Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling Collection
2.3. Statistical Analysis
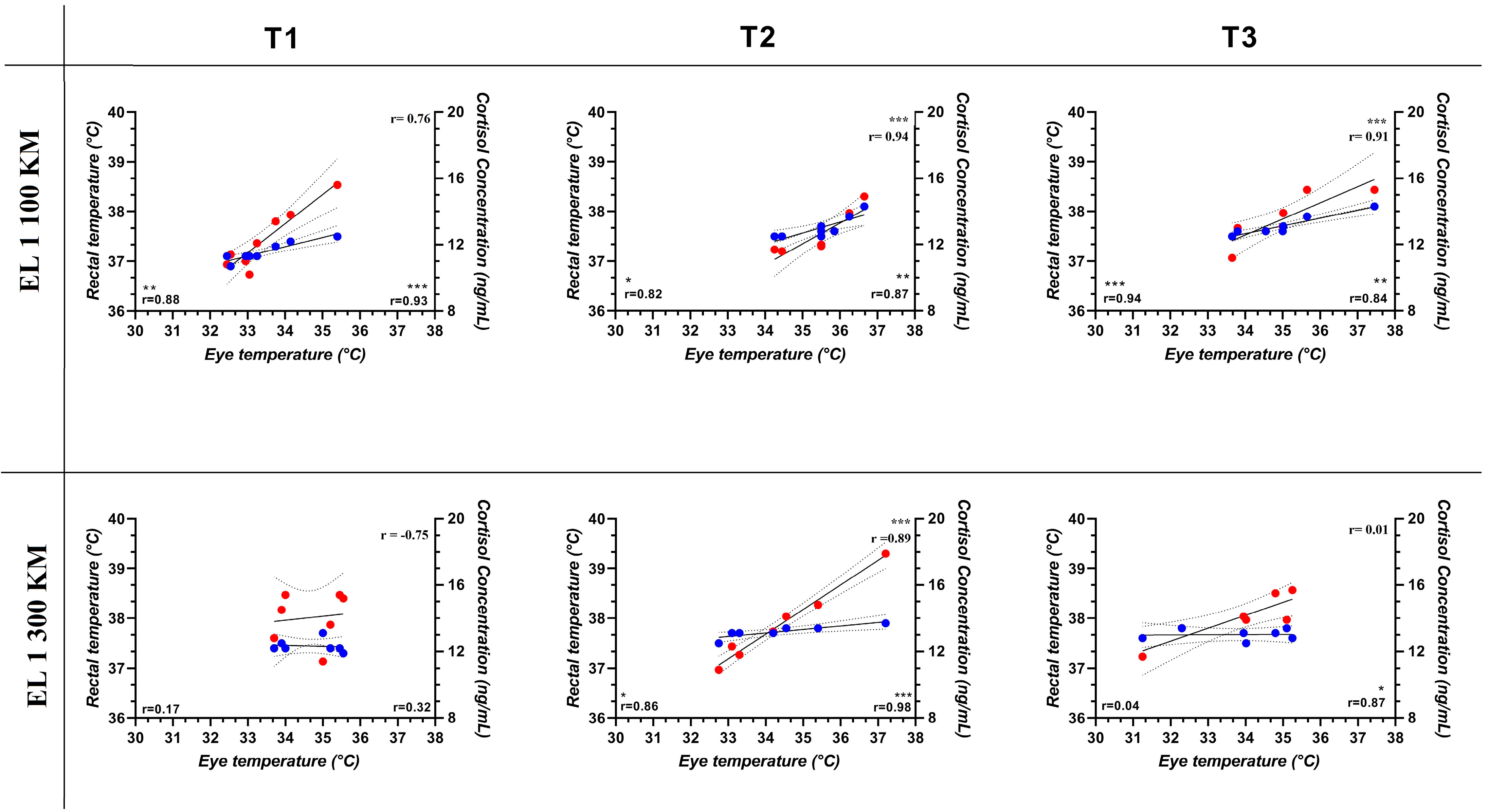
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fazio, E.; Medica, P.; Aronica, V.; Grasso, L.; Ferlazzo, A. Circulating β-Endorphin, Adrenocorticotrophic Hormone and Cortisol Levels of Stallions before and after Short Road Transport: Stress Effect of Different Distances. Acta Vet. Scand. 2008, 50, 6. [Google Scholar] [CrossRef]
- Piccione, G.; Bazzano, M.; Giannetto, C.; Panzera, M.; Fazio, F. Evaluation of Heart Rate as Marker of Stress during Road Transport in Horses. Acta Sci. Vet. 2013, 41, 1–5. [Google Scholar]
- Pryor, J.H. Transportation of horses by sea during the era of the crusades: Eighth century to 1285 A.D.: Part I: To c 1225. Mar. Mirror 1982, 68, 9–27. [Google Scholar] [CrossRef]
- Giovagnoli, G.; Trabalza Marinucci, M.; Bolla, A.; Borghese, A. Transport Stress in Horses: An Electromyographic Study on Balance Preservation. Livest. Prod. Sci. 2002, 73, 247–254. [Google Scholar] [CrossRef]
- Padalino, B. Effects of the Different Transport Phases on Equine Health Status, Behavior, and Welfare: A Review. J. Vet. Behav. 2015, 10, 272–282. [Google Scholar] [CrossRef]
- Judge, N.G. Transport of Horses. Aust. Vet. J. 1969, 45, 465–469. [Google Scholar] [CrossRef]
- Waran, N.K. The Behaviour of Horses during and after Transport by Road. Equine Vet. Educ. 1993, 5, 129–132. [Google Scholar] [CrossRef]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef]
- Leadon, D.; Waran, N.; Herholz, C.; Klay, M. Veterinary Management of Horse Transport. Vet. Ital. 2008, 44, 149–163. [Google Scholar]
- Soroko, M.; Howell, K.; Dudek, K.; Waliczek, A.; Micek, P.; Flaga, J. Relationship between Maximum Eye Temperature and Plasma Cortisol Concentration in Racehorses during Intensive Training. Pol. J. Vet. Sci. 2021, 24, 393–397. [Google Scholar] [CrossRef]
- Aragona, F.; Di Pietro, S.; Arfuso, F.; Fazio, F.; Piccione, G.; Giudice, E.; Giannetto, C. Correlation between Ocular and Rectal Temperature with Intra Ocular Pressure in Horse during Exercise. Animals 2022, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Aragona, F.; Arfuso, F.; Fazio, F.; De Caro, S.; Giudice, E.; Monteverde, V.; Piccione, G.; Giannetto, C. Circadian Variation of Peripheral Blood Cells in Horses Maintained in Different Environmental and Management Conditions. Animals 2023, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Rao, S.; Hussey, S.B.; Morley, P.S.; Traub-Dargatz, J.L. Thermographic Eye Temperature as an Index to Body Temperature in Ponies. J. Equine Vet. Sci. 2011, 31, 63–66. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of Environmental Factors on Infrared Eye Temperature Measurements in Cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhao, Q.; Chen, X.; Zhao, G.; Gu, X. Physiological Indicators and Production Performance of Dairy Cows With Tongue Rolling Stereotyped Behavior. Front. Vet. Sci. 2022, 9, 840726. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Casella, S.; Fazio, F.; Pennisi, P. Effect of Shearing on Some Haematochemical Parameters in Ewes. Czech J. Anim. Sci. 2008, 53, 106–111. [Google Scholar] [CrossRef]
