Genomic Selection for Weaning Weight in Alpine Merino Sheep Based on GWAS Prior Marker Information
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GWAS Model Analysis Methods
2.3. GS Model Analysis Method
2.4. Methods for Evaluating Predictive Accuracy of GS
3. Results
3.1. Statistics and Distribution of Phenotypic Data
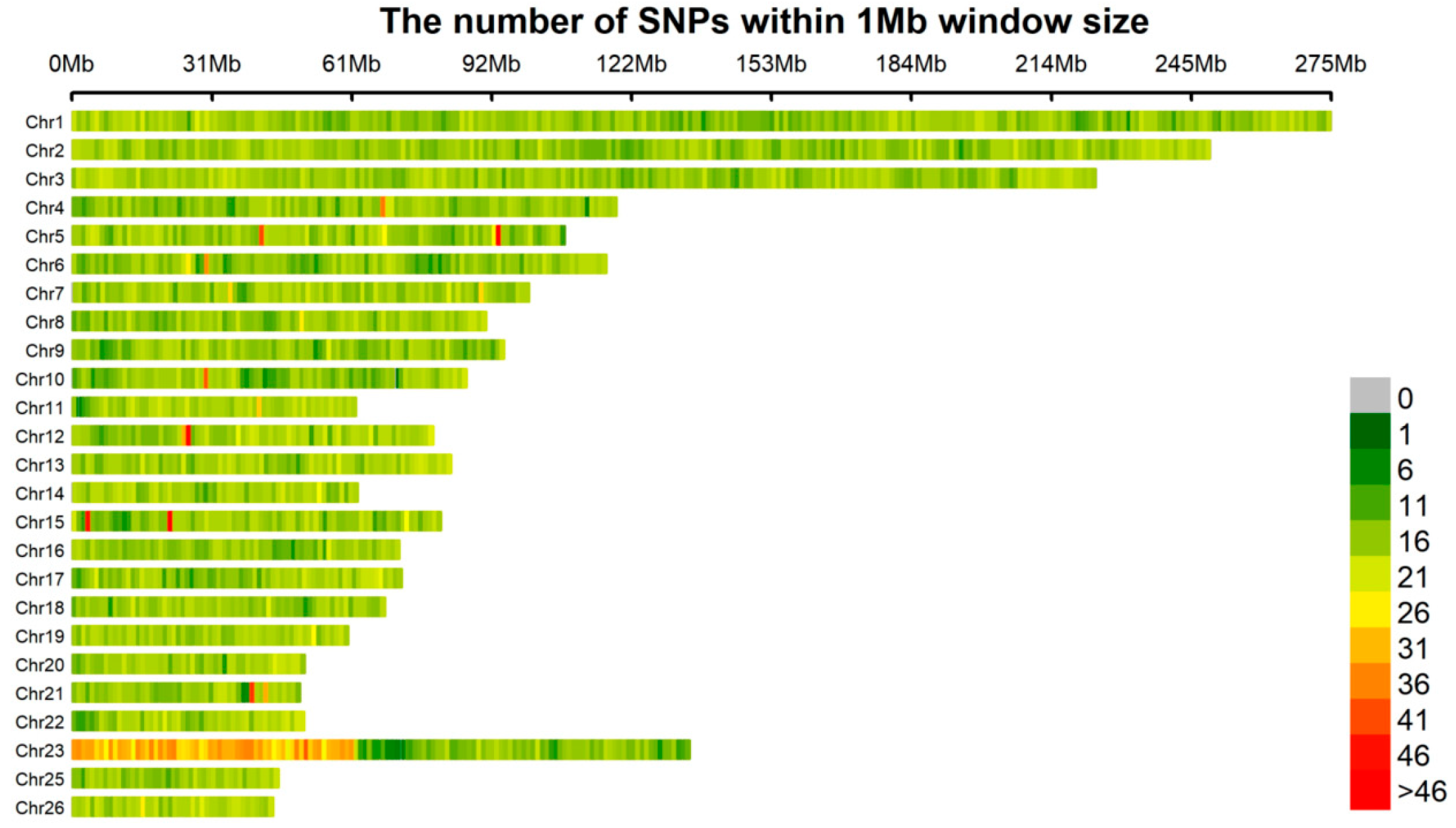
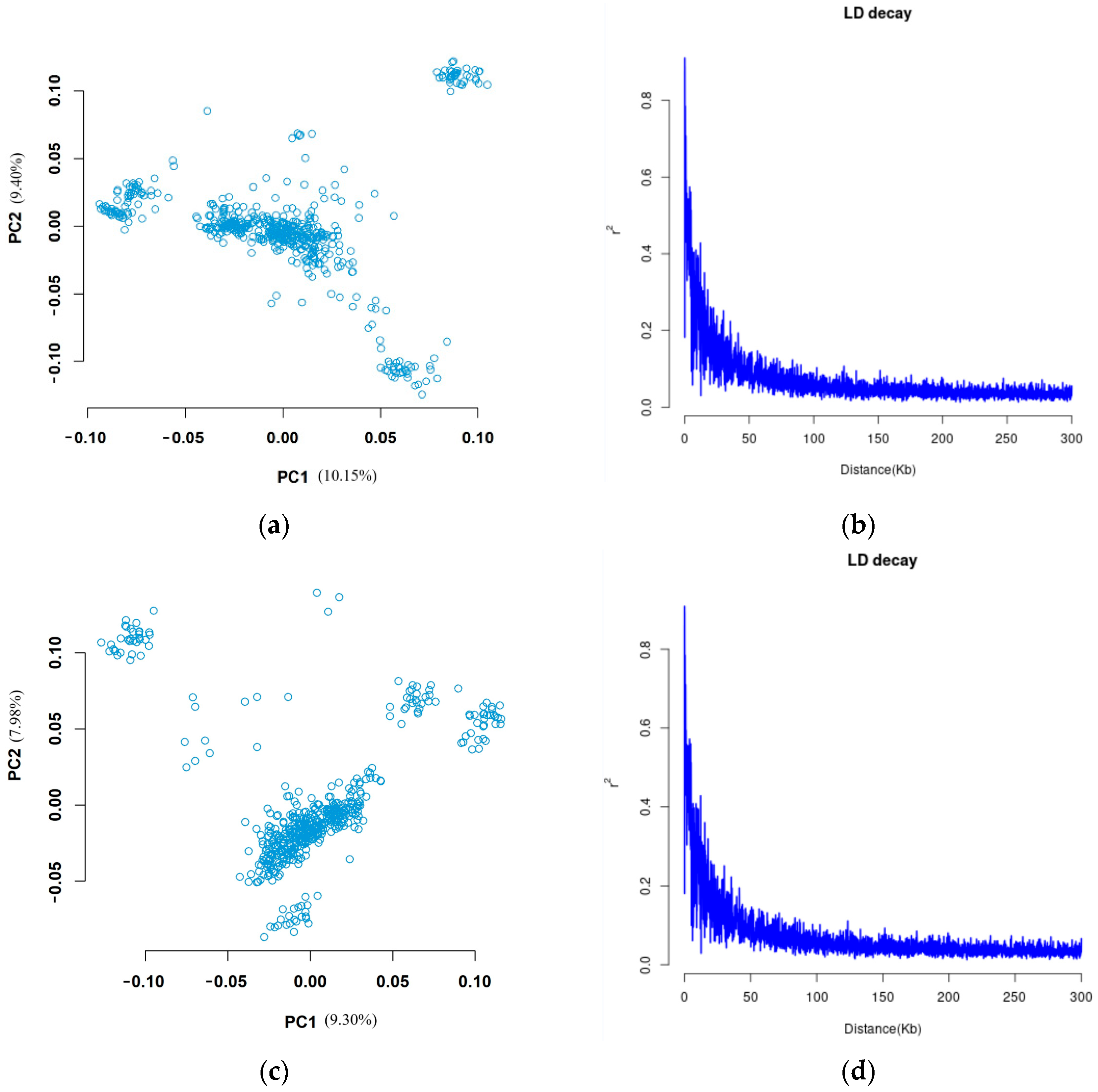
3.2. Genotype Data
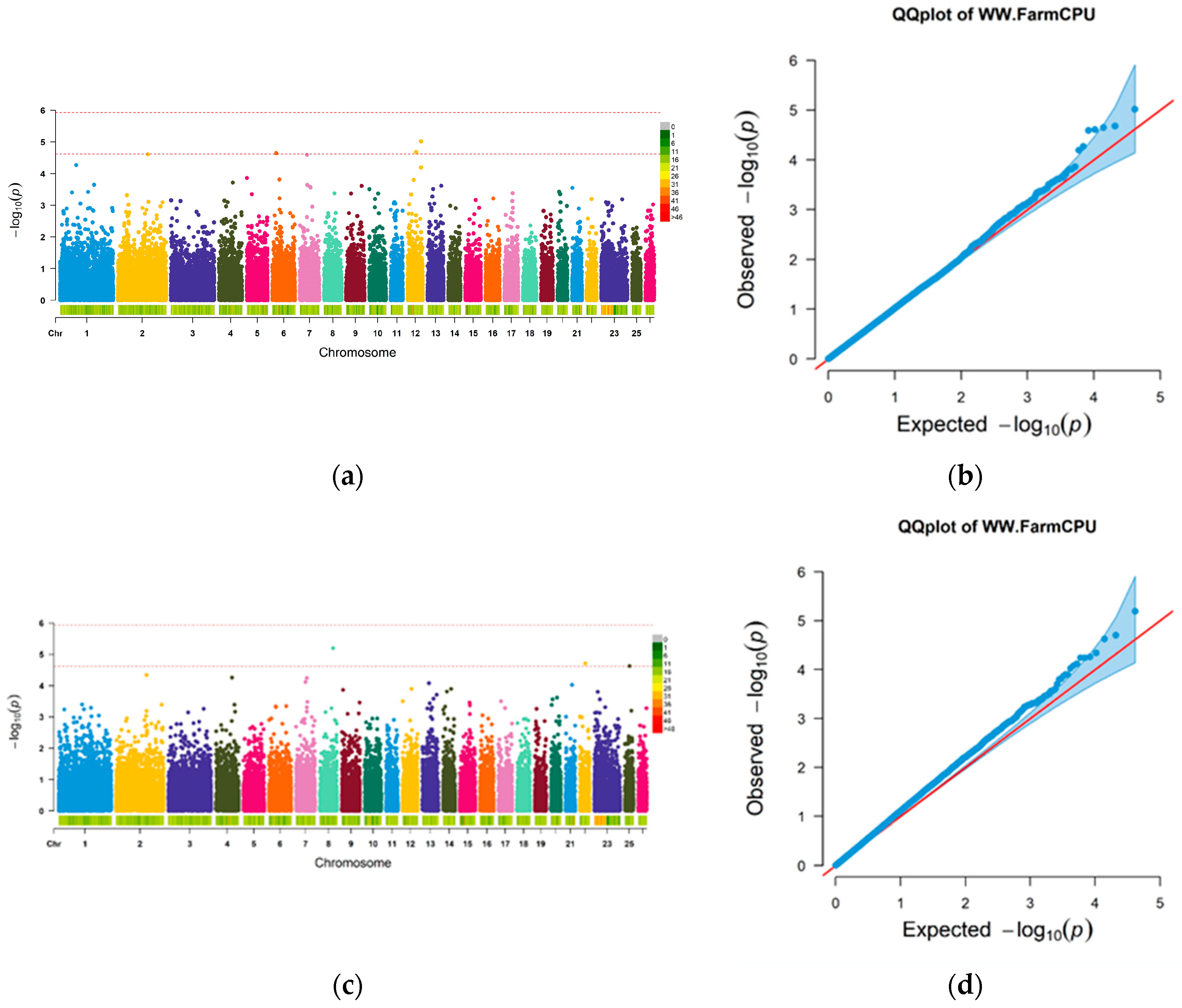
3.3. GWAS Analysis Results
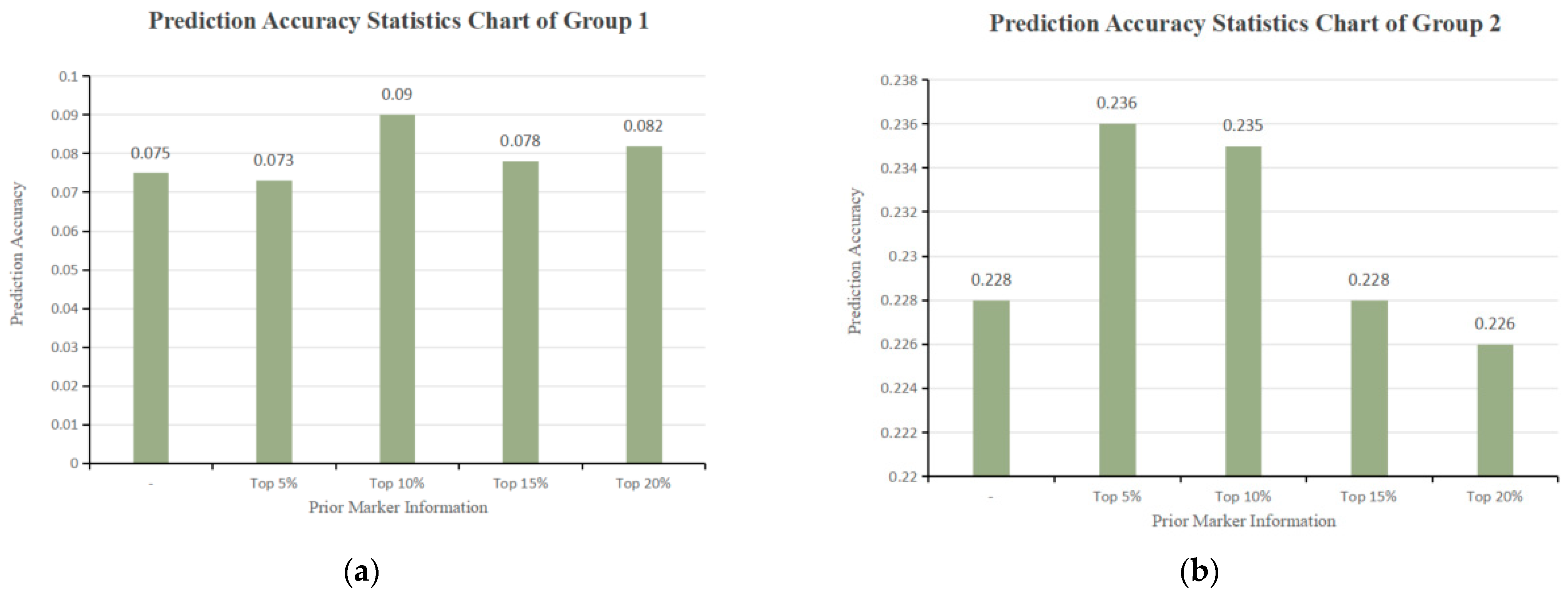
3.4. GS Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebreselassie, G.; Berihulay, H.; Jiang, L.; Ma, Y. Review on genomic regions and candidate genes associated with economically important production and reproduction traits in sheep (Ovies aries). Animals 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.; Hayes, B.; Goddard, M. Genomic selection: A paradigm shift in animal breeding. Anim. Front. 2016, 6, 6–14. [Google Scholar] [CrossRef]
- Heffner, E.L.; Sorrells, M.E.; Jannink, J.L. Genomic selection for crop improvement. Crop Sci. 2009, 49, 1–12. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.J.; Visscher, P.M.; Goddard, M.E. Increased accuracy of artificial selection by using the realized relationship matrix. Genet. Res. 2009, 91, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Habier, D.; Tetens, J.; Seefried, F.-R.; Lichtner, P.; Thaller, G. The impact of genetic relationship information on genomic breeding values in German Holstein cattle. Genet. Sel. Evol. 2010, 42, 5. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, L.R. Strategy for applying genome-wide selection in dairy cattle. J. Anim. Breed. Genet. 2006, 123, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.E.; Hayes, B.J. Genomic selection. J. Anim. Breed. Genet. 2007, 124, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.L.; Ma, Y.L.; Xiang, T.; Zhu, M.J.; Yu, M.; Li, X.Y.; Liu, X.L.; Zhao, S.H. The progress and prospect of genomic selection models. Acta Vet. Zootech. Sin. 2019, 50, 233–242. [Google Scholar]
