gollock, M.; Groves, L.; Bouchet, P.J.; Chapuis, L.; Vianna, G.M.S.; Kemp, K.; Koldewey, H.J. Assessing Pelagic Fish Populations: The Application of Demersal Video Techniques to the Mid-Water Environment. Methods Oceanogr. 2013, 8, 41–55. [Google Scholar] [CrossRef]
- Lewis, R.; Dawson, S.; Rayment, W. Size Structure of Broadnose Sevengill Sharks (Notorynchus cepedianus) in Sawdust Bay, Rakiura/Stewart Island, Estimated Using Underwater Stereo-Photogrammetry. N. Z. J. Mar. Freshw. Res. 2023, 57, 104–118. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Schifiliti, M.; Birt, M.J.; Bond, T.; McLean, D.L.; Barnes, P.B.; Langlois, T.J. A Novel Experimental Approach to Investigate the Potential for Behavioural Change in Sharks in the Context of Depredation. J. Exp. Mar. Biol. Ecol. 2020, 530–531, 151440. [Google Scholar] [CrossRef]
- Murray, R.; Conales, S.; Araujo, G.; Labaja, J.; Snow, S.J.; Pierce, S.J.; Songco, A.; Ponzo, A. Tubbataha Reefs Natural Park: The First Comprehensive Elasmobranch Assessment Reveals Global Hotspot for Reef Sharks. J. Asia-Pac. Biodivers. 2019, 12, 49–56. [Google Scholar] [CrossRef]
- O’Connell, C.P.; Payne, M.; Payne, S.; Eller, L.J.; Shaw, J.; McGregor, A.; Rerekura, A.; Stewart, M.; Fox, A. Observations of Multiple Young-of-the-Year to Juvenile White Sharks (Carcharodon carcharias) within South-West Australian Waters and Its Implications for a Potential Nursery Area(s). J. Mar. Sci. Eng. 2023, 11, 563. [Google Scholar] [CrossRef]
- de Oliveira-Junior, W.M.; Spaet, J.L.Y.; de, S. Rosa, R.; Santos, A. First Record of the Critically Endangered Great Hammerhead Shark (Sphyrna mokarran) in Its Natural Habitat in the Coast of Paraíba, Northeastern Brazil. Gaia Sci. 2022, 16, 1–15. [Google Scholar]
- Osgood, G.J.; McCord, M.E.; Baum, J.K. Using Baited Remote Underwater Videos (BRUVs) to Characterize Chondrichthyan Communities in a Global Biodiversity Hotspot. PLoS ONE 2019, 14, e0225859. [Google Scholar] [CrossRef] [PubMed]
- Ovegård, M.; Högvall, J.; Ovegård, M.; Wikström, A.; Wennhage, H. Previously Undocumented Relationship between Spiny Dogfish Squalus Acanthias and Juvenile Atlantic Horse Mackerel Trachurus Trachurus Revealed by Stereo-BRUV. Environ. Biol. Fishes 2022, 105, 453–458. [Google Scholar] [CrossRef]
- Parmegiani, A.; Gobbato, J.; Seveso, D.; Galli, P.; Montano, S. First Record of the Bull Shark Carcharhinus leucas (Valenciennes, 1839) from the Maldivian Archipelago, Central Indian Ocean. J. Fish Biol. 2023, 103, 1242–1247. [Google Scholar] [CrossRef]
- Parton, K.J.; Doherty, P.D.; Parrish, M.; Shearer, P.; Myrick, K.; Shipley, O.N.; Gallagher, A.J. Opportunistic Camera Surveys Provide Insight into Discrete Foraging Behaviours in Nurse Sharks (Ginglymostoma cirratum). Environ. Biol. Fishes 2023, 106, 19–30. [Google Scholar] [CrossRef]
- Prat-Varela, A.; Torres, A.; Cervantes, D.; Aquino-Baleytó, M.; Abril, A.-M.; Clua, E.E.G. Improved Baited Remote Underwater Video (BRUV) for 24 h Real-Time Monitoring of Pelagic and Demersal Marine Species from the Epipelagic Zone. J. Mar. Sci. Eng. 2023, 11, 1182. [Google Scholar] [CrossRef]
- Priede, I.G.; Bagley, P.M.; Smith, A.; Creasey, S.; Merrett, N.R. Scavenging Deep Demersal Fishes of the Porcupine Seabight, North-East Atlantic: Observations by Baited Camera, Trap and Trawl. J. Mar. Biol. Assoc. UK 1994, 74, 481–498. [Google Scholar] [CrossRef]
- Pimentel, C.R.; Andrades, R.; Ferreira, C.E.L.; Gadig, O.B.F.; Harvey, E.S.; Joyeux, J.; Giarrizzo, T. BRUVS Reveal Locally Extinct Shark and the Way for Shark Monitoring in Brazilian Oceanic Islands. J. Fish Biol. 2020, 96, 539–542. [Google Scholar] [CrossRef]
