Effects of Low-Salinity Stress on Histology and Metabolomics in the Intestine of Fenneropenaeus chinensis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Low Salinity Stress Experiment and Sampling
2.3. Histological Analyses of Intestine
2.4. Untargeted Metabonomic Analysis
2.5. Identification and Multivariate Statistical Analysis of Metabolites
3. Results
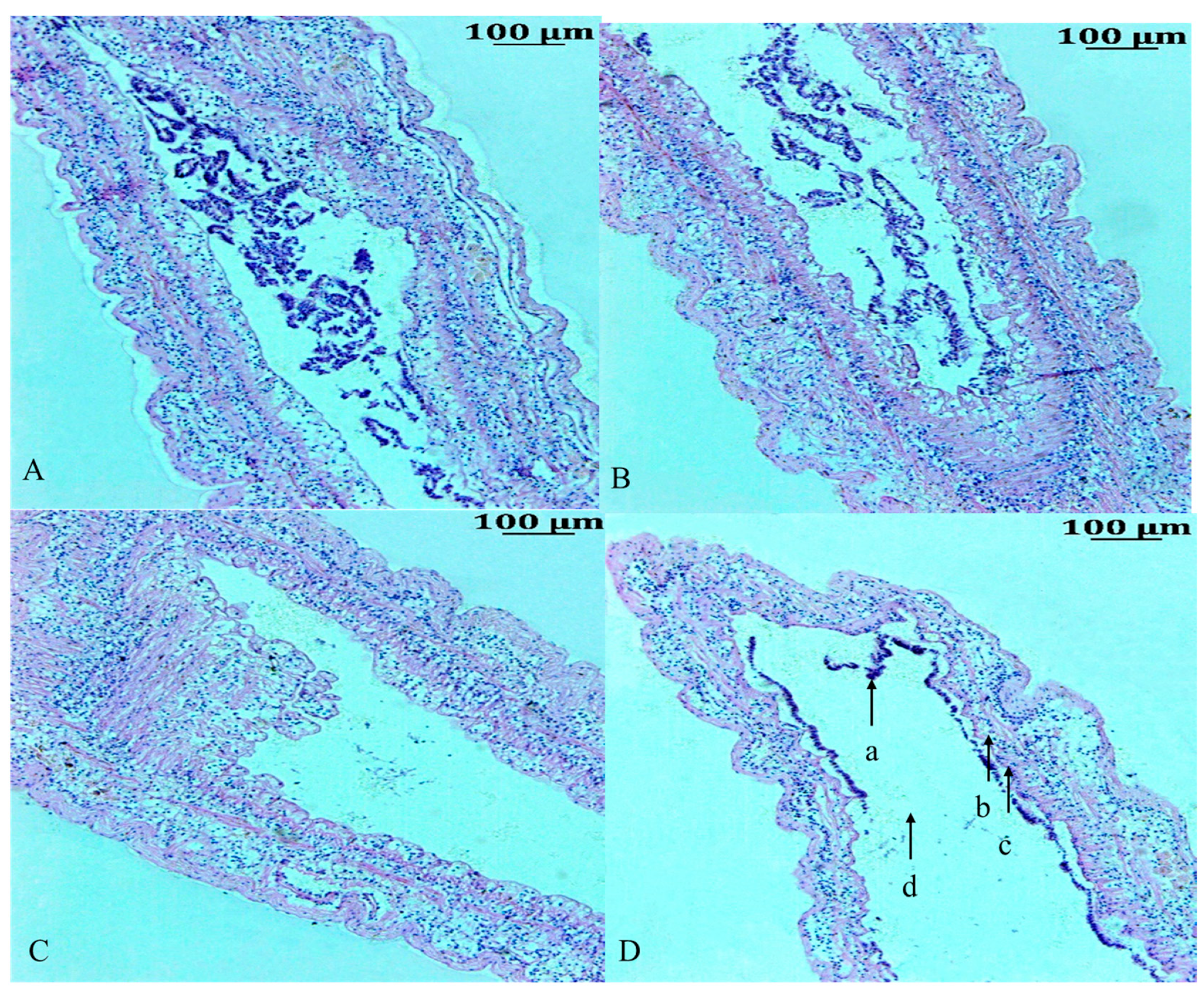
3.1. Histological Analysis of the Intestine
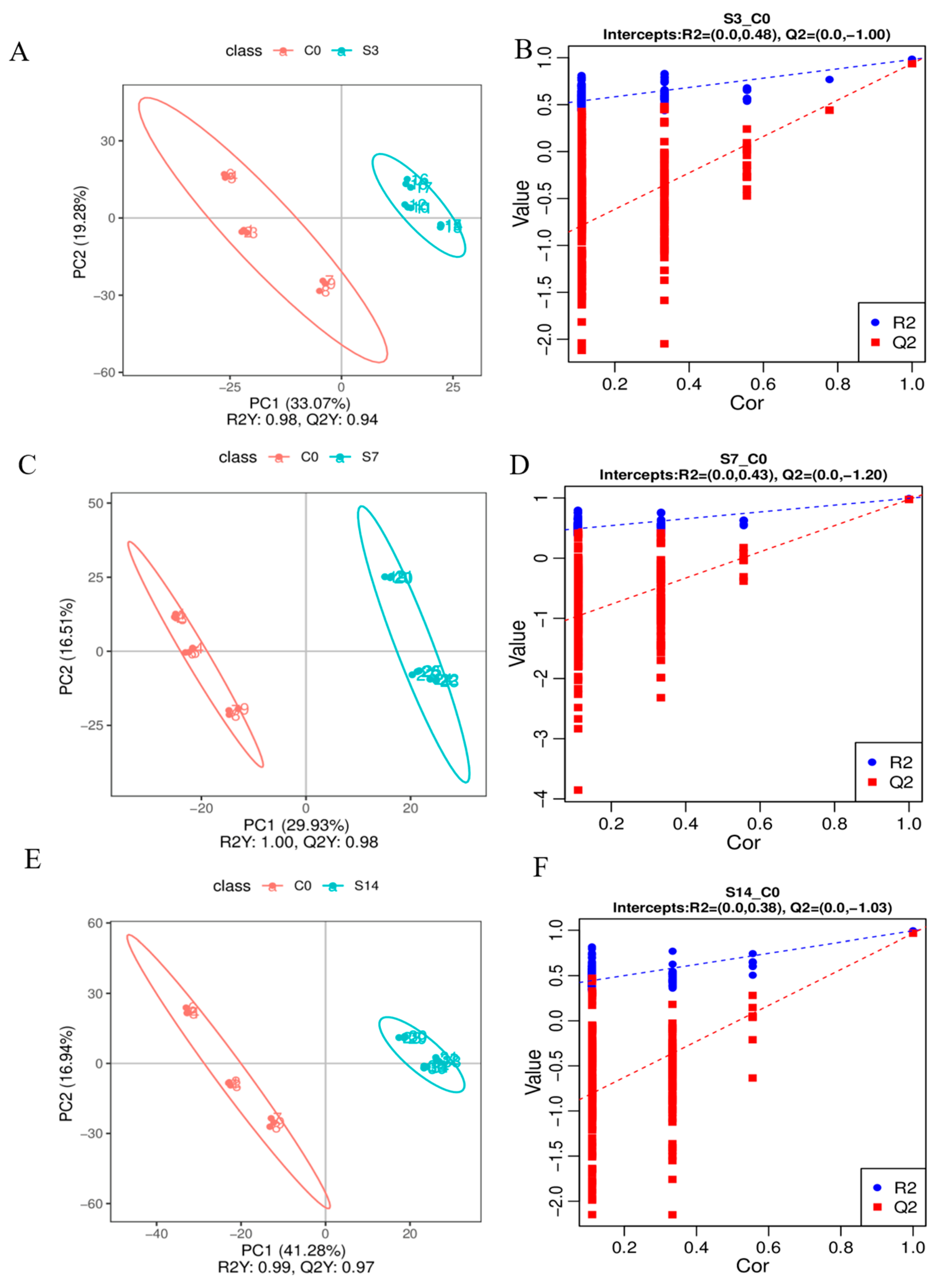
3.2. Identification of Metabolic Changes
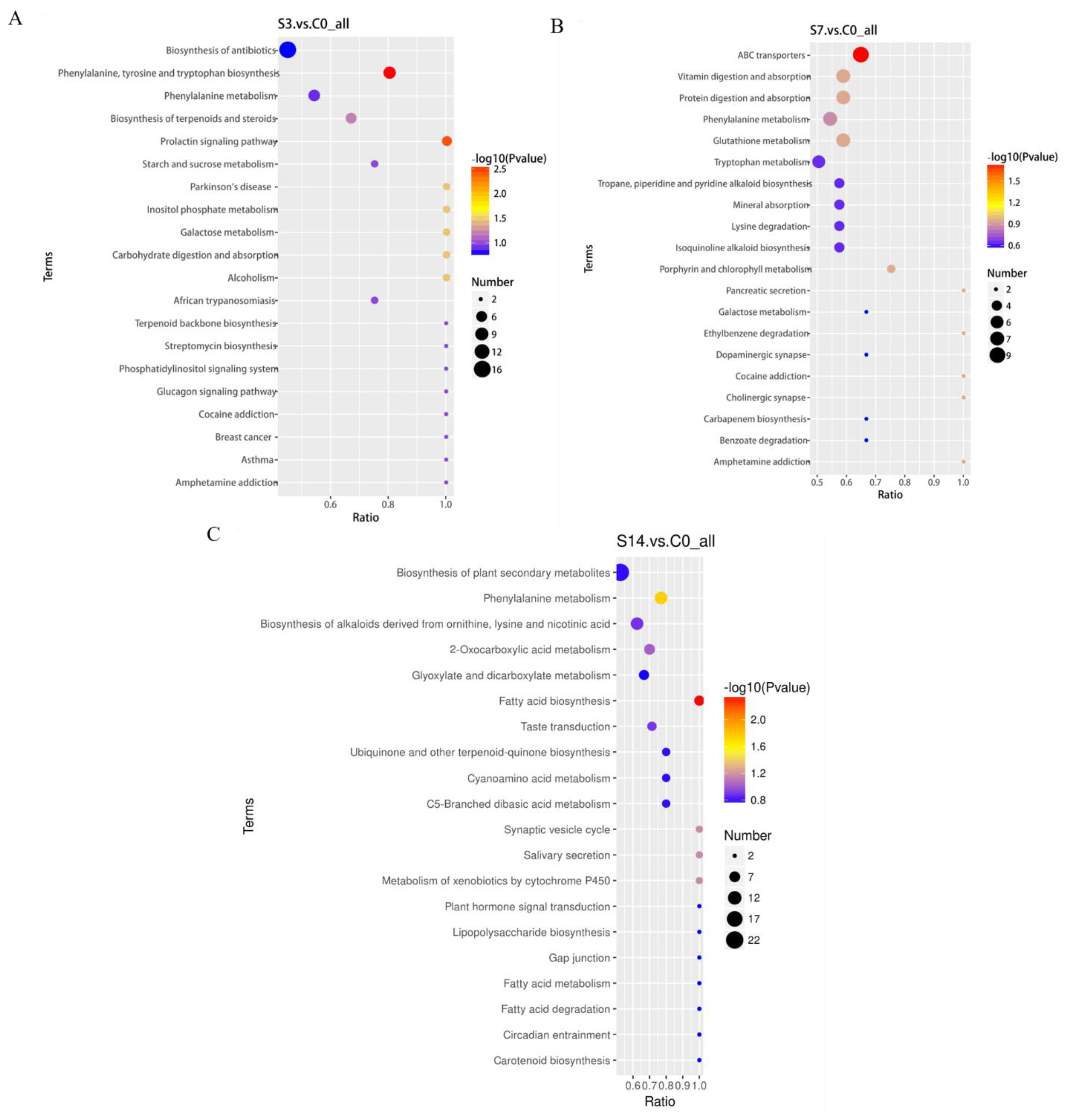
3.3. Identification and Enrichment Analysis of DEMs
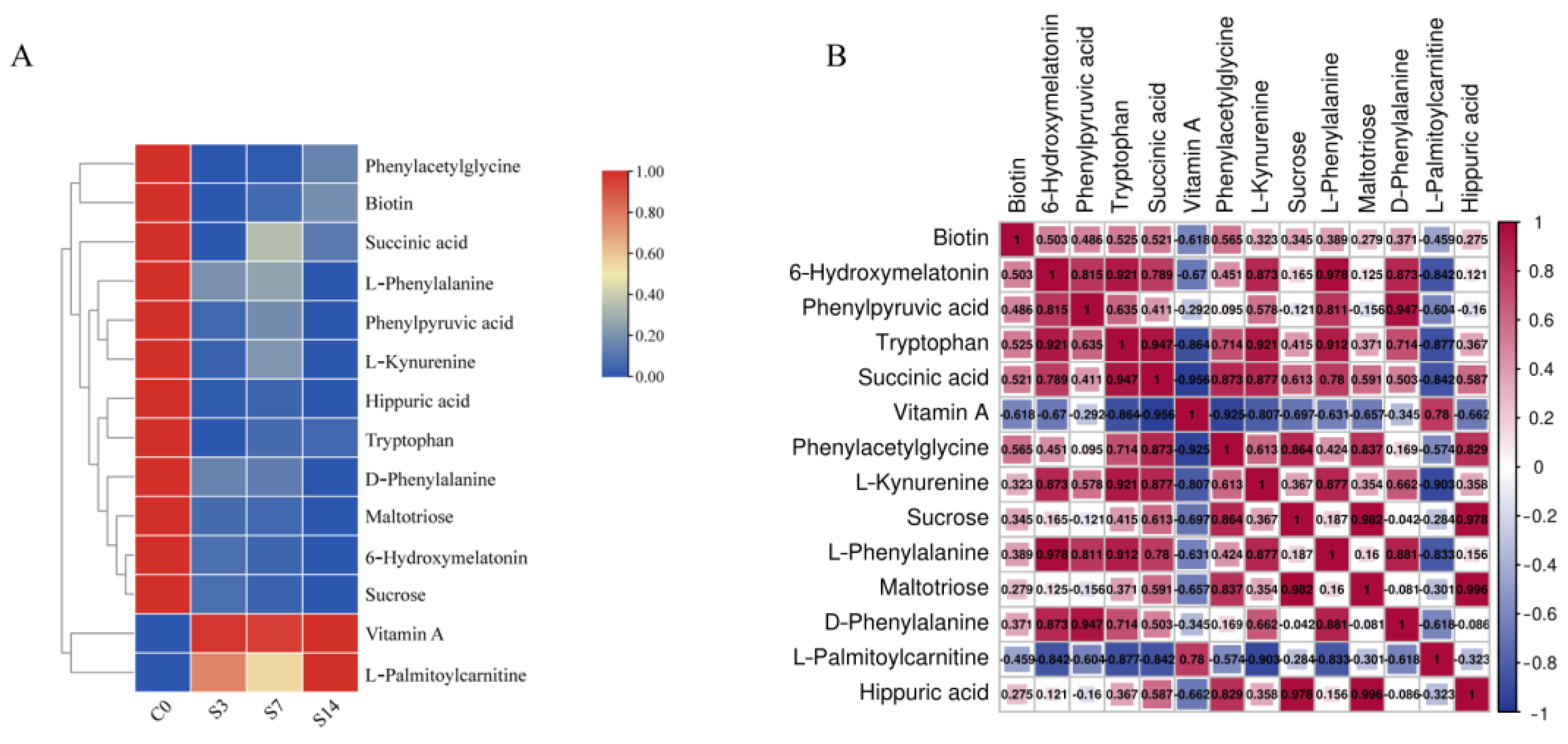
3.4. Identification and Analysis of 14 Key DEMs
4. Discussions
4.1. Low-Salinity Stress Induced Micromorphic and Metabolic Changes in Intestine
4.2. Effects of Low-Salinity Stress on Fatty Acid and Retinol Metabolism
4.3. Effects of Low-Salinity Stress on Phenylalanine, Tyrosine, and Tryptophan Biosynthesis
4.4. Effects of Low-Salinity Stress on ABC Transporters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, J.; Wang, Q.; He, Y.; Liu, P. Aflp-Based Genetic Linkage Map of Marine Shrimp Penaeus (Fenneropenaeus chinensis). Aquaculture 2006, 261, 463–472. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Luan, S.; Luo, K.; Cao, B.; Chen, B.; Kong, J. Isolation and Characterization of a Raf Gene from Chinese Shrimp Fenneropenaeus chinensis in Response to White Spot Syndrome Virus Infection. Fish Shellfish. Immunol. 2018, 83, 341–347. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Zhang, L.; Wang, Z.; Shen, J. New Insight on Vitality Differences for the Penaeid Shrimp, Fenneropenaeus chinensis, in Low Salinity Environment Through Transcriptomics. Front. Ecol. Evol. 2022, 10, 716018. [Google Scholar] [CrossRef]
- Gao, W.; Tian, L.; Huang, T.; Yao, M.; Hu, W.; Xu, Q. Effect of Salinity on the Growth Performance, Osmolarity and Metabolism-Related Gene Expression in White Shrimp Litopenaeus vannamei. Aquac. Rep. 2016, 4, 125–129. [Google Scholar] [CrossRef]
- Pan, L.Q.; Luan, Z.H.; Jin, C.X. Effects of Na+/K+ and Mg2+/Ca2+ Ratios in Saline Groundwaters On Na+–K+-Atpase Activity, Survival and Growth of Marsupenaeus Japonicus Postlarvae. Aquaculture 2006, 261, 1396–1402. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wei, J.; Hong, K.; Zhou, Q.; Liu, X.; Hong, X.; Li, W.; Liu, C.; Zhu, X.; et al. Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii. Antioxidants 2023, 12, 1836. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Ding, L.-G.; Huang, Z.-Y.; Xu, H.-Y.; Xu, Z. Commensal Bacteria-Immunity Crosstalk Shapes Mucosal Homeostasis in Teleost Fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Li, Y.; Wei, L.; Cao, J.; Qiu, L.; Jiang, X.; Li, P.; Song, Q.; Zhou, H.; Han, Q.; Diao, X. Oxidative Stress, Dna Damage and Antioxidant Enzyme Activities in the Pacific White Shrimp (Litopenaeus vannamei) When Exposed to Hypoxia and Reoxygenation. Chemosphere 2016, 144, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Ragunathan, M. Vicissitudes of Oxidative Stress Biomarkers in the Estuarine Crab Scylla Serrata with Reference to Dry and Wet Weather Conditions in Ennore Estuary, Tamil Nadu, India. Mar. Pollut. Bull. 2017, 116, 113–120. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.-X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative Physiological, Metabolomic, and Transcriptomic Analyses Reveal Mechanisms of Improved Abiotic Stress resistance in Bermudagrass [Cynodon dactylon (L). Pers.] by Exogenous Melatonin. J. Exp. Bot. 2014, 66, 681–694. [Google Scholar] [CrossRef]
- Fernández-García, J.; Altea-Manzano, P.; Pranzini, E.; Fendt, S.-M. Stable Isotopes for Tracing Mammalian-Cell Metabolism In Vivo. Trends Biochem. Sci. 2020, 45, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, F.; Heinken, A.; Thiele, I.; Lindenburg, P.W.; Harms, A.C.; Hankemeier, T. The Contribution of Gut Bacterial Metabolites in the Human Immune Signaling Pathway of Non-Communicable Diseases. Gut Microbes 2021, 13, 1882927. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The Intestinal Metabolome: An Intersection Between Microbiota and Host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef]
- Tian, C.; Wang, Q.; Wang, J.; Li, J.; Guan, C.; He, Y.; Gao, H. Integrated Analysis of the Intestinal Microbiota and Transcriptome of Fenneropenaeus chinensis Response to Low-Salinity Stress. Biology 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-B.; Peng, L.-N.; Yan, X.-H. Multi-Omics Responses of Red Algae Pyropia haitanensis to Intertidal Desiccation during Low Tides. Algal Res. 2021, 58, 102376. [Google Scholar] [CrossRef]
