Added Value of Sensor-Based Behavioural Monitoring in an Infectious Disease Study with Sheep Infected with Toxoplasma gondii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
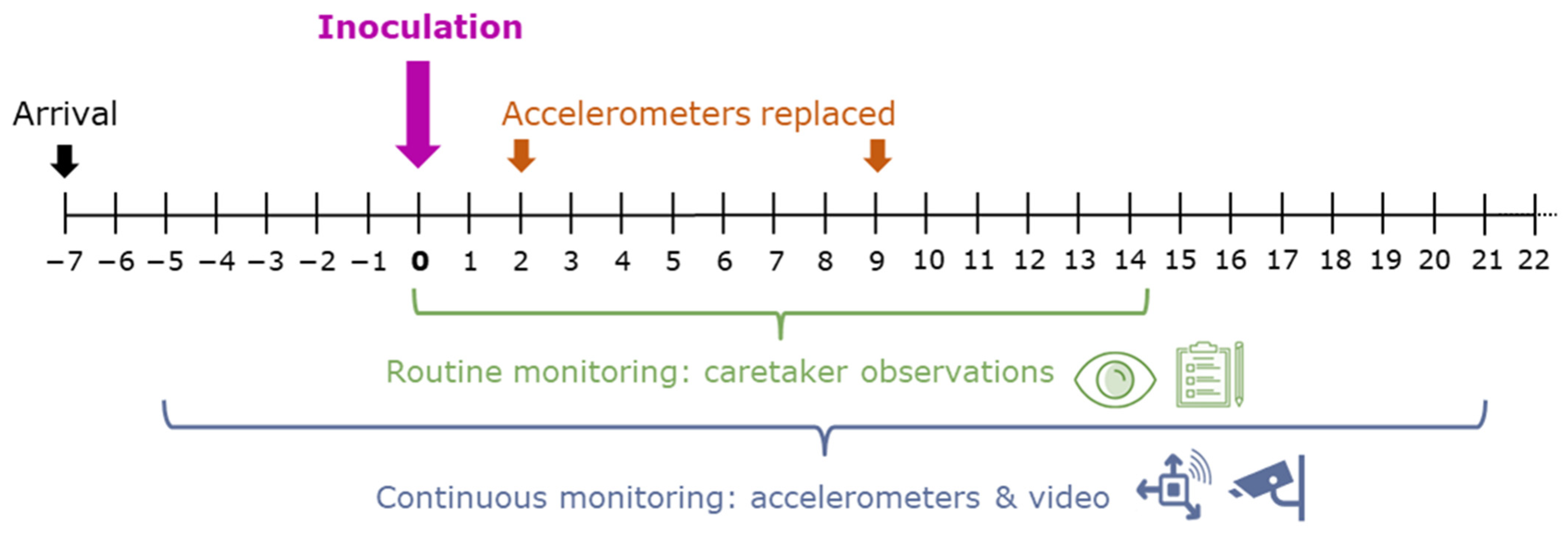
2.1. Animals and Experimental Treatment
2.2. Routine Monitoring: (Bi-)Daily Observations by Animal Caretakers
2.3. Activity Based on Accelerometers
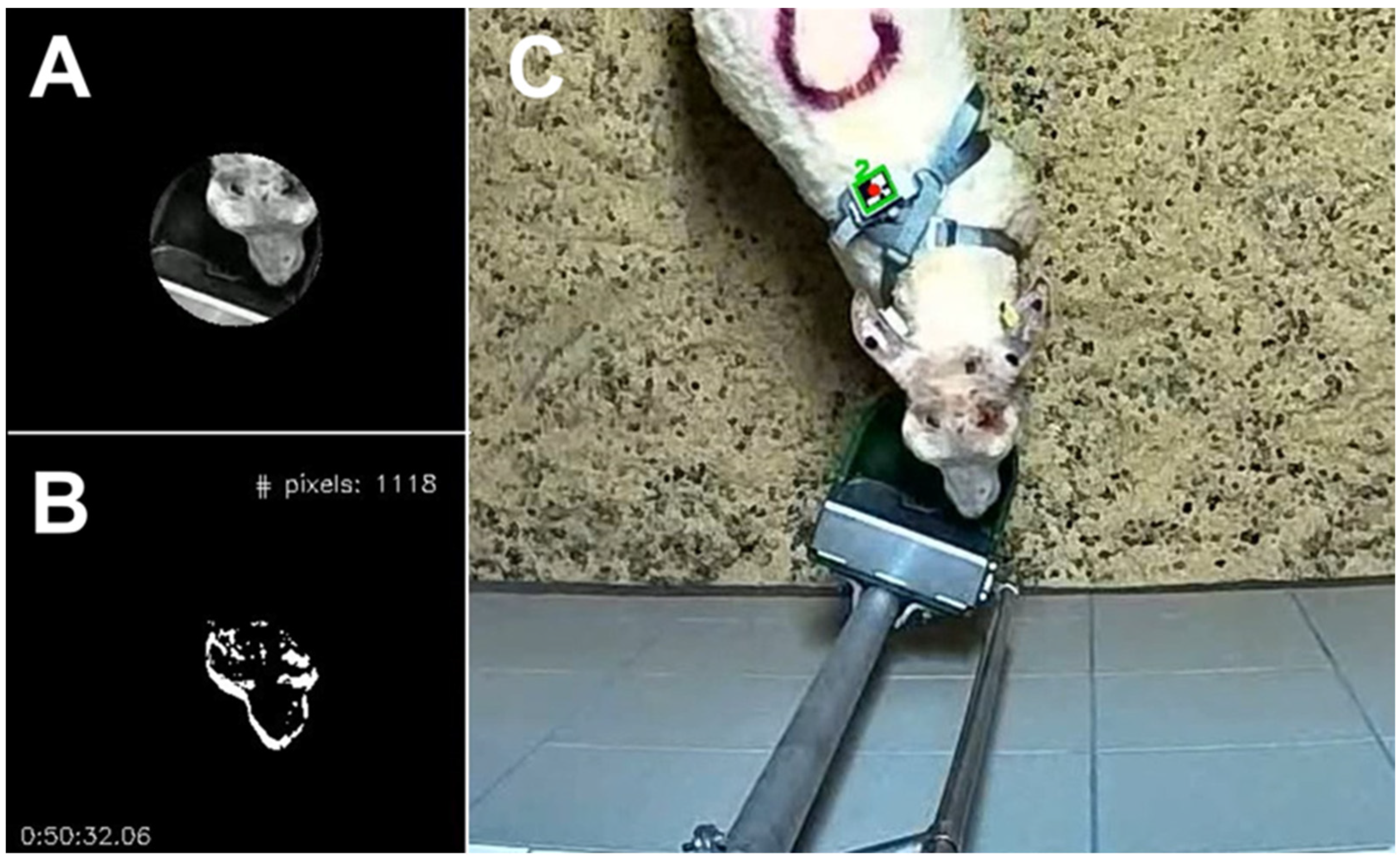
2.4. Time Spent at the Drinker Based on Video
2.5. Statistical Analysis
3. Results
3.1. Routine Monitoring Results
3.2. Activity Based on Accelerometers
3.3. Time Spent at the Drinker Based on Video
4. Discussion
4.1. Effects of T. gondii Infection on the Behaviour of Sheep
4.2. Added Value of Sensor Technologies
4.3. Challenges of Sensor Technologies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mähler, M.; Berar, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar]
- Ryan, M.; Waters, R.; Wolfensohn, S. Assessment of the welfare of experimental cattle and pigs using the Animal Welfare Assessment Grid. Animals 2021, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, C.; Morton, D.; Cussler, K. Use of humane endpoints to minimise suffering. In The COST Manual of Laboratory Animal Care and Use, 1st ed.; Howard, B., Nevalainen, T., Perretta, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 333–353. [Google Scholar]
- Escobar, J.; Van Alstine, W.G.; Baker, D.H.; Johnson, R.W. Behaviour of pigs with viral and bacterial pneumonia. Appl. Anim. Behav. Sci. 2007, 105, 42–50. [Google Scholar] [CrossRef]
- Munsterhjelm, C.; Nordgreen, J.; Aae, F.; Heinonen, M.; Valros, A.; Janczak, A.M. Sick and grumpy: Changes in social behaviour after a controlled immune stimulation in group-housed gilts. Physiol. Behav. 2019, 198, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carrión, E.; Martínez-Avilés, M.; Ivorra, B.; Martínez-López, B.; Ramos, Á.M.; Sánchez-Vizcaíno, J.M. Motion-based video monitoring for early detection of livestock diseases: The case of African swine fever. PLoS ONE 2017, 12, e0183793. [Google Scholar] [CrossRef]