- Rizzari, J.R.; Frisch, A.J.; Connolly, S.R. How Robust Are Estimates of Coral Reef Shark Depletion? Biol. Conserv. 2014, 176, 39–47. [Google Scholar] [CrossRef]
- Santana-Garcon, J.; Braccini, M.; Langlois, T.J.; Newman, S.J.; McAuley, R.B.; Harvey, E.S. Calibration of Pelagic Stereo- BRUV s and Scientific Longline Surveys for Sampling Sharks. Methods Ecol. Evol. 2014, 5, 824–833. [Google Scholar] [CrossRef]
- Santana-Garcon, J.; Newman, S.J.; Harvey, E.S. Development and Validation of a Mid-Water Baited Stereo-Video Technique for Investigating Pelagic Fish Assemblages. J. Exp. Mar. Biol. Ecol. 2014, 452, 82–90. [Google Scholar] [CrossRef]
- Sherman, C.S.; Chin, A.; Heupel, M.R.; Simpfendorfer, C.A. Are We Underestimating Elasmobranch Abundances on Baited Remote Underwater Video Systems (BRUVS) Using Traditional Metrics? J. Exp. Mar. Biol. Ecol. 2018, 503, 80–85. [Google Scholar] [CrossRef]
- Sherman, C.S.; Heupel, M.R.; Johnson, M.; Kaimuddin, M.; Qamar, L.M.S.; Chin, A.; Simpfendorfer, C.A. Repeatability of Baited Remote Underwater Video Station (BRUVS) Results within and between Seasons. PLoS ONE 2020, 15, e0244154. [Google Scholar] [CrossRef]
- Spaet, J.L.Y.; Nanninga, G.B.; Berumen, M.L. Ongoing Decline of Shark Populations in the Eastern Red Sea. Biol. Conserv. 2016, 201, 20–28. [Google Scholar] [CrossRef]
- Stobart, B.; García-Charton, J.A.; Espejo, C.; Rochel, E.; Goñi, R.; Reñones, O.; Herrero, A.; Crec’hriou, R.; Polti, S.; Marcos, C.; et al. A Baited Underwater Video Technique to Assess Shallow-Water Mediterranean Fish Assemblages: Methodological Evaluation. J. Exp. Mar. Biol. Ecol. 2007, 345, 158–174. [Google Scholar] [CrossRef]
- Taylor, M.D.; Baker, J.; Suthers, I.M. Tidal Currents, Sampling Effort and Baited Remote Underwater Video (BRUV) Surveys: Are We Drawing the Right Conclusions? Fish. Res. 2013, 140, 96–104. [Google Scholar] [CrossRef]
- Torres, A.; Abril, A.-M.; Clua, E.E.G. A Time-Extended (24 h) Baited Remote Underwater Video (BRUV) for Monitoring Pelagic and Nocturnal Marine Species. J. Mar. Sci. Eng. 2020, 8, 208. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Peters, J.R.; McCloskey, R.M.; Hinder, S.L. Optimising Stereo Baited Underwater Video for Sampling Fish and Invertebrates in Temperate Coastal Habitats. Estuar. Coast. Shelf Sci. 2014, 150, 281–287. [Google Scholar] [CrossRef]
- White, J.; Simpfendorfer, C.A.; Tobin, A.J.; Heupel, M.R. Application of Baited Remote Underwater Video Surveys to Quantify Spatial Distribution of Elasmobranchs at an Ecosystem Scale. J. Exp. Mar. Biol. Ecol. 2013, 448, 281–288. [Google Scholar] [CrossRef]
- Wraith, J.; Lynch, T.; Minchinton, T.; Broad, A.; Davis, A. Bait Type Affects Fish Assemblages and Feeding Guilds Observed at Baited Remote Underwater Video Stations. Mar. Ecol. Prog. Ser. 2013, 477, 189–199. [Google Scholar] [CrossRef]
- Zintzen, V.; Anderson, M.J.; Roberts, C.D.; Harvey, E.S.; Stewart, A.L.; Struthers, C.D. Diversity and Composition of Demersal Fishes along a Depth Gradient Assessed by Baited Remote Underwater Stereo-Video. PLoS ONE 2012, 7, e48522. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, H.; Gladstone, W.; Lindfield, S.; Wraith, J.; Lynch, T. Spatial and Temporal Variation in Reef Fish Assemblages of Marine Parks in New South Wales, Australia—Baited Video Observations. Mar. Ecol.-Prog. Ser. Mar. Ecol-Progr. Ser. 2007, 350, 277–290. [Google Scholar] [CrossRef]
- Anderson, S.D.; Goldman, K. Photographic Evidence of White Shark Movements in California Waters. Calif. Fish Game 1996, 82, 182–186. [Google Scholar]
