An Assessment of the Climate Change Impacts on the Distribution of the Glacial Relict Woodpecker Three-Toed Woodpecker Picoides tridactylus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Area
2.3. Occurrence Points
2.4. Environmental Variables
2.5. Species Distribution Modelling
3. Results
3.1. Maxent Statistics
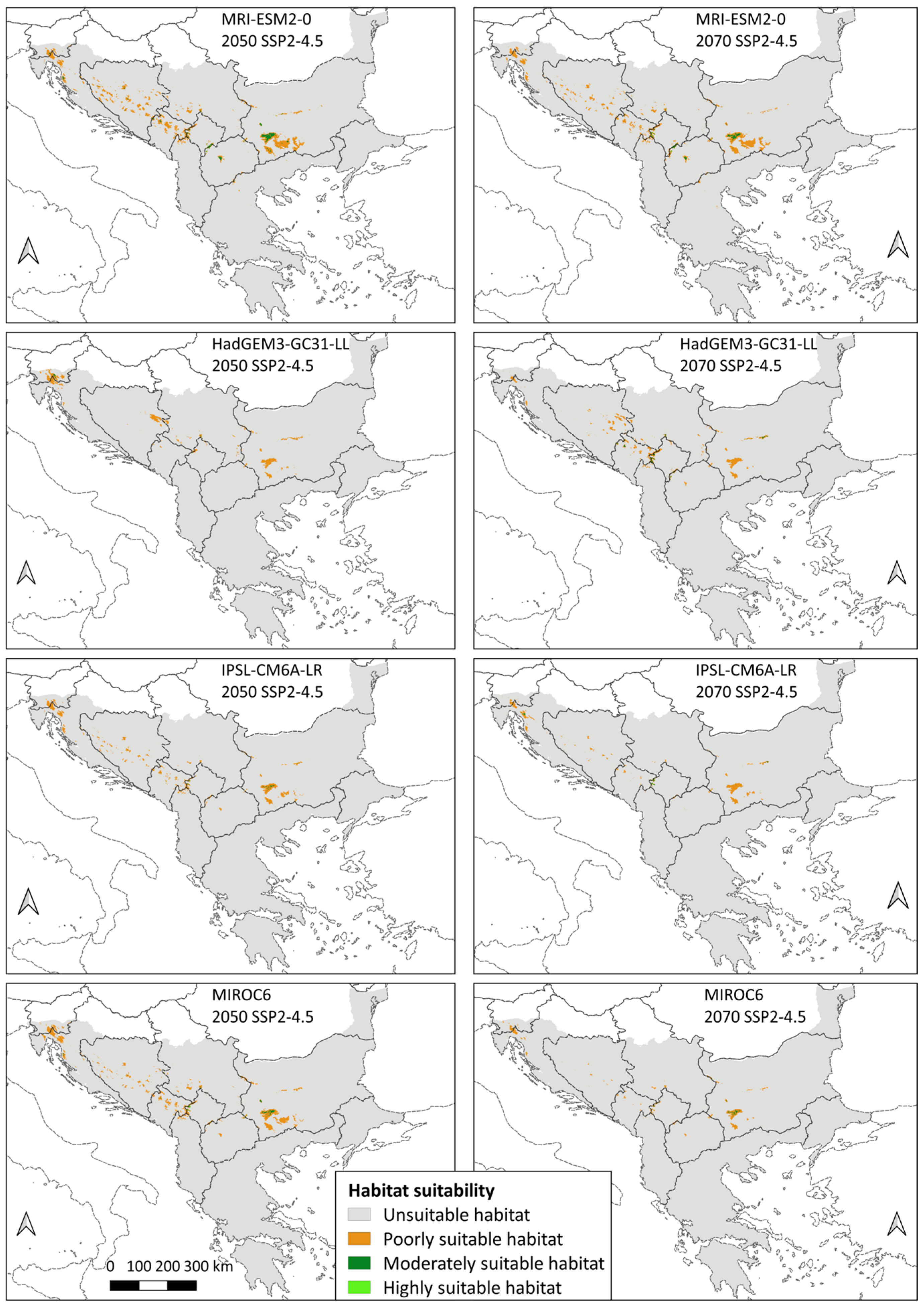
3.2. Species Distribution
4. Discussion
4.1. Environmental Determinants
4.2. Species Distribution
4.3. Conservation Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Bennett, K.D.; Tzedakis, P.C.; Willis, K.J. Quaternary refugia of north European trees. J. Biogeogr. 1991, 18, 103–115. [Google Scholar] [CrossRef]
- Willis, K.J. The vegetational history of the Balkans. Quat. Sci. Rev. 1994, 13, 769–788. [Google Scholar] [CrossRef]
- Hewitt, G.M. Speciation, hybrid zones and phylogeography—or seeing genes in space and time. Mol. Ecol. 2001, 10, 537–549. [Google Scholar] [CrossRef]
- Lambeck, K.; Yokoyama, Y.; Purcell, T. Into and out of the Last Glacial Maximum: Sea-level change during Oxygen Isotope Stages 3 and 2. Quat. Sci. Rev. 2002, 21, 343–360. [Google Scholar] [CrossRef]
- del Hoyo, J.; Collar, N.; Christie, D.A. Eurasian Three-toed Woodpecker (Picoides tridactylus), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Spiridonov, G.; Spassov, N.; Ivanov, V. New Records of Three Relict Bird Species in the Rhodopes. Hist. Nat. Bulg. 2008, 19, 181–182. Available online: https://www.nmnhs.com/historia-naturalis-bulgarica/article.php?id=000337000192008 (accessed on 12 January 2024).
