Effects of Lactoferrin and Lactobacillus Supplementation on Immune Function, Oxidative Stress, and Gut Microbiota in Kittens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Experimental Design
2.3. Diet
2.4. Sample Collection
2.4.1. Blood Collection and Analyses
2.4.2. Hair Collection and Analyses
Cat Hair Collection
Kitten Hair Scoring Basis and Method
Hair Sample Preparation
2.5. Analysis of 16S rRNA Gene Diversity of Microbiota in the Intestinal Tract of Kittens
2.6. Kitten Feces Scoring Basis and Method
2.7. Methodology for Evaluating Kitten Vitality
2.8. Statistical Analyses
3. Results
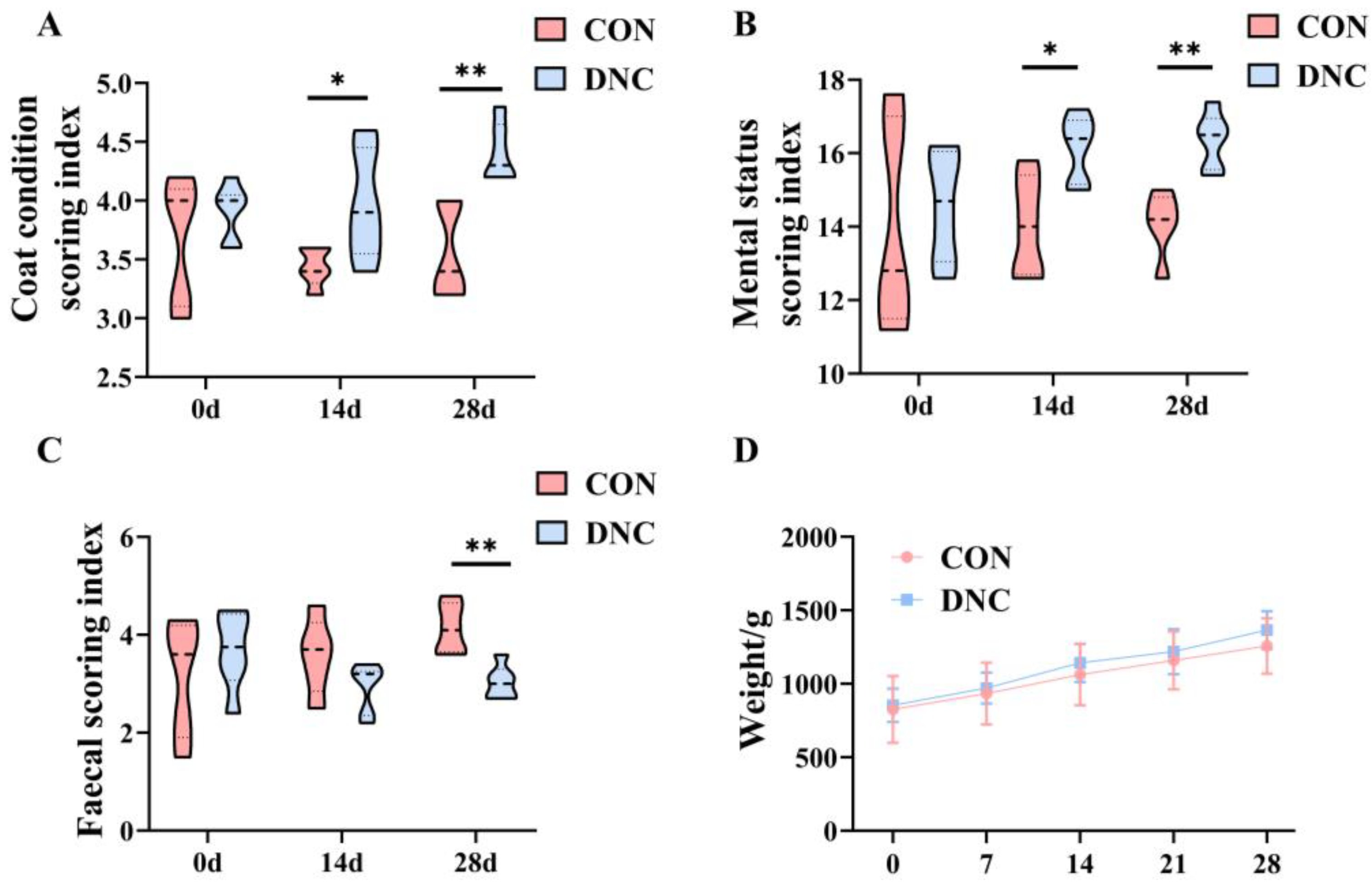