- Heithaus, M. The Biology of Tiger Sharks, Galeocerdo Cuvier, in Shark Bay, Western Australia: Sex Ratio, Size Distribution, Diet, and Seasonal Changes in Catch Rates. Environ. Biol. Fishes 2001, 61, 25–36. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction Risk and Conservation of the World’s Sharks and Rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.; Langlois, T.; Egli, D.; Harvey, E. Evidence of Artisanal Fishing Impacts and Depth Refuge in Assemblages of Fijian Reef Fish. Coral Reefs 2011, 30, 507–517. [Google Scholar] [CrossRef]
- Langlois, T.; Goetze, J.; Bond, T.; Monk, J.; Abesamis, R.; Asher, J.; Barrett, N.; Bernard, A.; Bouchet, P.; Birt, M.; et al. A Field and Video-annotation Guide for Baited Remote Underwater Stereo-video Surveys of Demersal Fish Assemblages. Methods Ecol. Evol. 2020, 11, 1401–1409. [Google Scholar] [CrossRef]
- Manduca, G.; Padovani, L.; Carosio, E.; Graziani, G.; Stefanini, C.; Romano, D. Development of an Autonomous Fish-Inspired Robotic Platform for Aquaculture Inspection and Management. In Proceedings of the 2023 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Pisa, Italy, 6–8 November 2023; pp. 188–193. [Google Scholar]
- Bayat, B.; Crespi, A.; Ijspeert, A. Envirobot: A Bio-Inspired Environmental Monitoring Platform. In Proceedings of the 2016 IEEE/OES Autonomous Underwater Vehicles (AUV), Tokyo, Japan, 6–9 November 2016; pp. 381–386. [Google Scholar]
- Harvey, E.S.; Cappo, M.; Butler, J.J.; Hall, N.; Kendrick, G.A. Bait Attraction Affects the Performance of Remote Underwater Video Stations in Assessment of Demersal Fish Community Structure. Mar. Ecol. Prog. Ser. 2007, 350, 245–254. [Google Scholar] [CrossRef]
- Parker, D.; DeMartini, E. Evaluation of a Video Camera Technique for Indexing Abundances of Juvenile Pink Snapper, Pristipomoides Filamentosus, and Other Hawaiian Insular Shelf Fishes. Fish. Bull. 1995, 93, 67–77. [Google Scholar]
- Simpfendorfer, C.; Heupel, M.; White, W.; Dulvy, N. The Importance of Research and Public Opinion to Conservation Management of Sharks and Rays: A Synthesis. Mar. Freshw. Res. 2011, 62, 518–527. [Google Scholar] [CrossRef]

| Family | n° Records |
|---|---|
| Carcharhinidae | 238 |
| Dasyatidae | 83 |
| Sphyrnidae | 37 |
| Scyliorhinidae | 32 |
| Triakidae | 32 |
| Myliobatidae | 28 |
| Ginglymostomatidae | 23 |
| Rhinobatidae | 19 |
| Rajidae | 13 |
| Lamnidae | 12 |
| Rhinidae | 11 |
| Squalidae | 11 |
| Hemigaleidae | 8 |
| Hexanchidae | 8 |
| Stegostomatidae | 8 |
| Hemiscylliidae | 7 |
| Mobulidae | 6 |
| Urotrygonidae | 6 |
| Alopiidae | 5 |
| Etmopteridae | 5 |
| Heterodontidae | 5 |
| Orectolobidae | 5 |
| Rhincodontidae | 5 |
| Aetobatidae | 4 |
| Glaucostegidae | 4 |
| Urolophidae | 4 |
| Chimaeridae | 3 |
| Gymnuridae | 3 |
| Narcinidae | 3 |
| Odontaspididae | 3 |
| Potamotrygonidae | 3 |
| Somniosidae | 3 |
| Callorhinchidae | 2 |
| Arhynchobatidae | 1 |
| Centrophoridae | 1 |
| Dalatiidae | 1 |
| Platyrhinidae | 1 |
| Pristidae | 1 |
| Pristiophoridae | 1 |
| Pseudotriakidae | 1 |
| Rhinochimaeridae | 1 |
| Squatinidae | 1 |
| Trygonorrhinidae | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonetti, F.L.; Bottaro, M.; Giglio, G.; Sperone, E. Studying Chondrichthyans Using Baited Remote Underwater Video Systems: A Review. Animals 2024, 14, 1875. https://doi.org/10.3390/ani14131875
Leonetti FL, Bottaro M, Giglio G, Sperone E. Studying Chondrichthyans Using Baited Remote Underwater Video Systems: A Review. Animals. 2024; 14(13):1875. https://doi.org/10.3390/ani14131875
Chicago/Turabian StyleLeonetti, Francesco Luigi, Massimiliano Bottaro, Gianni Giglio, and Emilio Sperone. 2024. "Studying Chondrichthyans Using Baited Remote Underwater Video Systems: A Review" Animals 14, no. 13: 1875. https://doi.org/10.3390/ani14131875