- Casella, S.; Vazzana, I.; Giudice, E.; Fazio, F.; Piccione, G. Relationship between Serum Cortisol Levels and Some Physiological Parameters Following Reining Training Session in Horse. Anim. Sci. J. 2016, 87, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Cerutti, R.; Scaglione, M.; Fazio, F.; Aragona, F.; Arfuso, F.; Zumbo, A.; Piccione, G. Simultaneous Recording of Subcutaneous Temperature and Total Locomotor Activity in Bos Taurus and Bos Indicus Raised in a Subtropical Region of Argentina. Trop. Anim. Health Prod. 2022, 54, 371. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Giudice, E.; Gianesella, M.; Fazio, F.; Panzera, M.; Piccione, G. Infrared Methodologies for the Assessment of Skin Temperature Daily Rhythm in Two Domestic Mammalian Species. J. Therm. Biol. 2020, 92, 102677. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, V.; Luzi, F.; Mazzola, S.; Bariffi, G.D.; Zappaterra, M.; Nanni Costa, L.; Padalino, B. The Use of Infrared Thermography (IRT) as Stress Indicator in Horses Trained for Endurance: A Pilot Study. Animals 2019, 9, 84. [Google Scholar] [CrossRef]
- Soroko, M.; Dudek, K.; Howell, K.; Jodkowska, E.; Henklewski, R. Thermographic Evaluation of Racehorse Performance. J. Equine Vet. Sci. 2014, 34, 1076–1083. [Google Scholar] [CrossRef]
- Jansson, A.; Lindgren, G.; Velie, B.D.; Solé, M. An Investigation into Factors Influencing Basal Eye Temperature in the Domestic Horse (Equus caballus) When Measured Using Infrared Thermography in Field Conditions. Physiol. Behav. 2021, 228, 113218. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.L.; Van Hoogmoed, L.M.; Snyder, J.R. The Role of Thermography in the Management of Equine Lameness. Vet. J. 2001, 162, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, E.; Sánchez, M.J.; Molina, A.; Schaefer, A.L.; Cervantes, I.; Valera, M. Using Eye Temperature and Heart Rate for Stress Assessment in Young Horses Competing in Jumping Competitions and Its Possible Influence on Sport Performance. Animals 2013, 7, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Riemer, S.; Assis, L.; Pike, T.W.; Mills, D.S. Dynamic Changes in Ear Temperature in Relation to Separation Distress in Dogs. Physiol. Behav. 2016, 167, 86–91. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared Thermography in Animal Production: An Overview. Comp. Electr. Agricult 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot Dogs: Thermography in the Assessment of Stress in Dogs (Canis familiaris)—A Pilot Study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared Thermal Imaging in Medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Giannetto, C.; Aragona, F.; Arfuso, F.; Piccione, G.; De Caro, S.; Fazio, F. Diurnal Variation in Rectal and Cutaneous Temperatures of Horses Housed under Different Management Conditions. Int. J. Biometeorol. 2022, 66, 1601–1611. [Google Scholar] [CrossRef]