- Vasić, V.; Grubač, B.; Raković, M.; Čović, S. Records of Three-toed Woodpecker Picoides tridactylus in Serbia. Ciconica 2009, 18, 147–155. Available online: https://pticesrbije.rs/wp-content/uploads/Ciconia_18.pdf (accessed on 20 December 2023).
- Pakkala, T.; Tiainen, J.; Piha, M.; Kouki, J. Nest tree characteristics of the old-growth specialist Three-toed Woodpecker Picoides tridactylus. Ornis Fennica 2018, 95, 89–102. [Google Scholar] [CrossRef]
- Shurulinkov, P.; Fedchuk, D. Observations of Pigmy Owl (Glaucidium passerinum) and Three-toed Woodpecker (Picoides tridactylus) in Mt. Durmitor, Montenegro. Hist. Nat. Bulg. 2015, 22, 101–103. [Google Scholar] [CrossRef]
- Obratil, S. Overview of research on the ornithofauna of Bosnia and Herzegovina Part VI. Glasnik Zemaljskog muzeja BiH u Sarajevu. Prirodne nauke 1977, 16, 203–223. Available online: https://www.wild-herzegovina.com/bibliography/Obratil-1977.pdf (accessed on 10 December 2023).
- Ćiković, D.; Barišić, S.; Tutiš, V.; Kralj, J. Woodpeckers in the Croatian Karst Mountains. Bird Census News 2008, 21, 2–15. Available online: https://www.ebcc.info/wp-content/uploads/2020/06/bcn-21-1.pdf (accessed on 10 January 2024).
- Denac, K.; Mihelič, T.; Božič, L.; Kmecl, P.; Jančar, T.; Figelj, J.; Rubinić, B. Strokovni Predlog za Revizijo Posebnih Območij Varstva (SPA) z Uporabo Najnovejših Kriterijev za Določitev Mednarodno Pomembnih Območij za Ptice (IBA). Končno poročilo (dopolnjena verzija); DOPPS—BirdLife: Ljubljana, Slovenia, 2011; p. 245. Available online: https://www.ptice.si/wp-content/uploads/2014/03/201110_denac_revizija_iba_porocilo_28102011_dopolnjena_verzija.pdf (accessed on 1 June 2024).