- Morton, D.B. A systematic approach for establishing humane endpoints. ILAR J. 2000, 41, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bokkers, E.; De Vries, M.; Antonissen, I.; De Boer, I. Inter-and intra-observer reliability of experienced and inexperienced observers for the Qualitative Behaviour Assessment in dairy cattle. Anim. Welf. 2012, 21, 307–318. [Google Scholar] [CrossRef]
- Tuyttens, F.; de Graaf, S.; Heerkens, J.L.; Jacobs, L.; Nalon, E.; Ott, S.; Stadig, L.; Van Laer, E.; Ampe, B. Observer bias in animal behaviour research: Can we believe what we score, if we score what we believe? Anim. Behav. 2014, 90, 273–280. [Google Scholar] [CrossRef]
- Iredale, S.K.; Nevill, C.H.; Lutz, C.K. The influence of observer presence on baboon (Papio spp.) and rhesus macaque (Macaca mulatta) behavior. Appl. Anim. Behav. Sci. 2010, 122, 53–57. [Google Scholar] [CrossRef]
- Leruste, H.; Bokkers, E.; Sergent, O.; Wolthuis-Fillerup, M.; Van Reenen, C.; Lensink, B. Effects of the observation method (direct v. from video) and of the presence of an observer on behavioural results in veal calves. Animal 2013, 7, 1858–1864. [Google Scholar] [CrossRef]
- Ellen, E.D.; Van Der Sluis, M.; Siegford, J.; Guzhva, O.; Toscano, M.J.; Bennewitz, J.; van der Zande, L.E.; van der Eijk, J.A.J.; de Haas, E.N.; Norton, T.; et al. Review of sensor technologies in animal breeding: Phenotyping behaviors of laying hens to select against feather pecking. Animals 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.P.; Börger, L.; Holton, M.D.; Scantlebury, D.M.; Gómez-Laich, A.; Quintana, F.; Rosell, F.; Graf, P.M.; Williams, H.; Gunner, R.; et al. Estimates for energy expenditure in free-living animals using acceleration proxies: A reappraisal. J. Anim. Ecol. 2020, 89, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Rushen, J.; Chapinal, N.; de Passile, A. Automated monitoring of behavioural-based animal welfare indicators. Anim. Welf. 2012, 21, 339–350. [Google Scholar] [CrossRef]
- Chapa, J.M.; Maschat, K.; Iwersen, M.; Baumgartner, J.; Drillich, M. Accelerometer systems as tools for health and welfare assessment in cattle and pigs–a review. Behav. Process 2020, 181, 104262. [Google Scholar] [CrossRef]
- Redfern, W.S.; Tse, K.; Grant, C.; Keerie, A.; Simpson, D.J.; Pedersen, J.C.; Rimmer, V.; Leslie, L.; Klein, S.K.; Karp, N.A.; et al. Automated recording of home cage activity and temperature of individual rats housed in social groups: The Rodent Big Brother project. PLoS ONE 2017, 12, e0181068. [Google Scholar] [CrossRef] [PubMed]