- Esfandyari, H.; Sørensen, A.C.; Bijma, P. A crossbred reference population can improve the response to genomic selection for crossbred performance. Genet. Sel. Evol. 2015, 47, 76. [Google Scholar] [CrossRef]
- Song, H.; Ye, S.; Jiang, Y.; Zhang, Z.; Zhang, Q.; Ding, X. Using imputation-based whole-genome sequencing data to improve the accuracy of genomic prediction for combined populations in pigs. Genet. Sel. Evol. 2019, 51, 58. [Google Scholar] [CrossRef]
- Lourenco, D.A.; Tsuruta, S.; Fragomeni, B.O.; Masuda, Y.; Aguilar, I.; Legarra, A.; Bertrand, J.K.; Amen, T.S.; Wang, L.; Moser, D.W.; et al. Genetic evaluation using single-step genomic best linear unbiased predictor in American Angus. J. Anim. Sci. 2015, 93, 2653–2662. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Zhang, L. Applications of genome selection in sheep breeding. Yi Chuan 2019, 41, 293–303. [Google Scholar]
- Wolc, A.; Zhao, H.H.; Arango, J.; Settar, P.; Fulton, J.E.; O’sullivan, N.P.; Preisinger, R.; Stricker, C.; Habier, D.; Fernando, R.L. Response and inbreeding from a genomic selection experiment in layer chickens. Genet. Sel. Evol. 2015, 47, 59. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.E.; Kemper, K.E.; MacLeod, I.M.; Chamberlain, A.J.; Hayes, B.J. Genetics of complex traits: Prediction of phenotype, identification of causal polymorphisms and genetic architecture. Proc. Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, J.; Goddard, M.E.; Wray, N.R.; Visscher, P.M. Concepts, estimation and interpretation of SNP-based heritability. Nat. Genet. 2017, 49, 1304–1310. [Google Scholar] [CrossRef]