- Spiridonov, G.; Velev, K.; Nikolov, S.C. Three-toed Woodpecker. In Atlas of the Breeding Birds in Bulgaria; Iankov, P., Ed.; Bulgarian Society for the Protection of Birds: Sofia, Bulgaria, 2007; pp. 374–375. ISBN 978-954-91421-7-4. [Google Scholar]
- Brambilla, M.; Rubolini, D.; Appukuttan, O.; Calvi, G.; Karger, D.N.; Kmecl, P.; Mihelič, T.; Sattler, T.; Seaman, B.; Teufelbauer, N.; et al. Identifying climate refugia for high-elevation Alpine birds under current climate warming predictions. Glob. Change Biol. 2022, 28, 4276–4291. [Google Scholar] [CrossRef] [PubMed]
- Diaz, H.F.; Grosjean, M.; Graumlich, L. Climate Variability and Change in High Elevation Regions: Past, Present and Future. Clim. Change 2003, 59, 1–4. [Google Scholar] [CrossRef]
- Djurdjevic, V.; Trbić, G.; Krzic, A.; Bozanic, D. Projected Changes in Multi-day Extreme Precipitation Over the Western Balkan Region. In Climate Change Adaptation in Eastern Europe: Managing Risks and Building Resilience to Climate Change; Leal Filho, W., Trbic, G., Filipovic, D., Eds.; Springer: Cham, Switzerland, 2019; pp. 15–28. ISBN 978-3-030-03383-5. [Google Scholar]
- Kurnik, B. Changes in the climate system. In Climate Change, Impacts and Vulnerability in Europe 2016: An Indicator-Based Report; Publications Office of the European Union: Luxembourg, Luxembourg, 2017; p. 73. Available online: https://op.europa.eu/en/publication-detail/-/publication/794dcba3-e922-11e6-ad7c-01aa75ed71a1/language-en (accessed on 2 June 2024).
- Peneva, E.; Matov, M.; Tsekov, M. Mediterranean Influence on the Climatic Regime over the Balkan Peninsula from 1901–2021. Climate 2023, 11, 68. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Giorgi, F. Climate change hotspots in the CMIP5 global climate model ensemble. Clim. Chang. 2012, 114, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 6, 12, 1450. ISBN 9781009157896. [Google Scholar]
- Fiedler, W. Bird Ecology as an Indicator of Climate and Global Change. In Climate Change: Observed Impacts on Planet Earth; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 181–195. ISBN 978-0-444-53301-2. [Google Scholar]
- Huntley, B.; Collingham, Y.C.; Green, R.E.; Hilton, G.M.; Rahbek, C.; Willis, S.G. Potential impacts of climatic change upon geographical distributions of birds. Ibis 2006, 148, 8–28. [Google Scholar] [CrossRef]
- Wormworth, J.; Mallon, K. Bird Species and Climate Change; WWF: Sydney, Australia, 2006; Available online: http://assets.wwf.org.uk/downloads/birdandclimatechangesummary.pdf (accessed on 20 December 2023).
- Trautmann, S. Climate Change Impacts on Bird Species. In Bird Species: How They Arise, Modify and Vanish; Tietze, D.T., Ed.; Springer: Cham, Switzerland, 2018; pp. 217–234. ISBN 978-3-319-91689-7. [Google Scholar]
- Jiguet, F.; Gadot, A.-S.; Julliard, R.; Newson, S.E.; Couvet, D. Climate envelope, life history traits and the resilience of birds facing global change. Glob. Change Biol. 2007, 13, 1672–1684. [Google Scholar] [CrossRef]
- BirdLife International. Picoides tridactylus. The IUCN Red List of Threatened Species 2016. Available online: https://www.iucnredlist.org/species/22727137/87304270 (accessed on 11 April 2024).
- Biodiversity Information System for Europe. Three-Toed Woodpecker—Picoides tridactylus. Available online: https://biodiversity.europa.eu/species/1217 (accessed on 11 April 2024).
- BirdLife International. European Red List of Birds; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-45974-3. [Google Scholar]
- BirdLife International. Species Factsheet: Picoides tridactylus. Available online: https://datazone.birdlife.org/species/factsheet/three-toed-woodpecker-picoides-tridactylus (accessed on 11 April 2024).
- Saveljić, D.; Zeković, B.; Šoškić Popović, M.; Novović, N.; Drobnjak, J. Red List of Birds of Montenegro; Environmental Protection Agency of Montenegro: Podgorica, Montenegro, 2022; ISBN 978-9940-9924-4-6.