- Hobson, L.; Bains, R.S.; Greenaway, S.; Wells, S.; Nolan, P.M. Phenotyping in mice using continuous home cage monitoring and ultrasonic vocalization recordings. Curr. Protoc. Mouse Biol. 2020, 10, e80. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, Y.; Orsini, C.; Bolhuis, J.E.; de Vlieg, J.; Bijma, P.; de With, P.H. Enhanced camera-based individual pig detection and tracking for smart pig farms. Comput. Electron. Agric. 2023, 211, 108009. [Google Scholar] [CrossRef]
- Alarcón-Nieto, G.; Graving, J.M.; Klarevas-Irby, J.A.; Maldonado-Chaparro, A.A.; Mueller, I.; Farine, D.R. An automated barcode tracking system for behavioural studies in birds. Methods Ecol. Evol. 2018, 9, 1536–1547. [Google Scholar] [CrossRef]
- Coello, C.; Nordbø, Ø.; Sagevik, R.; Cheikh, F.; Ullah, M.; Martinsen, K.H.; Grindflek, E. Extracting video-based phenotypes in a pig breeding programme. In Book of Abstracts of the 74th Annual Meeting of the European Federation of Animal Science, Lyon, France, 26 August–1 September 2023; Wageningen Academic Publishers: Wageningen, The Netherlands, 2023; p. 379. [Google Scholar]
- Doornweerd, J.; Kootstra, G.; Veerkamp, R.; de Klerk, B.; Fodor, I.; van der Sluis, M.; Bouwman, A.; Ellen, E. Passive radio frequency identification and video tracking for the determination of location and movement of broilers. Poult. Sci. 2023, 102, 102412. [Google Scholar] [CrossRef]
- Wang, Z.; Langenhuizen, P.; Visser, B.; Doekes, H.P.; Bijma, P.; De With, P.H.N. Multi-camera tracking of turkeys in large groups using instance segmentation. In Book of Abstracts of the 74th Annual Meeting of the European Federation of Animal Science, Lyon, France, 26 August–1 September 2023; Wageningen Academic Publishers: Wageningen, The Netherlands, 2023; p. 381. [Google Scholar]
- Hajimohammadi, B.; Ahmadian, S.; Firoozi, Z.; Askari, M.; Mohammadi, M.; Eslami, G.; Askari, V.; Loni, E.; Barzegar-Bafrouei, R.; Boozhmehrani, M.J. A meta-analysis of the prevalence of toxoplasmosis in livestock and poultry worldwide. EcoHealth 2022, 19, 55–74. [Google Scholar] [CrossRef]