- Lopes, M.S.; Bovenhuis, H.; van Son, M.; Nordbø, Ø.; Grindflek, E.H.; Knol, E.F.; Bastiaansen, J.W. Using markers with large effect in genetic and genomic predictions. J. Anim. Sci. 2017, 95, 59–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Ober, U.; Erbe, M.; Zhang, H.; Gao, N.; He, J.; Li, J.; Simianer, H. Improving the accuracy of whole genome prediction for complex traits using the results of genome wide association studies. PLoS ONE 2014, 9, e93017. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Deniskova, T.E.; Yudin, N.S.; Dotsev, A.V.; Khamiruev, T.N.; Selionova, M.I.; Egorov, S.V.; Reyer, H.; Wimmers, K.; Brem, G.; et al. High-density genotyping reveals signatures of selection related to acclimation and economically important traits in 15 local sheep breeds from Russia. BMC Genom. 2019, 20, 294. [Google Scholar] [CrossRef]
- Ghasemi, M.; Zamani, P.; Vatankhah, M.; Abdoli, R. Genome-wide association study of birth weight in sheep. Animal 2019, 13, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef] [PubMed]
- VanRaden, P.M. Efficient methods to compute genomic predictions. J. Dairy. Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Reif, J.C.; Jiang, Y. On the use of GBLUP and its extension for GWAS with additive and epistatic effects. G3 Genes Genomes Genet. 2021, 11, jkab122. [Google Scholar] [CrossRef] [PubMed]
- Browne, N. Cross-Validation Methods. J. Math. Psychol. 2000, 44, 108–132. [Google Scholar] [CrossRef] [PubMed]
- Mehrban, H.; Lee, D.H.; Moradi, M.H.; IlCho, C.; Naserkheil, M.; Ibáñez-Escriche, N. Predictive performance of genomic selection methods for carcass traits in Hanwoo beef cattle: Impacts of the genetic architecture. Genet. Sel. Evol. 2017, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Gregersen, P.K.; Seldin, M.F. Accounting for ancestry: Population substructure and genome-wide association studies. Hum. Mol. Genet. 