- Tutiš, V.; Kralj, J.; Radović, D.; Ćiković, D.; Barišić, S. Red Data Book of Birds of Croatia; Ministry of Environmental and Nature Protection, State Institute for Nature Protection: Zagreb, Croatia, 2013; ISBN 9789537169909. [Google Scholar]
- Škrijelj, R.; Lelo, S.; Drešković, N.; Sofradžija, A.; Trožić-Borovac, S.; Korjenić, E.; Lukić-Bilela, L.; Mitrašinović-Brulić, M.; Kotrošan, D.; Šljuka, S.; et al. Red List of Fauna of the Federation of Bosnia and Herzegovina. Draft Report—Proposal; EU Greenway Sarajevo, Prirodno-Matematički Fakultet Sarajevo: Sarajevo, Bosnia and Herzegovina, 2013. Available online: https://www.fmoit.gov.ba/upload/file/okolis/Crvena%20lista%20Faune%20FBiH.pdf (accessed on 10 April 2024).
- Rajković, D.; Vasić, V. Three-Toed Woodpecker. In Red Book of Fauna of Serbia III—Birds; Radišić, D., Vasić, V., Puzović, S., Ružić, M., Šćiban, M., Grubač, B., Vujić, A., Eds.; Zavod za Zaštitu Prirode Srbije, Univerzitet u Novom Sadu, Prirodno-Matematički fakultet, Departman za Biologiju i Ekologiju, Društvo za Zaštitu i Proučavanje ptica Srbije: Belgrade, Serbia, 2018; pp. 282–284. ISBN 978-86-80877-60-0. [Google Scholar]
- Michev, T.; Boev, Z.; Kambourova, N. Red List of the Birds of Bulgaria. In Proceedings of the Jubilee National Scientific Conference with International Participation “The Man and the Universe”, Smolyan, Bulgaria, 6–8 October 2011; Available online: https://www.academia.edu/33196426/Red_List_of_the_Birds_of_Bulgaria (accessed on 12 April 2024).
- Legakis, A.; Maragos, P. The Red Book of Endangered Animals of Greece; Hellenic Zoological Society: Athens, Greece, 2009; ISBN 978-960-85298-8-5. [Google Scholar]
- Ćurčić, S.; Pavićević, D.; Radović, D.; Vesović, N.; Bekchiev, R.; Ćurčić, N.; Guéorguiev, B. Current and predicted distribution of the rare and threatened beetle Bolbelasmus (Bolbelasmus) unicornis (Coleoptera: Geotrupidae) in Serbia. Eur. J. Entomol. 2019, 116, 413–424. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Fløjgaard, C.; Marske, K.A.; Nógues-Bravo, D.; Normand, S. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 2011, 30, 2930–2947. [Google Scholar] [CrossRef]
- Romero-Calcerrada, R.; Luque, S. Habitat quality assessment using Weights-of-Evidence based GIS modelling: The case of Picoides tridactylus as species indicator of the biodiversity value of the Finnish forest. Ecol. Model. 2006, 196, 62–76. [Google Scholar] [CrossRef]
- Virkkala, R.; Leikola, N.; Kujala, H.; Kivinen, S.; Hurskainen, P.; Kuusela, S.; Valkama, J.; Heikkinen, R.K. Developing fine-grained nationwide predictions of valuable forests using biodiversity indicator bird species. Ecol. Appl. 2022, 32, e2505. [Google Scholar] [CrossRef] [PubMed]
- Stachura-Skierczyńska, K.; Tumiel, T.; Skierczyński, M. Habitat prediction model for three-toed woodpecker and its implications for the conservation of biologically valuable forests. For. Ecol. Manag. 2009, 258, 697–703. [Google Scholar] [CrossRef]
- Bollmann, K.; Braunisch, V.; Arlettaz, R. Predicted Effects of Climate Change on Indicator Species of Structural and Biological Diversity in Mountain Forests: Towards Adaptive Forest Management in the Face of Environmental Uncertainty. Available online: https://www.research-collection.ethz.ch/handle/20.500.11850/311144 (accessed on 10 January 2024).
- Virkkala, R.; Heikkinen, R.K.; Fronzek, S.; Kujala, H.; Leikola, N. Does the protected area network preserve bird species of conservation concern in a rapidly changing climate? Biodivers. Conserv. 2013, 22, 459–482. [Google Scholar] [CrossRef]
- Pearson, R.G. Climate change and the migration capacity of species. Trends Ecol. Evol. 2006, 21, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Shurulinkov, P.; Stoyanov, G.; Komitov, E.; Daskalova, G.; Ralev, A. Contribution to the Knowledge on Distribution, Number and Habitat Preferences of Rare and Endangered Birds in Western Rhodopes Mts, Southern Bulgaria. Strigiformes and Piciformes. Acta Zool. Bulg. 2012, 64, 43–56. Available online: https://www.acta-zoologica-bulgarica.eu/downloads/acta-zoologica-bulgarica/2012/64-1-043-056.pdf (accessed on 10 February 2024).