- Opsteegh, M.; Cuperus, T.; van Buuren, C.; Dam-Deisz, C.; van Solt-Smits, C.; Verhaegen, B.; Joeres, M.; Schares, G.; Koudela, B.; Egberts, F.; et al. In vitro assay to determine inactivation of Toxoplasma gondii in meat samples. Int. J. Food Microbiol. 2024, 416, 110643. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Aubert, D.; Escotte-Binet, S.; Durand, B.; Robert, C.; Geers, R.; Alliot, A.; Belbis, G.; Villena, I.; Blaga, R. Anatomical distribution of Toxoplasma gondii in naturally and experimentally infected lambs. Parasite 2022, 29, 3. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J. Toxoplasmosis in sheep—The last 20 years. Vet. Parasitol. 2009, 163, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.P.; White, C.R.; Quintana, F.; Halsey, L.G.; Liebsch, N.; Martin, G.R.; Butler, P.J. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: The case of the cormorant. J. Anim. Ecol. 2006, 75, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Qasem, L.; Cardew, A.; Wilson, A.; Griffiths, I.; Halsey, L.G.; Shepard, E.L.; Gleiss, A.C.; Wilson, R. Tri-axial dynamic acceleration as a proxy for animal energy expenditure; should we be summing values or calculating the vector? PLoS ONE 2012, 7, e31187. [Google Scholar] [CrossRef] [PubMed]
- Gleiss, A.C.; Wilson, R.P.; Shepard, E.L. Making overall dynamic body acceleration work: On the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2011, 2, 23–33. [Google Scholar] [CrossRef]
- Mulvenna, C.C.; Marks, N.J.; Wilson, R.P.; Halsey, L.G.; Scantlebury, D.M. Can metrics of acceleration provide accurate estimates of energy costs of locomotion on uneven terrain? Using domestic sheep (Ovis aries) as an example. Anim. Biotelemetry 2022, 10, 8. [Google Scholar] [CrossRef]
- Conklin, E.E.; Lee, K.L.; Schlabach, S.A.; Woods, I.G. VideoHacking: Automated tracking and quantification of locomotor behavior with open source software and off-the-shelf video equipment. J. Undergrad. Neurosci. Educ. 2015, 13, A120. [Google Scholar]
- Garrido-Jurado, S.; Muñoz-Salinas, R.; Madrid-Cuevas, F.J.; Marín-Jiménez, M.J. Automatic generation and detection of highly reliable fiducial markers under occlusion. Pattern Recognit. 2014, 47, 2280–2292. [Google Scholar] [CrossRef]