- Wang, T.; Long, X.; Chen, X.; Liu, Y.; Liu, Z.; Han, S.; Yan, S. Integrated Transcriptome, Proteome and Physiology Analysis of Epinephelus coioides After Exposure to Copper Nanoparticles Or Copper Sulfate. Nanotoxicology 2017, 11, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J. Effects of Saline and Alkaline Stress on Growth, Reproduction and Immune Function of Fenneropenaeus chinensis. Master’s Thesis, Dalian Ocean University, Dalian, China. 2023.
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics Reveals Significant Variations in Metabolites and Correlations Regarding the Maturation of Walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Heischmann, S.; Quinn, K.; Cruickshank-Quinn, C.; Liang, L.-P.; Reisdorph, R.; Reisdorph, N.; Patel, M. Exploratory Metabolomics Profiling in the Kainic Acid Rat Model Reveals Depletion of 25-Hydroxyvitamin D3 during Epileptogenesis. Sci. Rep. 2016, 6, 31424. [Google Scholar] [CrossRef]
- Boulesteix, A.-L.; Strimmer, K. Partial Least Squares: A Versatile Tool for the Analysis of High-Dimensional Genomic Data. Brief. Bioinform. 2006, 8, 32–44. [Google Scholar] [CrossRef]
- Wang, J.-B.; Pu, S.-B.; Sun, Y.; Li, Z.-F.; Niu, M.; Yan, X.-Z.; Zhao, Y.-L.; Wang, L.-F.; Qin, X.-M.; Ma, Z.-J.; et al. Metabolomic Profiling of Autoimmune Hepatitis: The Diagnostic Utility of Nuclear Magnetic Resonance Spectroscopy. J. Proteome Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. Metax: A Flexible and Comprehensive Software for Processing Metabolomics Data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.A.; Davis, D.A.; Saoud, I.P.; Boyd, C.A.; Pine, H.J.; Boyd, C.E. Shrimp Culture in Inland Low Salinity Waters. Rev. Aquac. 2010, 2, 191–208. [Google Scholar] [CrossRef]
- Andrews, N. Intestinal Iron Absorption: Current Concepts Circa 2000. Dig. Liver Dis. 2000, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, Y.; Liu, Q.; Zhang, J.; Xiong, D. Changes in the Intestine Barrier Function of Litopenaeus vannamei in Response to pH Stress. Fish Shellfish. Immunol. 2019, 88, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, P.; Wen, H.; Xue, M.; Wang, Q.; He, J.; He, C.; Su, S.; Li, J.; Yu, F.; et al. Hypothermia-Mediated Oxidative Stress Induces Immunosuppression, Morphological Impairment and Cell Fate Disorder in the Intestine of Freshwater Drum, Aplodinotus grunniens. Aquaculture 2023, 575, 739805. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Li, J.; Lv, W.; Zou, J.; Fan, L. A New Insight into the Intestine of Pacific White Shrimp: Regulation of Intestinal Homeostasis and Regeneration in Litopenaeus vannamei during Temperature Fluctuation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 35, 100687. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dong, H.; Wang, Y.; Li, H.; Liu, Q.; Zhang, Y.; Zhang, J. Intestine oxidative stress and immune response to sulfide stress in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2017, 63, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Rath, E.; Haller, D. Intestinal Epithelial Cell Metabolism at the Interface of Microbial Dysbiosis and Tissue Injury. Mucosal Immunol. 2022, 15, 595–604. [Google Scholar] [CrossRef]
- Tang, D.; Wu, Y.; Huang, S.; Wu, L.; Luo, Y.; Wang, Z. Transcriptome Reveals the Mechanism of Immunity in the Low Salinity Stress of the Chinese Shrimp (Fenneropenaeus chinensis). Thalass. Int. J. Mar. Sci. 2022, 38, 977–987. [Google Scholar] [CrossRef]
- Lu, H.; Chen, W.; Peng, K.; Huang, M.; Zhao, J.; Chen, X.; Sun, Y.; Ruan, Z.; Li, C.; Liu, D.; et al. Rapid Adaptive and Acute Stimulatory Responses to Low Salinity Stress in Pacific White Shrimp (Litopenaeus vannamei): Insights from Integrative Transcriptomic and Proteomic Analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101149. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.; Bing, H.; Li, L.; Sun, P.; Li, T.; Du, D.; Zhao, J.; Wang, X.; Xiang, W. Integrated Physiological, Transcriptomic, and Metabolomic Analysis Reveals the Mechanism of Guvermectin Promoting Seed Germination in Direct-Seeded Rice under Chilling Stress. J. Agric. Food Chem. 2023, 71, 7348–7358. [Google Scholar] [CrossRef]
- Meng, X.; Jayasundara, N.; Zhang, J.; Ren, X.; Gao, B.; Li, J.; Liu, P. Integrated physiological, transcriptome and metabolome analyses of the hepatopancreas of the female swimming crab Portunus trituberculatus under ammonia exposure. Ecotoxicol. Environ. Saf. 2021, 228, 113026. [Google Scholar] [CrossRef]
- Li, E.; Wang, X.; Chen, K.; Xu, C.; Qin, J.G.; Chen, L. Physiological Change and Nutritional Requirement of Pacific White Shrimp Litopenaeus vannamei at Low Salinity. Rev. Aquac. 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Sato, T.; Yamamoto, H.; Sawada, N.; Nashiki, K.; Tsuji, M.; Muto, K.; Kume, H.; Sasaki, H.; Arai, H.; Nikawa, T.; et al. Restraint Stress Alters the Duodenal Expression of Genes Important for Lipid Metabolism in Rat. Toxicology 2006, 227, 248–261. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Liu, Y.; Wang, X.; Qin, J.G.; Chen, L. Comparative Proteome Analysis of the Hepatopancreas from the Pacific White Shrimp Litopenaeus vannamei under Long-Term Low Salinity Stress. J. Proteom. 2017, 162, 1–10. [Google Scholar] [CrossRef]