- Adriaan Bouwknecht, J.; Olivier, B.; Paylor, R.E. The Stress-Induced Hyperthermia Paradigm as a Physiological Animal Model for Anxiety: A Review of Pharmacological and Genetic Studies in the Mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- de Freitas, A.C.B.; Quirino, C.R.; Bartholazzi Junior, A.; Vega, W.H.O.; David, C.M.G.; Geraldo, A.T.; Rua, M.A.S.; Rojas, L.F.C.; de Almeida Filho, J.E.; Dias, A.J.B. Surface Temperature in Different Anatomical Regions of Ewes Measured by Infrared Thermography. Livestock Sci. 2018, 216, 84–87. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Mora-Medina, P.; Casas-Alvarado, A.; Rios-Sandoval, J.; de Mira Geraldo, A.; Wang, D. Thermal Imaging to Assess the Health Status in Wildlife Animals under Human Care: Limitations and Perspectives. Animals 2022, 12, 3558. [Google Scholar] [CrossRef] [PubMed]
- Vianna, D.M.L.; Carrive, P. Changes in Cutaneous and Body Temperature during and after Conditioned Fear to Context in the Rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Cogi, N.H.; Heinzl, E.U.L.; Dalla Costa, E.; Canali, E.; Minero, M. Validation of a Fear Test in Sport Horses Using Infrared Thermography. J. Vet. Behav. 2015, 10, 128–136. [Google Scholar] [CrossRef]
- Jerem, P.; Jenni-Eiermann, S.; Herborn, K.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye Region Surface Temperature Reflects Both Energy Reserves and Circulating Glucocorticoids in a Wild Bird. Sci. Rep. 2018, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.; Warren-Smith, A.; Guisard, Y. The Effect of Double Bridles and Jaw-Clamping Crank Nosebands on Temperature of Eyes and Facial Skin of Horses. J. Vet. Behav. 2012, 7, 142–148. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-Invasive Measurement of Stress in Dairy Cows Using Infrared Thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of Infrared Thermography as a Non-Invasive Method of Measuring the Autonomic Nervous Response in Sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef]
- Giannetto, C.; Fazio, F.; Alberghina, D.; Assenza, A.; Panzera, M.; Piccione, G. Different Daily Patterns of Serum Cortisol and Locomotor Activity Rhythm in Horses under Natural Photoperiod. J. Vet. Behav. Clin. Appl. Res. 2014, 10, 118–121. [Google Scholar] [CrossRef]
- Boucher, P.; Plusquellec, P. Acute Stress Assessment From Excess Cortisol Secretion: Fundamentals and Perspectives. Front. Endocrinol. 2019, 10, 749. [Google Scholar] [CrossRef]
- Rizzo, M.; Arfuso, F.; Giannetto, C.; Giudice, E.; Longo, F.; Di Pietro, S.; Piccione, G. Cortisol Levels and Leukocyte Population Values in Transported and Exercised Horses after Acupuncture Needle Stimulation. J. Vet. Behav. 2017, 18, 56–61. [Google Scholar] [CrossRef]