- Virkkala, R.; Rajasärkkä, A.; Väisänen, R.A.; Vickholm, M.; Virolainen, E. Conservation Value of Nature Reserves: Do Hole-nesting Birds Prefer Protected Forests in Southern Finland? Ann. Zool. Fennici 1994, 31, 173–186. Available online: http://www.jstor.org/stable/23735509 (accessed on 10 January 2024).
- Balasso, M. Ecological Requirements of the Threetoed Woodpecker (Picoides tridactylus L.) in Boreal Forests of Northern Sweden. Master’s Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 2016. Available online: https://stud.epsilon.slu.se/8777/ (accessed on 10 December 2023).
- Bock, C.E.; Bock, J.H. On the Geographical Ecology and Evolution of the Three-toed Woodpeckers, Picoides tridactylus and P. arcticus. Am. Midl. Nat. J. 1974, 92, 397–405. [Google Scholar] [CrossRef]
- Zellweger, F.; Braunisch, V.; Baltensweiler, A.; Bollmann, K. Remotely sensed forest structural complexity predicts multi species occurrence at the landscape scale. For. Ecol. Manag. 2013, 307, 303–312. [Google Scholar] [CrossRef]
- Askeyev, A.; Askeyev, O.; Askeyev, I. Long-Term Woodpecker Winter Population Dynamics in Tatarstan Republic. Vogelwelt 2017, 137, 130–133. Available online: https://www.researchgate.net/publication/315047843_Long-term_woodpecker_winter_population_dynamics_in_the_Tatarstan_Republic (accessed on 1 June 2024).
- Rubinić, B.; Sackl, P.; Gramatikov, M. Conserving of Wild Birds in Montenegro: The First Inventory of Potential Special Protection Areas in Montenegro; AAM Consulting: Budapest, Hungary, 2019; p. 309. ISBN 978-9940-9924-1-5. [Google Scholar]
- Doyon, F.; Higgelke, P.E.; MacLeod, H.L. Three-Toed Woodpecker (Picoides tridactylus); KBM Forestry Consultants Inc: Thunder Bay, ON, Canada, 2000; Available online: https://isfort.uqo.ca/wp-content/uploads/2020/11/Doyon-et-al.-2000.-Three-toed-woodpecker-Picoides-tridactylus.pdf (accessed on 11 January 2024).
- Pechacek, P.; d’Oleire-Oltmanns, W. Habitat use of the three-toed woodpecker in central Europe during the breeding period. Biol. Conserv. 2004, 116, 333–341. [Google Scholar] [CrossRef]
- Bütler, R.; Angelstam, P.; Ekelund, P.; Schlaepfer, R. Dead wood threshold values for the three-toed woodpecker presence in boreal and sub-Alpine forest. Biol. Conserv. 2004, 119, 305–318. [Google Scholar] [CrossRef]
- Cvijić, J. Balkan Peninsula and the South Slavic Lands. Državna štamparija Kraljevine Srba, Hrvata i Slovenaca. Available online: https://www.zbor.rs/wp-content/uploads/2019/11/jovan-cvijic-balkansko-poluostrvo.pdf (accessed on 20 March 2024).
- Strid, A.; Andonoski, A.; Andonovski, V. The High Mountain Vegetation of the Balkan Peninsula. In Alpine Biodiversity in Europe; Nagy, L., Grabherr, G., Körner, C., Thompson, D.B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 113–121. ISBN 978-3-642-18967-8. [Google Scholar]
- Telbisz, T.; Tóth, G.; Ruban, D.A.; Gutak, J.M. Notable Glaciokarsts of the World. In Glaciokarsts; Veress, M., Telbisz, T., Tóth, G., Lóczy, D., Ruban, D.A., Gutak, J.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 373–499. ISBN 978-3-319-97292-3. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System; QGIS Association: Grüt, Switzerland, 2022; Available online: https://www.qgis.org (accessed on 29 April 2022).