- Nałęcz, K.A.; Szczepankowska, D.; Czeredys, M.; Kulikova, N.; Grześkiewicz, S. Palmitoylcarnitine Regulates Estrification of Lipids and Promotes Palmitoylation of Gap-43. FEBS Lett. 2007, 581, 3950–3954. [Google Scholar] [CrossRef]
- Chai, Z.; Lyu, Y.; Chen, Q.; Wei, C.-H.; Snyder, L.M.; Weaver, V.; Sebastian, A.; Albert, I.; Li, Q.; Cantorna, M.T.; et al. RNAseq Studies Reveal Distinct Transcriptional Response to Vitamin A Deficiency in Small Intestine Versus Colon, Uncovering Novel Vitamin A-Regulated Genes. J. Nutr. Biochem. 2021, 98, 108814. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Sato, Y.; Obeng, K.A.; Suzuki, D.; Komiya, Y.; Adachi, S.-I.; Yoshizawa, F.; Sato, Y. Energy Metabolism Profile of the Effects of Amino Acid Treatment On Skeletal Muscle Cells: Leucine Inhibits Glycolysis of Myotubes. Nutrition 2020, 77, 110794. [Google Scholar] [CrossRef]
- Suzuki, A.; Iwata, J. Amino Acid Metabolism and Autophagy in Skeletal Development and Homeostasis. Bone 2021, 146, 115881. [Google Scholar] [CrossRef]
- Jarvis, P.L.; Ballantyne, J.S. Metabolic Responses to Salinity Acclimation in Juvenile Shortnose Sturgeon Acipenser Brevirostrum. Aquaculture 2003, 219, 891–909. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Wang, Q.; Li, L.; Ji, C.; Liu, X.; Zhao, J.; Yin, X. A Metabolomic Investigation On Arsenic-Induced Toxicological Effects in the Clam Ruditapes philippinarum under Different Salinities. Ecotoxicol. Environ. Saf. 2013, 90, 1–6. [Google Scholar] [CrossRef]
- Dai, X.; Liu, M.; Xu, S.; Zhao, H.; Li, X.; Bai, Y.; Zou, Y.; An, Y.; Fan, F.; Zhang, J.; et al. Metabolomics Profile of Plasma in Acute Diquat-Poisoned Patients Using Gas Chromatography-Mass Spectrometry. Food Chem. Toxicol. 2023, 176, 113765. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, J.; Teng, J.; Ren, J.; Shan, E.; Zhu, X.; Zhang, W.; Wang, L.; Hou, C.; Wang, Q. Combined Effects of Salinity and Polystyrene Microplastics Exposure On the Pacific Oysters Crassostrea gigas: Oxidative Stress and Energy Metabolism. Mar. Pollut. Bull. 2023, 193, 115153. [Google Scholar] [CrossRef]
- Mani, K.; Vitenberg, T.; Ben-Mordechai, L.; Schweitzer, R.; Opatovsky, I. Comparative Untargeted Metabolic Analysis of Natural- And Laboratory-Reared Larvae of Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2023, 266, 110851. [Google Scholar] [CrossRef]