- Valera, M.; Bartolomé, E.; Sánchez Guerrero, M.; Molina Alcalá, A.; Cook, N.; Schaefer, A. Changes in Eye Temperature and Stress Assessment in Horses During Show Jumping Competitions. J. Equine Vet. Sci. 2012, 32, 827–830. [Google Scholar] [CrossRef]
- Martin, S.; Kline, A. Can there be a standard for temperature measurement in the pediatric intensive care unit? AACN Adv. Crit. Care 2004, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Cho, G.-J. Validation of Eye Temperature Assessed Using Infrared Thermography as an Indicator of Welfare in Horses. Appl. Sci. 2021, 11, 7186. [Google Scholar] [CrossRef]
- Arfuso, F.; Giudice, E.; Panzera, M.; Rizzo, M.; Fazio, F.; Piccione, G.; Giannetto, C. Interleukin-1Ra (Il-1Ra) and Serum Cortisol Level Relationship in Horse as Dynamic Adaptive Response during Physical Exercise. Vet. Immunol. Immunopathol. 2022, 243, 110368. [Google Scholar] [CrossRef]
- Aragona, F.; Fazio, F.; Piccione, G.; Giannetto, C. Chronophysiology of Domestic Animals. Chronobiol. Int. 2024, 41, 888–903. [Google Scholar] [CrossRef]
- Aragona, F.; Arfuso, F.; Rizzo, M.; Fazio, F.; Acri, G.; Piccione, G.; Giannetto, C. Using Infrared Thermography for the Evaluation of Road Transport Thermal Homeostasis in Athletic Horse. J. Equine Vet. Sci. 2024, 138, 105102. [Google Scholar] [CrossRef] [PubMed]
- Gjendal, K.; Franco, N.; Ottesen, J.; Sørensen, D.; Olsson, A. Eye, Body or Tail? Thermography as a Measure of Stress in Mice. Physiol. Behav. 2018, 196, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Stull, C.L. Responses of Horses to Trailer Design, Duration, and Floor Area during Commercial Transportation to Slaughter. J. Anim. Sci. 1999, 77, 2925–2933. [Google Scholar] [CrossRef]
- Moe, R.O.; Bohlin, J.; Flø, A.; Vasdal, G.; Stubsjøen, S.M. Hot Chicks, Cold Feet. Physiol. Behav. 2017, 179, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep. Animals 2018, 8, 146. [Google Scholar] [CrossRef]
- Chen, X.; Ogdahl, W.; Hulsman Hanna, L.L.; Dahlen, C.R.; Riley, D.G.; Wagner, S.A.; Berg, E.P.; Sun, X. Evaluation of Beef Cattle Temperament by Eye Temperature Using Infrared Thermography Technology. Comp. Electr. Agricult 2021, 188, 106321. [Google Scholar] [CrossRef]
- de Mira, M.C.; Lamy, E.; Santos, R.; Williams, J.; Pinto, M.V.; Martins, P.S.; Rodrigues, P.; Marlin, D. Salivary Cortisol and Eye Temperature Changes during Endurance Competitions. BMC Vet. Res. 2021, 17, 329. [Google Scholar] [CrossRef]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol Release and Heart Rate Variability in Horses during Road Transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef]
- Bruschetta, G.; Zanghì, G.; Giunta, R.P.; Ferlazzo, A.M.; Satué, K.; D’Ascola, A.; Fazio, E. Short Road Transport and Slaughter Stress Affects the Expression Profile of Serotonin Receptors, Adrenocortical, and Hematochemical Responses in Horses. Vet. Sci. 2024, 11, 113. [Google Scholar] [CrossRef]