- Blatteis, C.M. Fever: Pathological or physiological, injurious or beneficial? J. Therm. Biol. 2003, 28, 1–13. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A. Sickness behavior in animals: Implications for health and wellness. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Morelle, K.; Barasona, J.A.; Bosch, J.; Heine, G.; Daim, A.; Arnold, J.; Bauch, T.; Kosowska, A.; Cadenas-Fernández, E.; Aviles, M.M.; et al. Accelerometer-based detection of African swine fever infection in wild boar. Proc. R. Soc. B 2023, 290, 20231396. [Google Scholar] [CrossRef] [PubMed]
- Vichaya, E.G.; Dantzer, R. Inflammation-induced motivational changes: Perspective gained by evaluating positive and negative valence systems. Curr. Opin. Behav. Sci. 2018, 22, 90–95. [Google Scholar] [CrossRef]
- Miller, N.E. Some psychophysiological studies of motivation and of the behavioral-effects of illness. Bull. Br. Psychol. Soc. 1963, 16, 1–20. [Google Scholar]
- Verheijden, J.; Pijpers, A.; Schoevers, E.; van Gogh, H.; van Leengoed, L.; Visser, I.; van Miert, A.; Verheijden, J. The influence of disease on feed and water consumption and on pharmacokinetics of. J. Anim. Sci. 1991, 69, 2947–2954. [Google Scholar]
- Ahmed, S.; Mun, H.-S.; Yoe, H.; Yang, C.-J. Monitoring of behavior using a video-recording system for recognition of Salmonella infection in experimentally infected growing pigs. Animal 2015, 9, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Cardot, V.; Le Roux, Y.; Jurjanz, S. Drinking behavior of lactating dairy cows and prediction of their water intake. J. Dairy. Sci. 2008, 91, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, J.A.; Houpt, T.R. Feeding and drinking patterns in young pigs. Physiol. Behav. 1988, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P. The effect of Toxoplasma gondii on animal behavior: Playing cat and mouse. Schizophr. Bull. 2007, 33, 752–756. [Google Scholar] [CrossRef]
- Johnson, S.K.; Johnson, P.T. Toxoplasmosis: Recent advances in understanding the link between infection and host behavior. Annu. Rev. Anim. Biosci. 2021, 9, 249–264. [Google Scholar] [CrossRef]
- Tong, W.H.; Pavey, C.; O’Handley, R.; Vyas, A. Behavioral biology of Toxoplasma gondii infection. Parasit. Vectors 2021, 14, 77. [Google Scholar] [CrossRef]