- Rocchetti, G.; Bhumireddy, S.R.; Giuberti, G.; Mandal, R.; Lucini, L.; Wishart, D.S. Edible Nuts Deliver Polyphenols and their Transformation Products to the Large Intestine: An in Vitro Fermentation Model Combining Targeted/Untargeted Metabolomics. Food Res. Int. 2019, 116, 786–794. [Google Scholar] [CrossRef]
- Valerio, F.; Di Biase, M.; Lattanzio, V.M.; Lavermicocca, P. Improvement of the Antifungal Activity of Lactic Acid Bacteria by Addition to the Growth Medium of Phenylpyruvic Acid, a Precursor of Phenyllactic Acid. Int. J. Food Microbiol. 2016, 222, 1–7. [Google Scholar] [CrossRef]
- Duan, Y.F.; Wang, Y.; Zhang, J.S.; Sun, X.X.Y.; Wang, J. Dietary Effects of Succinic Acid On the Growth, Digestive Enzymes, Immune Response and Resistance to Ammonia Stress of Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 78, 10–17. [Google Scholar] [CrossRef]
- Cheng, S.; Zhu, Z.; Li, H.; Wang, W.; Jiang, Z.; Pan, F.; Liu, D.; Ho, R.C.; Ho, C.S. Rifaximin Ameliorates Depression-Like Behaviour in Chronic Unpredictable Mild Stress Rats by Regulating Intestinal Microbiota and Hippocampal Tryptophan Metabolism. J. Affect. Disord. 2023, 329, 30–41. [Google Scholar] [CrossRef]
- Liu, Y.; Pei, Z.; Pan, T.; Wang, H.; Chen, W.; Lu, W. Indole Metabolites and Colorectal Cancer: Gut Microbial Tryptophan Metabolism, Host Gut Microbiome Biomarkers, and Potential Intervention Mechanisms. Microbiol. Res. 2023, 272, 127392. [Google Scholar] [CrossRef]
- Parolisi, S.; Montanari, C.; Borghi, E.; Cazzorla, C.; Zuvadelli, J.; Tosi, M.; Barone, R.; Bensi, G.; Bonfanti, C.; Vici, C.D.; et al. Possible Role of Tryptophan Metabolism Along the Microbiota-Gut-Brain Axis On Cognitive & Behavioral Aspects in Phenylketonuria. Pharmacol. Res. 2023, 197, 106952. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan Metabolism in Health and Disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Sakano, K.; Oikawa, S.; Hiraku, Y.; Kawanishi, S. Oxidative Dna Damage Induced by a Melatonin Metabolite, 6-Hydroxymelatonin, Via a Unique Non-O-Quinone Type of Redox Cycle. Biochem. Pharmacol. 2004, 68, 1869–1878. [Google Scholar] [CrossRef]
- Ma, X.; Zou, Y.; Tang, Y.; Wang, D.; Zhou, W.; Yu, S.; Qiu, L. High-throughput analysis of total homocysteine and methylmalonic acid with the efficiency to separate succinic acid in serum and urine via liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2022, 1193, 123135. [Google Scholar] [CrossRef]