- Demek, J.; Gams, I.; Vaptsarov, I. Balkan Peninsula. In Geomorphology of Europe; Embleton, C., Ed.; Palgrave: London, UK, 1984; pp. 374–386. ISBN 978-1-349-17346-4. [Google Scholar]
- Skoulikidis, N. Balkan Rivers—Pressing Environmental Challenges. In Proceedings of the International Wild Rivers Science Symposium, Tirana, Albania, 17–20 October 2019; Available online: https://balkanrivers.net/sites/default/files/Nikos%20Skoulikidis_Balkan%20Rivers_.pdf (accessed on 20 March 2024).
- Đurđević, D.; Vasić, M.; Ogrin, M.; Savić, S.; Milošević, D.; Dunjić, J.; Šećerov, I.; Žgela, M.; Boras, M.; Herceg Bulić, I.; et al. Long-Term Assessment of Bioclimatic Conditions at Micro and Local Scales in the Cities of the Western Part of the Balkan Peninsula during the 21st Century. Sustainability 2023, 15, 15286. [Google Scholar] [CrossRef]
- Maheras, P.; Kolyva-Machera, F. Temporal and spatial characteristics of annual precipitation over the Balkans in the twentieth century. Int. J. Climatol. 1990, 10, 495–504. [Google Scholar] [CrossRef]
- Reed, J.M.; Kryštufek, B.; Eastwood, W.J. The Physical Geography of the Balkans and Nomenclature of Place Names. In Balkan Biodiversity; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 9–22. ISBN 978-1-4020-2854-0. [Google Scholar]
- Institute for Nature Conservation of Serbia. Data from the Electronic Database Collected as Part of the Projects “Creation of the Red Book of Flora, Fauna and Fungi in the Republic of Serbia”, “Establishment of an Ecological Network on the Territory of the Republic of Serbia” and “Establishment of the Ecological Network of the European Union NATURA 2000 as Part of the Ecological Network of the Republic of Serbia” and Field Research of the Institute for Nature Conservation of Serbia; Institute for Nature Conservation of Serbia: Belgrade, Serbia, 2023. [Google Scholar]
- Global Biodiversity Information Facility. GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0130746-230530130749713 (accessed on 2 August 2023).
- Popović, M.; Vasić, N.; Koren, T.; Burić, I.; Živanović, N.; Kulijer, D.; Golubović, A. Biologer: An open platform for collecting biodiversity data. Biodivers. Data J. 2020, 8, e53014. [Google Scholar] [CrossRef]
- Reddy, S.; Dávalos, L.M. Geographical sampling bias and its implications for conservation priorities in Africa. J. Biogeogr. 2003, 30, 1719–1727. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 30 April 2022).
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Jha, K.K.; Jha, R. Study of Vulture Habitat Suitability and Impact of Climate Change in Central India Using MaxEnt. J. Resour. Ecol. 2021, 12, 30–42. [Google Scholar] [CrossRef]
- Salas, E.A.L.; Seamster, V.A.; Boykin, K.G.; Harings, N.M.; Dixon, K.W. Modeling the impacts of climate change on Species of Concern (birds) in South Central U.S. based on bioclimatic variables. AIMS Environ. Sci. 2017, 4, 358–385. [Google Scholar] [CrossRef]
- Sutton, L.J.; McClure, C.J.W.; Kini, S.; Leonardi, G. Climatic Constraints on Laggar Falcon (Falco jugger) Distribution Predicts Multidirectional Range Movements under Future Climate Change Scenarios. J. Raptor Res. 2020, 54, 1–17. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- WorldClim. Bioclimatic Variables. Available online: https://www.worldclim.org/data/bioclim.html (accessed on 20 February 2024).
- WorldClim. Historical Climate Data. Available online: https://www.worldclim.org/data/worldclim21.html (accessed on 20 February 2024).