- Worth, A.R.; Thompson, R.A.; Lymbery, A.J. Reevaluating the evidence for Toxoplasma gondii-induced behavioural changes in rodents. Adv. Parasitol. 2014, 85, 109–142. [Google Scholar] [PubMed]
- Shamsi, S.; Fahey, H.; Rast, L.; Freire, R. Is Toxoplasma gondii infection related to spatial problem solving and fear response in sheep? Appl. Anim. Behav. Sci. 2023, 263, 105933. [Google Scholar] [CrossRef]
- Hoy, R.A.; Brereton, J.E. Does Observer Presence Modify the Behavior and Enclosure Use of Captive Edwards’ Pheasants? J. Zool. Bot. Gard. 2022, 3, 147–157. [Google Scholar] [CrossRef]
- Barwick, J.; Lamb, D.W.; Dobos, R.; Welch, M.; Schneider, D.; Trotter, M. Identifying sheep activity from tri-axial acceleration signals using a moving window classification model. Remote Sens. 2020, 12, 646. [Google Scholar] [CrossRef]
- Do, J.P.; Defensor, E.B.; Ichim, C.V.; Lim, M.A.; Mechanic, J.A.; Rabe, M.D.; Schaevitz, L.R. Automated and continuous monitoring of animal welfare through digital alerting. Comp. Med. 2020, 70, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, A.; Giersberg, M.F.; Meijboom, F.L.B. Do we improve any aspects of animal welfare by implementing Computer Vision in livestock farming? In Transforming Food Systems: Ethics, Innovation and Responsibility; Wageningen Academic Publishers: Wageningen, The Netherlands, 2022; pp. 481–486. [Google Scholar]
- Tuyttens, F.A.; Molento, C.F.; Benaissa, S. Twelve threats of precision livestock farming (PLF) for animal welfare. Front. Vet. Sci. 2022, 9, 889623. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.M. Guidelines for the instrumentation of wild birds and mammals. Anim. Behav. 2009, 78, 1477–1483. [Google Scholar] [CrossRef]
- Bossert, L.; Hagendorff, T. Animals and, A.I. The role of animals in AI research and application–An overview and ethical evaluation. Technol. Soc. 2021, 67, 101678. [Google Scholar] [CrossRef]
- Halsey, L.; Green, J.; Wilson, R.; Frappell, P. Accelerometry to estimate energy expenditure during activity: Best practice with data loggers. Physiol. Biochem. Zool. 2009, 82, 396–404. [Google Scholar] [CrossRef]
- Yu, H.; Muijres, F.T.; te Lindert, J.S.; Hedenström, A.; Henningsson, P. Accelerometer sampling requirements for animal behaviour classification and estimation of energy expenditure. Anim. Biotelemetry 2023, 11, 28. [Google Scholar] [CrossRef]