2008, 17, R143–R150. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Shi, G. On rare variants in principal component analysis of population stratification. BMC Genet. 2020, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Alemu, A.; El Baouchi, A.; El Hanafi, S.; Kehel, Z.; Eddakhir, K.; Tadesse, W. Genetic analysis of grain protein content and dough quality traits in elite spring bread wheat (Triticum aestivum) lines through association study. J. Cereal Sci. 2021, 100, 103214. [Google Scholar] [CrossRef]
- Kusmec, A.; Schnable, P.S. Farm CPU pp: Efficient large-scale genomewide association studies. Plant Direct. 2018, 2, e00053. [Google Scholar] [CrossRef]
- Ren, D.; An, L.; Li, B.; Qiao, L.; Liu, W. Efficient weighting methods for genomic best linear-unbiased prediction (BLUP) adapted to the genetic architectures of quantitative traits. Heredity 2020, 126, 320–334. [Google Scholar] [CrossRef]
- Edwards, S.M.; Sørensen, I.F.; Sarup, P.; Mackay, T.F.; Sørensen, P. Genomic Prediction for Quantitative Traits Is Improved by Mapping Variants to Gene Ontology Categories in Drosophila melanogaster. Genetics 2016, 203, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, I.; Bowman, P.; Vander Jagt, C.; Haile-Mariam, M.; Kemper, K.; Chamberlain, A.; Schrooten, C.; Hayes, B.J.; Goddard, M. Exploiting biological priors and sequence variants enhances QTL discovery and genomic prediction of complex traits. BMC Genom. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Ding, X.; Bijma, P.; de Koning, D.-J.; Zhang, Q. Best linear unbiased prediction of genomic breeding values using a trait-specific marker-derived relationship matrix. PLoS ONE 2010, 5, e12648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Erbe, M.; He, J.; Ober, U.; Gao, N.; Zhang, H.; Simianer, H.; Li, J. Accuracy of whole-genome prediction using a genetic architecture-enhanced variance-covariance matrix. G3 Genes Genomes Genet. 