- Henckel, L.; Bradter, U.; Jönsson, M.; Isaac, N.J.B.; Snäll, T. Assessing the usefulness of citizen science data for habitat suitability modelling: Opportunistic reporting versus sampling based on a systematic protocol. Divers. Distrib. 2020, 26, 1276–1290. [Google Scholar] [CrossRef]
- Luoto, M.; Virkkala, R.; Heikkinen, R.K. The role of land cover in bioclimatic models depends on spatial resolution. Glob. Ecol. Biogeogr. 2007, 16, 34–42. [Google Scholar] [CrossRef]
- Virkkala, R.; Rajasärkkä, A. Northward density shift of bird species in boreal protected areas due to climate change. Boreal Environ. Res. 2011, 16, 2–13. Available online: http://hdl.handle.net/10138/232780 (accessed on 20 March 2024).
- Virkkala, R.; Pöyry, J.; Heikkinen, R.K.; Lehikoinen, A.; Valkama, J. Protected areas alleviate climate change effects on northern bird species of conservation concern. Ecol. Evol. 2014, 4, 2991–3003. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, J.; Perez, L.; Harati, S. Towards Modelling Future Trends of Quebec’s Boreal Birds’ Species Distribution under Climate Change. ISPRS Int. J. Geo-Inf. 2018, 7, 335. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- De Marco, P.J.; Nóbrega, C.C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Groen, T.A. VIF_Calculation_Function-R.R. Available online: https://github.com/icipe-official/COMBAT/blob/main/VIF_calculation_function-R.R (accessed on 10 January 2024).
- European Space Agency. Copernicus DEM—Global and European Digital Elevation Model (COP-DEM). Available online: https://spacedata.copernicus.eu/collections/copernicus-digital-elevation-model (accessed on 10 January 2024).
- European Environment Agency. CORINE Land Cover 2018 (Raster 100 m), Europe, 6-Yearly—Version 2020_20u1, May 2020. Available online: https://sdi.eea.europa.eu/catalogue/copernicus/api/records/960998c1-1870-4e82-8051-6485205ebbac?language=all (accessed on 10 January 2024).
- WorldClim. WorldClim 1.4 Downscaled Paleo Climate. Available online: https://www.worldclim.org/data/v1.4/paleo1.4.html (accessed on 12 January 2024).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Merrifield, A.L.; Brunner, L.; Lorenz, R.; Humphrey, V.; Knutti, R. Climate model Selection by Independence, Performance, and Spread (ClimSIPS v1.0.1) for regional application. Geosci. Model Dev. 2023, 16, 4715–4747. [Google Scholar] [CrossRef]
- Goberville, E.; Beaugrand, G.; Hautekèete, N.-C.; Piquot, Y.; Luczak, C. Uncertainties in the projection of species distributions related to general circulation models. Ecol. Evol. 2015, 5, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, M.; Bergero, V.; Bassi, E.; Falco, R. Current and future effectiveness of Natura 2000 network in the central Alps for the conservation of mountain forest owl species in a warming climate. Eur. J. Wildl. Res. 2015, 61, 35–44. [Google Scholar] [CrossRef]
- Subedi, T.R.; Peréz-García, J.M.; Gurung, S.; Baral, H.S.; Virani, M.Z.; Sah, S.A.M.; Anadón, J.D. Global range dynamics of the Bearded Vulture (Gypaetus barbatus) from the Last Glacial Maximum to climate change scenarios. Ibis 2023, 165, 403–419. [Google Scholar] [CrossRef]
- Hausfather, Z. CMIP6: The Next Generation of Climate Models Explained. Available online: https://www.carbonbrief.org/cmip6-the-next-generation-of-climate-models-explained/ (accessed on 11 February 2024).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.4). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 30 April 2022).
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 20 December 2023).
- Potts, A.J.; Hedderson, T.A.; Franklin, J.; Cowling, R.M. The Last Glacial Maximum distribution of South African subtropical thicket inferred from community distribution modelling. J. Biogeogr. 2013, 40, 310–322. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Change Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Cerman, K.; Rajković, D.; Topić, B.; Topić, G.; Shurulinkov, P.; Mihelič, T.; Delgado, J.D. Environmental Niche Modelling Predicts a Contraction in the Potential Distribution of Two Boreal Owl Species under Different Climate Scenarios. Animals 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, B.; Zou, B.; Xu, Y.; Yang, B.; Yang, N.; Ran, J. Assessment of habitat suitability of a high-mountain Galliform species, buff-throated partridge (Tetraophasis szechenyii). Glob. Ecol. Conserv. 2020, 24, e01230. [Google Scholar] [CrossRef]
- Copernicus Land Monitoring Service. 1.4.2 Sport and Leisure Facilities. Available online: https://land.copernicus.eu/content/corine-land-cover-nomenclature-guidelines/html/index-clc-142.html (accessed on 20 February 2024).