- Shen, W.; Sun, Y.; Zhang, Y.; Fu, X.; Hou, H.; Kou, S.; Zhang, Y. Automatic recognition method of cow ruminating behaviour based on edge computing. Comput. Electron. Agric. 2021, 191, 106495. [Google Scholar] [CrossRef]
- Van Der Sluis, M.; De Klerk, B.; Ellen, E.D.; De Haas, Y.; Hijink, T.; Rodenburg, T.B. Validation of an ultra-wideband tracking system for recording individual levels of activity in broilers. Animals 2019, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Maselyne, J.; Van Nuffel, A.; Vangeyte, J.; Sonck, B. Tracking group housed sows with an ultra-wideband indoor positioning system: A feasibility study. Biosyst. Eng. 2020, 200, 176–187. [Google Scholar] [CrossRef]
- Alvarez, J.C.; Álvarez, D.; López, A.M. Accelerometry-based distance estimation for ambulatory human motion analysis. Sensors 2018, 18, 4441. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.; Shepard, E.; Quintana, F.; Laich, A.G.; Green, J.; Wilson, R.P. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2009, 152, 197–202. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Purswell, J.L.; Du, Q.; Chesser Jr, G.D.; Lowe, J.W. Analysis of feeding and drinking behaviors of group-reared broilers via image processing. Comput. Electron. Agric. 2020, 175, 105596. [Google Scholar] [CrossRef]


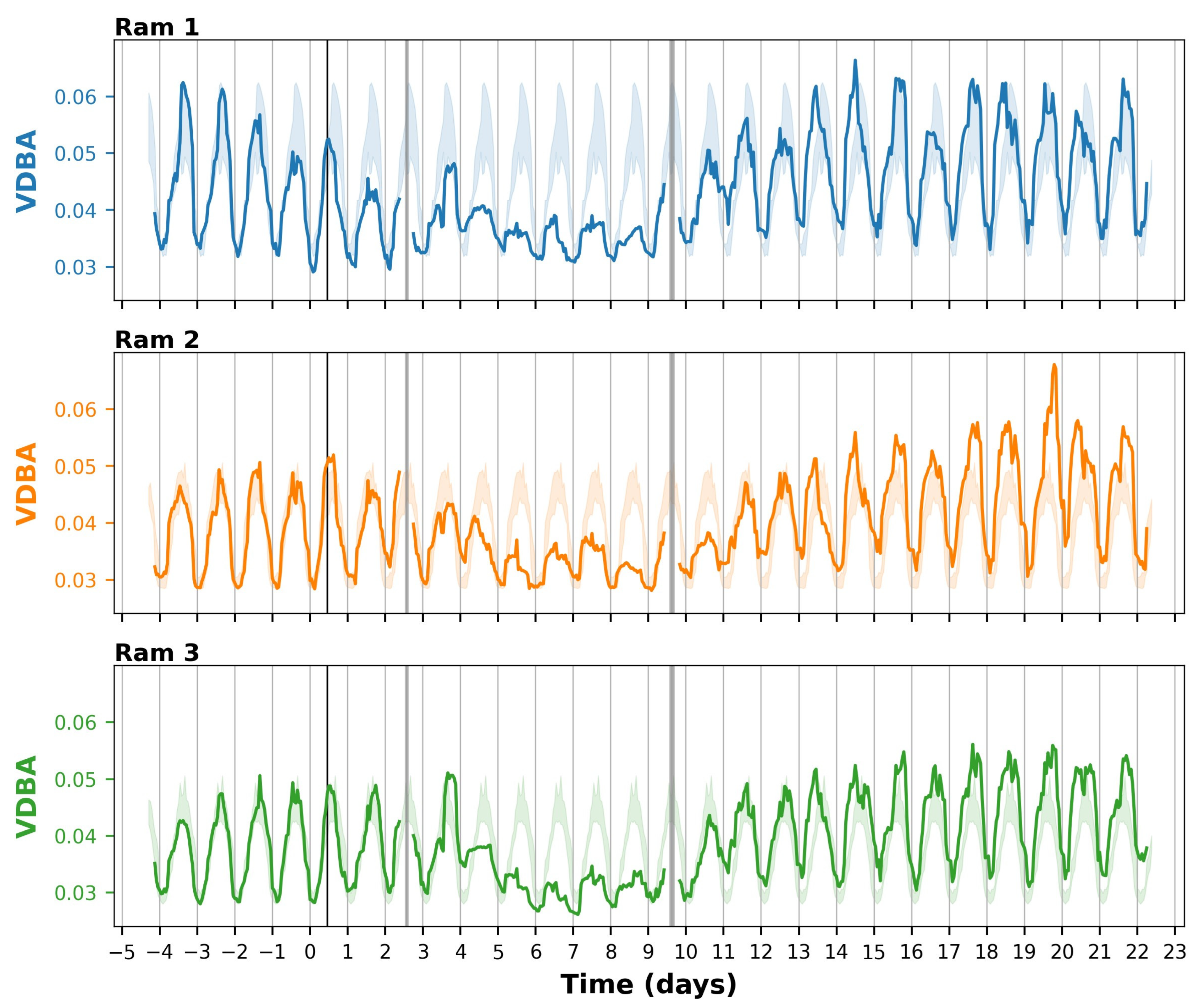
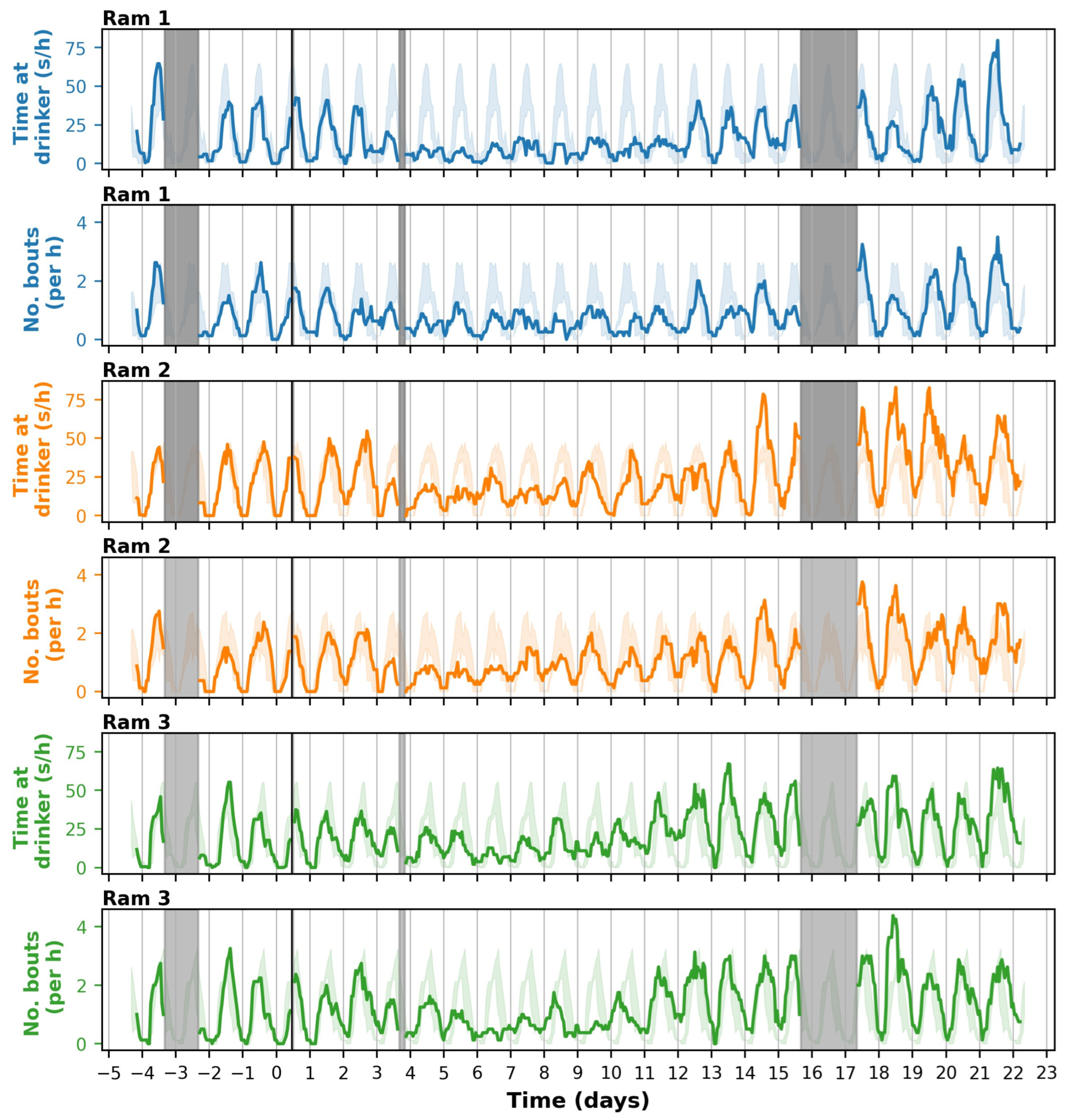
| VDBA | Time at Drinker (s/h) | No. Drinking Bouts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | Delta | Delta % | p-Value | Delta | Delta % | p-Value | Delta | Delta % | p-Value |
| 0 | −0.0011 | −2 | 0.259 | −2.5 | −13 | 0.177 | −0.181 | −17 | 0.047 |
| 1 | −0.0016 | −3 | 0.297 | 0.9 | 4 | 0.247 | −0.028 | −2 | 0.315 |
| 2 | NA * | NA * | NA * | 3.0 | 14 | 0.208 | 0.181 | 16 | 0.233 |
| 3 | −0.0003 | 0 | 0.349 | NA * | NA * | NA * | NA * | −45 | NA * |
| 4 | −0.0031 | −7 | 0.084 | −7.1 | −38 | 0.021 | −0.319 | −33 | 0.014 |
| 5 | −0.0078 | −19 | 0.002 | −8.8 | −48 | 0.020 | −0.403 | −40 | 0.003 |
| 6 | −0.0084 | −21 | 0.007 | −8.9 | −49 | 0.015 | −0.514 | −51 | 0.015 |
| 7 | −0.0069 | −17 | 0.009 | −6.9 | −37 | 0.002 | −0.264 | −25 | 0.109 |
| 8 | −0.0081 | −20 | 0.003 | −6.7 | −36 | 0.063 | −0.347 | −35 | 0.017 |