- Maharaj, D.S.; Walker, R.B.; Glass, B.D.; Daya, S. 6-Hydroxymelatonin protects against cyanide induced oxidative stress in rat brain homogenates. J. Chem. Neuroanat. 2003, 26, 103–107. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of Photosynthesis, Nitrogen and Proline Metabolism to Salinity Stress in Solanum lycopersicum under Different Levels of Nitrogen Supplementation. Plant Physiol. Biochem. 2016, 109, 72–83. [Google Scholar] [CrossRef]
- Tseng, Y.L.; Hwang, P. Some Insights Into Energy Metabolism for Osmoregulation in Fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 419–429. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.; Li, T.; Xu, C.; Wang, X.; Lin, H.; Qin, J.G.; Chen, L. Transcriptome and Molecular Pathway Analysis of the Hepatopancreas in the Pacific White Shrimp Litopenaeus vannamei under Chronic Low-Salinity Stress. PLoS ONE 2015, 10, e0131503. [Google Scholar] [CrossRef]
- Moore, J.M.; Bell, E.L.; Hughes, R.O.; Garfield, A.S. ABC Transporters: Human Disease and Pharmacotherapeutic Potential. Trends Mol. Med. 2023, 29, 152–172. [Google Scholar] [CrossRef]
- Moons, A. Ospdr9, Which Encodes a Pdr-Type Abc Transporter, is Induced by Heavy Metals, Hypoxic Stress and Redox Perturbations in Rice Roots11the Nucleotide Sequence Reported in this Paper Has Been Submitted to the Embl, Genbank and Ddbj Nucleotide Sequence Databases with the Accession Number Ay271618. FEBS Lett. 2003, 553, 370–376. [Google Scholar] [PubMed]
- Nguyen, V.N.T.; Moon, S.; Jung, K.-H. Genome-Wide Expression Analysis of Rice Abc Transporter Family Across Spatio-Temporal Samples and in Response to Abiotic Stresses. J. Plant Physiol. 2014, 171, 1276–1288. [Google Scholar] [CrossRef]
- Yazaki, K. Abc Transporters Involved in the Transport of Plant Secondary Metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef]
- Messer, M.; Kerry, K.R. Intestinal Digestion of Maltotriose in Man. Biochim. Biophys. Acta Enzymol. 1967, 132, 432–443. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Biotin Functionalized Pegylated Poly(Amidoamine) Dendrimer Conjugate for Active Targeting of Paclitaxel in Cancer. Int. J. Pharm. 2019, 557, 329–341. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Li, C.; Zhang, K.; Gao, X.; Shen, J.; Wang, Y.; Cheng, D.; Lv, J.; Sun, J. Based On 1H Nmr and Lc-Ms Metabolomics Reveals Biomarkers with Neuroprotective Effects in Multi-Parts Ginseng Powder. Arab. J. Chem. 2023, 16, 104840. [Google Scholar] [CrossRef]