- Tadich, T.; Leal, F.; Gallo, C. Preliminary Study on the Effects of Long Distance Road Transport on Some Blood Constituents in Recently Weaned Thoroughbred Foals. J. Equine Vet. Sci. 2015, 35, 697–699. [Google Scholar] [CrossRef]
- Tateo, A.; Padalino, B.; Boccaccio, M.; Maggiolino, A.; Centoducati, P. Transport Stress in Horses: Effects of Two Different Distances. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 162–169. [Google Scholar] [CrossRef]
- Borstel, U.K.V.; Visser, E.K.; Hall, C. Indicators of Stress in Equitation. Appl. Anim. Behav. Sci. 2017, 190, 43–56. [Google Scholar] [CrossRef]

| Environmental Factors | Experimental Conditions | Max. | Mean | Min. |
|---|---|---|---|---|
| Ambient temperature (°C) | 100 km | 24.75 | 21 | 18.13 |
| 300 km | 27.6 | 22 | 20.7 | |
| Relative humidity (%) | 100 km | 79.30 | 67.50 | 51.64 |
| 300 km | 80.32 | 63.80 | 51.96 | |
| Temperature–humidity index (THI) | 100 km | 66.21 | ||
| 300 km | 69.19 | |||
| Parameters | 100 km | 300 km | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | |||
| eye temperature (°C) | EL1 | max | 35.3 ± 1 | 36.8 ± 0.8 * | 36.7 ± 0.7 * | 36.7 ± 0.60 | 36.3 ± 1.42 | 35.9 ± 1 |
| avg | 33.4 ± 1 | 35.5 ± 0.8 ** | 35 ± 1.2 * | 34.7 ± 0.79 | 34.4 ± 1.56 | 33.8 ± 1.51 | ||
| min | 31.7 ± 1.3 | 33.4 ± 0.7 * | 33.2 ± 1.1 * | 33.1 ± 0.60 | 32.7 ± 1.38 | 31.6 ± 1.79 | ||
| EL2 | max | 33.7 ± 1.3 | 34.9 ± 1.1 * | 35.1 ± 0.9 * | 35 ± 1.16 | 35.1 ± 1.46 | 34.3 ± 1.29 | |
| avg | 32.3 ± 1.2 | 33.6 ± 0.9 * | 33.9 ± 1.2 ** | 33.4 ± 1.04 | 33.4 ± 1.21 | 32.6 ± 1.29 | ||
| min | 31.3 ± 1.8 | 33 ± 0.9 * | 32.9 ± 1.3 * | 32.4 ± 1.21 | 32.3 ± 1.37 | 31.6 ± 1.46 | ||
| EL3 | max | 34.5 ± 1 | 35.7 ± 1 * | 35.8 ± 1 * | 35.4 ± 0.98 | 35.6 ± 1.25 | 35.2 ± 0.65 | |
| avg | 33.1 ± 0.9 | 34.6 ± 1.1 ** | 33.5 ± 1.3 * | 34 ± 1.35 | 34.3 ± 1 | 33.7 ± 0.88 | ||
| min | 31.7 ± 1 | 33.4 ± 1.1 ** | 34.5 ± 1.1 * | 32.8 ± 1.44 | 33.1 ± 1.20 | 32.3 ± 1.12 | ||
| rectal temperature (°C) | 37.5 ± 0.1 | 37.7 ± 0.2 * | 37.7 ± 0.2 * | 37.4 ± 0.13 | 37.7 ± 0.13 * | 37.7 ± 0.11 | ||
| cortisol (ng/mL) | 12.3 ± 1.8 | 12.7 ± 1.2 | 13.6 ± 1.4 | 13.8 ± 1.53 | 13.6 ± 2.33 | 14 ± 1.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, F.; Rizzo, M.; Arfuso, F.; Acri, G.; Fazio, F.; Piccione, G.; Giannetto, C. Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses. Animals 2024, 14, 1877. https://doi.org/10.3390/ani14131877
Aragona F, Rizzo M, Arfuso F, Acri G, Fazio F, Piccione G, Giannetto C. Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses. Animals. 2024; 14(13):1877. https://doi.org/10.3390/ani14131877
Chicago/Turabian StyleAragona, Francesca, Maria Rizzo, Francesca Arfuso, Giuseppe Acri, Francesco Fazio, Giuseppe Piccione, and Claudia Giannetto. 2024. "Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses" Animals 14, no. 13: 1877. https://doi.org/10.3390/ani14131877