| 9 | NA * | NA * | NA * | −4.5 | −25 | 0.019 | −0.194 | −17 | 0.246 |
| 10 | −0.0021 | −5 | 0.093 | −3.0 | −18 | 0.226 | −0.278 | −29 | 0.080 |
| 11 | 0.0018 | 4 | 0.081 | −0.9 | −5 | 0.346 | 0.056 | 3 | 0.339 |
| 12 | 0.0025 | 6 | 0.022 | 7.6 | 41 | 0.123 | 0.375 | 34 | 0.146 |
| 13 | 0.0036 | 9 | 0.021 | 5.6 | 30 | 0.177 | 0.139 | 12 | 0.266 |
| 14 | 0.0059 | 15 | <0.001 | 8.9 | 45 | 0.083 | 0.431 | 43 | 0.050 |
| 15 | 0.0054 | 14 | <0.001 | NA * | NA * | NA * | NA * | NA * | NA * |
| 16 | 0.0034 | 9 | 0.028 | NA * | NA * | NA * | NA * | NA * | NA * |
| 17 | 0.0070 | 18 | <0.001 | NA * | NA * | NA * | NA * | NA * | NA * |
| 18 | 0.0072 | 18 | 0.003 | 11.3 | 55 | 0.167 | 0.708 | 67 | 0.116 |
| 19 | 0.0075 | 19 | 0.013 | 13.1 | 67 | 0.087 | 0.403 | 41 | 0.018 |
| 20 | 0.0072 | 18 | 0.014 | 9.4 | 50 | 0.006 | 0.625 | 64 | 0.007 |
| 21 | 0.0055 | 14 | 0.002 | 18.2 | 98 | 0.002 | 0.694 | 71 | 0.001 |
| 22 | 0.0006 | 2 | 0.256 | −3.9 | −22 | 0.030 | −0.148 | −14 | 0.327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doekes, H.P.; Petie, R.; de Jong, R.; Adriaens, I.; Wisselink, H.J.; Stockhofe-Zurwieden, N. Added Value of Sensor-Based Behavioural Monitoring in an Infectious Disease Study with Sheep Infected with Toxoplasma gondii. Animals 2024, 14, 1908. https://doi.org/10.3390/ani14131908
Doekes HP, Petie R, de Jong R, Adriaens I, Wisselink HJ, Stockhofe-Zurwieden N. Added Value of Sensor-Based Behavioural Monitoring in an Infectious Disease Study with Sheep Infected with Toxoplasma gondii. Animals. 2024; 14(13):1908. https://doi.org/10.3390/ani14131908
Chicago/Turabian StyleDoekes, Harmen P., Ronald Petie, Rineke de Jong, Ines Adriaens, Henk J. Wisselink, and Norbert Stockhofe-Zurwieden. 2024. "Added Value of Sensor-Based Behavioural Monitoring in an Infectious Disease Study with Sheep Infected with Toxoplasma gondii" Animals 14, no. 13: 1908. https://doi.org/10.3390/ani14131908
APA StyleDoekes, H. P., Petie, R., de Jong, R., Adriaens, I., Wisselink, H. J., & Stockhofe-Zurwieden, N. (2024). Added Value of Sensor-Based Behavioural Monitoring in an Infectious Disease Study with Sheep Infected with Toxoplasma gondii. Animals, 14(13), 1908. https://doi.org/10.3390/ani14131908