| Mode | Name | Up/Down | Formula | RT [min] | m/z | p Value in S3, S7, S14 | ||
|---|---|---|---|---|---|---|---|---|
| Pos | Hippuric acid (HA) | Down | C9H9NO3 | 5.131 | 180.0657 | 0.008725318 | 0.016019771 | 0.005866128 |
| Phenylpyruvic acid (PPA) | Down | C9H8O3 | 5.035 | 165.0547 | 2.66021 × 10−5 | 0.00059833 | 1.03938 × 10−5 | |
| L-Phenylalanine (L-Phe) | Down | C9H11NO2 | 5.012 | 166.0865 | 0.002437022 | 0.005238281 | 8.70015 × 10−5 | |
| D-Phenylalanine (D-Phe) | Down | C9H11NO2 | 10.229 | 166.0868 | 0.00534596 | 0.003718651 | 0.000552025 | |
| 6-Hydroxymelatonin (6-OHM) | Down | C13H16N2O3 | 5.442 | 249.1245 | 1.4535 × 10−6 | 3.12169 × 10−7 | 1.54119 × 10−7 | |
| L-Kynurenine (L-Kyn) | Down | C10H12N2O3 | 5.272 | 209.0925 | 0.001332862 | 0.010433043 | 0.0011305 | |
| Vitamin A | Up | C20H30O | 9.36 | 287.2369 | 0.000773157 | 0.001374898 | 0.004364512 | |
| L-Palmitoylcarnitine (L-Pcar) | Up | C23H45NO4 | 10.234 | 400.3429 | 0.007961563 | 0.010861824 | 0.001583441 | |
| Neg | Succinic acid (SA) | Down | C4H6O4 | 2.469 | 117.0182 | 0.000417082 | 0.023997658 | 0.002328271 |
| Phenylacetylglycine (PAG) | Down | C10H11NO3 | 5.606 | 192.066 | 0.000971555 | 0.0008622 | 0.005389868 | |
| Tryptophan (Trp) | Down | C11H12N2O2 | 5.219 | 203.0823 | 0.000142969 | 0.000869403 | 0.000486337 | |
| Maltotriose (Mal) | Down | C18H32O16 | 1.385 | 539.1381 | 0.004708651 | 0.007046087 | 0.001962056 | |
| Sucrose (SUC) | Down | C12H22O11 | 1.364 | 341.1101 | 0.002328382 | 0.001675135 | 0.000357143 | |
| Biotin | Down | C10H16N2O3S | 5.394 | 243.0809 | 1.13455 × 10−6 | 3.62306 × 10−5 | 5.47528 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Wang, Q.; Gao, T.; Sun, H.; Li, J.; He, Y. Effects of Low-Salinity Stress on Histology and Metabolomics in the Intestine of Fenneropenaeus chinensis. Animals 2024, 14, 1880. https://doi.org/10.3390/ani14131880
Tian C, Wang Q, Gao T, Sun H, Li J, He Y. Effects of Low-Salinity Stress on Histology and Metabolomics in the Intestine of Fenneropenaeus chinensis. Animals. 2024; 14(13):1880. https://doi.org/10.3390/ani14131880
Chicago/Turabian StyleTian, Caijuan, Qiong Wang, Tian Gao, Huarui Sun, Jitao Li, and Yuying He. 2024. "Effects of Low-Salinity Stress on Histology and Metabolomics in the Intestine of Fenneropenaeus chinensis" Animals 14, no. 13: 1880. https://doi.org/10.3390/ani14131880