2015, 5, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Wang, H.; Zhang, R.; An, X.; Yuan, C.; Guo, T.; Yue, Y. Genomic Selection for Live Weight in the 14th Month in Alpine Merino Sheep Combining GWAS Information. Animals 2023, 13, 3516. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sunduimijid, B.; Xiang, R.; Behrendt, R.; Knight, M.I.; Mason, B.A.; Reich, C.M.; Prowse-Wilkins, C.; Vander Jagt, C.J.; Chamberlain, A.J.; et al. Expression quantitative trait loci in sheep liver and muscle contribute to variations in meat traits. Genet. Sel. Evol. 2021, 53, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, C.; Shi, L.; He, Y.; Hu, M.; Sun, H.; Peng, H.; Lai, W.; Jiao, S.; Zhao, Z.; et al. Detection of selection signatures in South African Mutton Merino sheep using whole-genome sequencing data. Anim. Genet. 2022, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Choi, T.-J.; Kim, Y.-S.; Lee, Y.-M.; Zahangir Alam, M.; Jung, J.-H.; Choe, H.-S.; Kim, J.-J. Comparison of genomic predictions for carcass and reproduction traits in Berkshire, Duroc and Yorkshire populations in Korea. Anim. Biosci. 2019, 32, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Bolormaa, S.; Gore, K.; van der Werf, J.H.J.; Hayes, B.J.; Daetwyler, H.D. Design of a low-density SNP chip for the main Australian sheep breeds and its effect on imputation and genomic prediction accuracy. Anim. Genet. 2015, 46, 544–556. [Google Scholar] [CrossRef]
- Naserkheil, M.; Lee, D.H.; Mehrban, H. Improving the accuracy of genomic evaluation for linear body measurement traits using single-step genomic best linear unbiased prediction in Hanwoo beef cattle. BMC Genet. 2020, 21, 144. [Google Scholar] [CrossRef]