- Koç, D.E.; Biltekin, D.; Ustaoğlu, B. Modelling potential distribution of Carpinus betulus in Anatolia and its surroundings from the Last Glacial Maximum to the future. Arab. J. Geosci. 2021, 14, 1186. [Google Scholar] [CrossRef]
- Andersen, K.K.; Azuma, N.; Barnola, J.-M.; Bigler, M.; Biscaye, P.; Caillon, N.; Chappellaz, J.; Clausen, H.B.; Dahl-Jensen, D.; Fischer, H.; et al. High-resolution record of Northern Hemisphere climate extending into the last interglacial period. Nature 2004, 431, 147–151. [Google Scholar] [CrossRef]
- European Environment Agency. Picoides tridactylus. Available online: https://forum.eionet.europa.eu/article-12-birds-directive/library/2008-2012-reporting/species-factsheets/picoides-tridactylus (accessed on 10 March 2024).
- Arcilla, N.; Strazds, M. Ten Principles for Bird-Friendly Forestry: Conservation Approaches in Natural Forests Used for Timber Production. Birds 2023, 4, 245–261. [Google Scholar] [CrossRef]
- Ilić, T.; Kuzmanović, N.; Vukojičić, S.; Lakušić, D. Phytogeographic Characteristics of Montane Coniferous Forests of the Central Balkan Peninsula (SE Europe). Plants 2022, 11, 3194. [Google Scholar] [CrossRef]
- Esseen, P.-A.; Ehnström, B.; Ericson, L.; Sjöberg, K. Boreal Forests. Ecol. Bull. 1997, 46, 16–47. Available online: http://www.jstor.org/stable/20113207 (accessed on 10 January 2024).
- Doderović, M.; Burić, D.; Ducić, V.; Mijanović, I. Recent and future air temperature and precipitation changes in the mountainous north of Montenegro. J. Geogr. Inst. Jovan Cvijić SASA 2020, 70, 189–201. [Google Scholar] [CrossRef]
- Quante, M. The Changing Climate: Past, Present, Future. In Relict Species: Phylogeography and Conservation Biology; Habel, J.C., Assmann, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–56. ISBN 978-3-540-92160-8. [Google Scholar]
- Princé, K.; Lorrillière, R.; Barbet-Massin, M.; Léger, F.; Jiguet, F. Forecasting the Effects of Land Use Scenarios on Farmland Birds Reveal a Potential Mitigation of Climate Change Impacts. PLoS ONE 2015, 10, e0117850. [Google Scholar] [CrossRef] [PubMed]
- Scridel, D.; Brambilla, M.; Martin, K.; Lehikoinen, A.; Iemma, A.; Matteo, A.; Jähnig, S.; Caprio, E.; Bogliani, G.; Pedrini, P.; et al. A review and meta-analysis of the effects of climate change on Holarctic mountain and upland bird populations. Ibis 2018, 160, 489–515. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, T.; Ćurčić, N.B.; Đurđić, S.; Stanojević, G.; Raković, M. An Assessment of the Climate Change Impacts on the Distribution of the Glacial Relict Woodpecker Three-Toed Woodpecker Picoides tridactylus. Animals 2024, 14, 1879. https://doi.org/10.3390/ani14131879
Popović T, Ćurčić NB, Đurđić S, Stanojević G, Raković M. An Assessment of the Climate Change Impacts on the Distribution of the Glacial Relict Woodpecker Three-Toed Woodpecker Picoides tridactylus. Animals. 2024; 14(13):1879. https://doi.org/10.3390/ani14131879
Chicago/Turabian StylePopović, Teodora, Nina B. Ćurčić, Snežana Đurđić, Gorica Stanojević, and Marko Raković. 2024. "An Assessment of the Climate Change Impacts on the Distribution of the Glacial Relict Woodpecker Three-Toed Woodpecker Picoides tridactylus" Animals 14, no. 13: 1879. https://doi.org/10.3390/ani14131879