- Schrag, T.A.; Westhues, M.; Schipprack, W.; Seifert, F.; Thiemann, A.; Scholten, S.; Melchinger, A.E. Beyond Genomic Prediction: Combining Different Types of omics Data Can Improve Prediction of Hybrid Performance in Maize. Genetics 2018, 208, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Teng, J.; Ye, S.; Yuan, X.; Huang, S.; Zhang, H.; Zhang, X.; Li, J.; Zhang, Z. Genomic Prediction of Complex Phenotypes Using Genic Similarity Based Relatedness Matrix. Front. Genet. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, H.; Zhou, X.; Yuan, X.; Zhao, S.; Li, X.; Liu, X. KAML: Improving genomic prediction accuracy of complex traits using machine learning determined parameters. Genome Biol. 2020, 21, 146. [Google Scholar] [CrossRef]
| Item | Number | Mean | Sd | Median | Trimmed | Med | Min | Max | Se |
|---|---|---|---|---|---|---|---|---|---|
| All | 1007 | 28.27 | 3.19 | 28.00 | 28.21 | 2.97 | 18.00 | 37.60 | 0.10 |
| Group 1 | 503 | 28.12 | 3.18 | 28.00 | 28.07 | 2.97 | 18.00 | 37.60 | 0.14 |
| Group 2 | 504 | 28.42 | 3.19 | 28.20 | 28.34 | 3.26 | 19.00 | 37.00 | 0.14 |
| Prior Marker Information | Matrix | Genetic Variance | Environmental Variance | Heritability | Weight | Prediction Accuracy | Promotion |
|---|---|---|---|---|---|---|---|
| - | G | 1.135 | 8.153 | 0.122 | - | 0.075 | - |
| Top 5% | G1 | 1.022 | 8.275 | 0.110 | 0.476 | - | - |
| G2 | 1.124 | 8.166 | 0.121 | 0.533 | - | - | |
| G3 | 1.161 | 8.121 | 0.125 | - | 0.073 | -2.67% | |
| Top 10% | G1 | 1.369 | 7.930 | 0.147 | 0.558 | - | - |
| G2 | 1.083 | 8.207 | 0.117 | 0.442 | - | - | |
| G3 | 1.319 | 7.962 | 0.142 | - | 0.090 | +20.00% | |
| Top 15% | G1 | 1.085 | 8.207 | 0.117 | 0.492 | - | - |
| G2 | 1.121 | 8.169 | 0.121 | 0.508 | - | - | |
| G3 | 1.143 | 8.136 | 0.123 | - | 0.078 | +4.00% | |
| Top 20% | G1 | 1.282 | 8.009 | 0.138 | 0.546 | - | - |
| G2 | 1.068 | 8.223 | 0.115 | 0.454 | - | - | |
| G3 | 1.228 | 8.050 | 0.132 | - | 0.082 | +9.33% |
| Prior Marker Information | Matrix | Genetic Variance | Environmental Variance | Heritability | Weight | Prediction Accuracy | Promotion |
|---|---|---|---|---|---|---|---|
| - | G | 3.932 | 6.045 | 0.394 | - | 0.228 | - |
| Top 5% | G1 | 2.853 | 7.063 | 0.288 | 0.427 | - | - |
| G2 | 3.849 | 6.131 | 0.386 | 0.573 | - | - | |
| G3 | 3.922 | 6.011 | 0.395 | - | 0.236 | +3.51% | |
| Top 10% | G1 | 3.337 | 6.590 | 0.336 | 0.469 | - | - |
| G2 | 3.789 | 6.187 | 0.380 | 0.531 | - | - | |
| G3 | 4.049 | 5.894 | 0.407 | - | 0.235 | +3.07% | |
| Top 15% | G1 | 3.406 | 6.529 | 0.343 | 0.472 | - | - |
| G2 | 3.824 | 6.156 | 0.383 | 0.528 | - | - | |
| G3 | 3.937 | 6.000 | 0.396 | - | 0.228 | 0 | |
| Top 20% | G1 | 3.430 | 6.506 | 0.345 | 0.472 | - | - |
| G2 | 3.849 | 6.135 | 0.386 | 0.528 | - | - | |
| G3 | 3.899 | 6.036 | 0.392 | - | 0.226 | −0.88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, C.; Li, J.; Zhang, R.; An, X.; Yuan, C.; Guo, T.; Yue, Y. Genomic Selection for Weaning Weight in Alpine Merino Sheep Based on GWAS Prior Marker Information. Animals 2024, 14, 1904. https://doi.org/10.3390/ani14131904
Wang H, Li C, Li J, Zhang R, An X, Yuan C, Guo T, Yue Y. Genomic Selection for Weaning Weight in Alpine Merino Sheep Based on GWAS Prior Marker Information. Animals. 2024; 14(13):1904. https://doi.org/10.3390/ani14131904
Chicago/Turabian StyleWang, Haifeng, Chenglan Li, Jianye Li, Rui Zhang, Xuejiao An, Chao Yuan, Tingting Guo, and Yaojing Yue. 2024. "Genomic Selection for Weaning Weight in Alpine Merino Sheep Based on GWAS Prior Marker Information" Animals 14, no. 13: 1904. https://doi.org/10.3390/ani14131904