3.1. Physical Condition Assessment
3.2. Hematological Indicators
3.2.1. Complete Blood Count
3.2.2. Serum Biochemical Indices
3.2.3. Immunoglobulin Parameters
3.2.4. Antioxidant Parameters
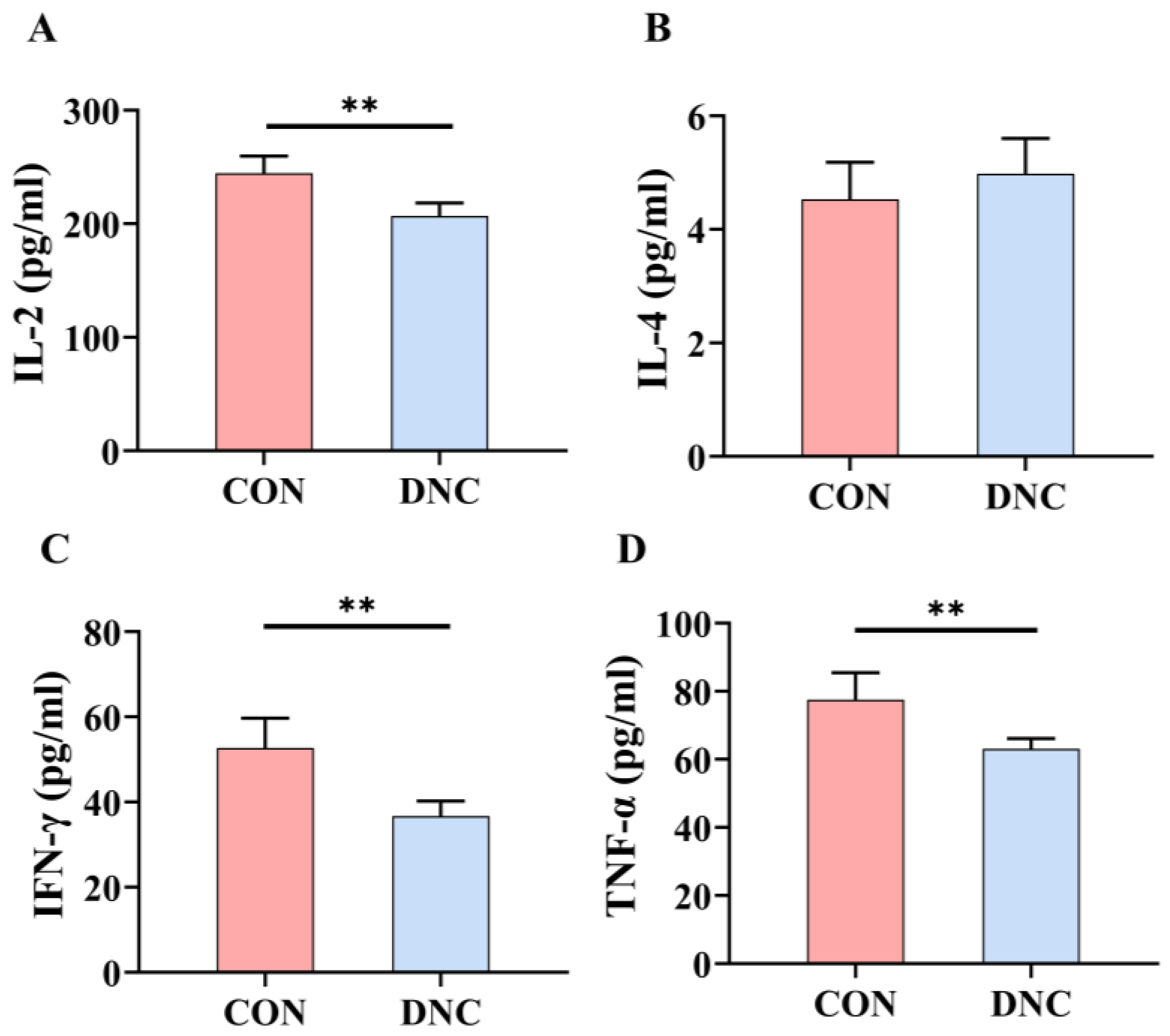
3.2.5. Cytokine Levels
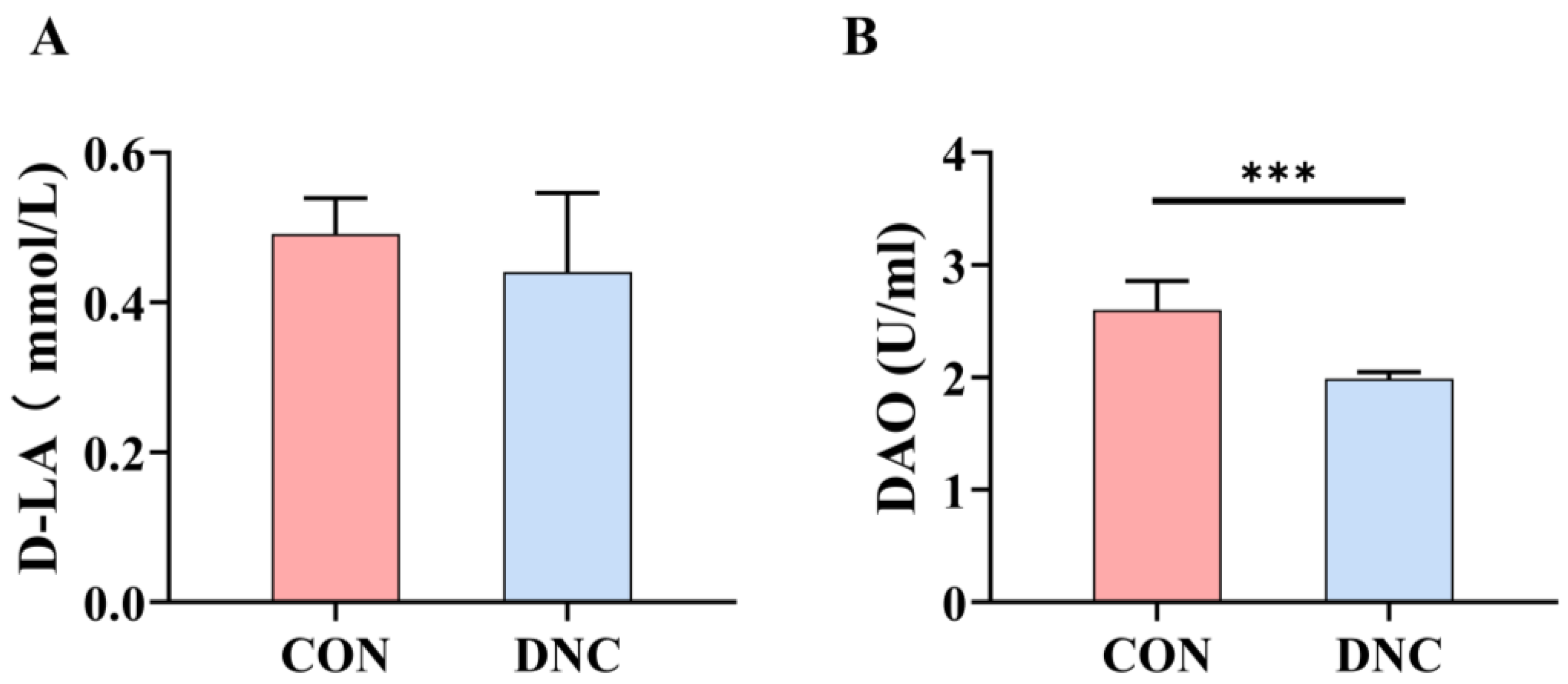
3.2.6. Gut Barrier Function Parameters
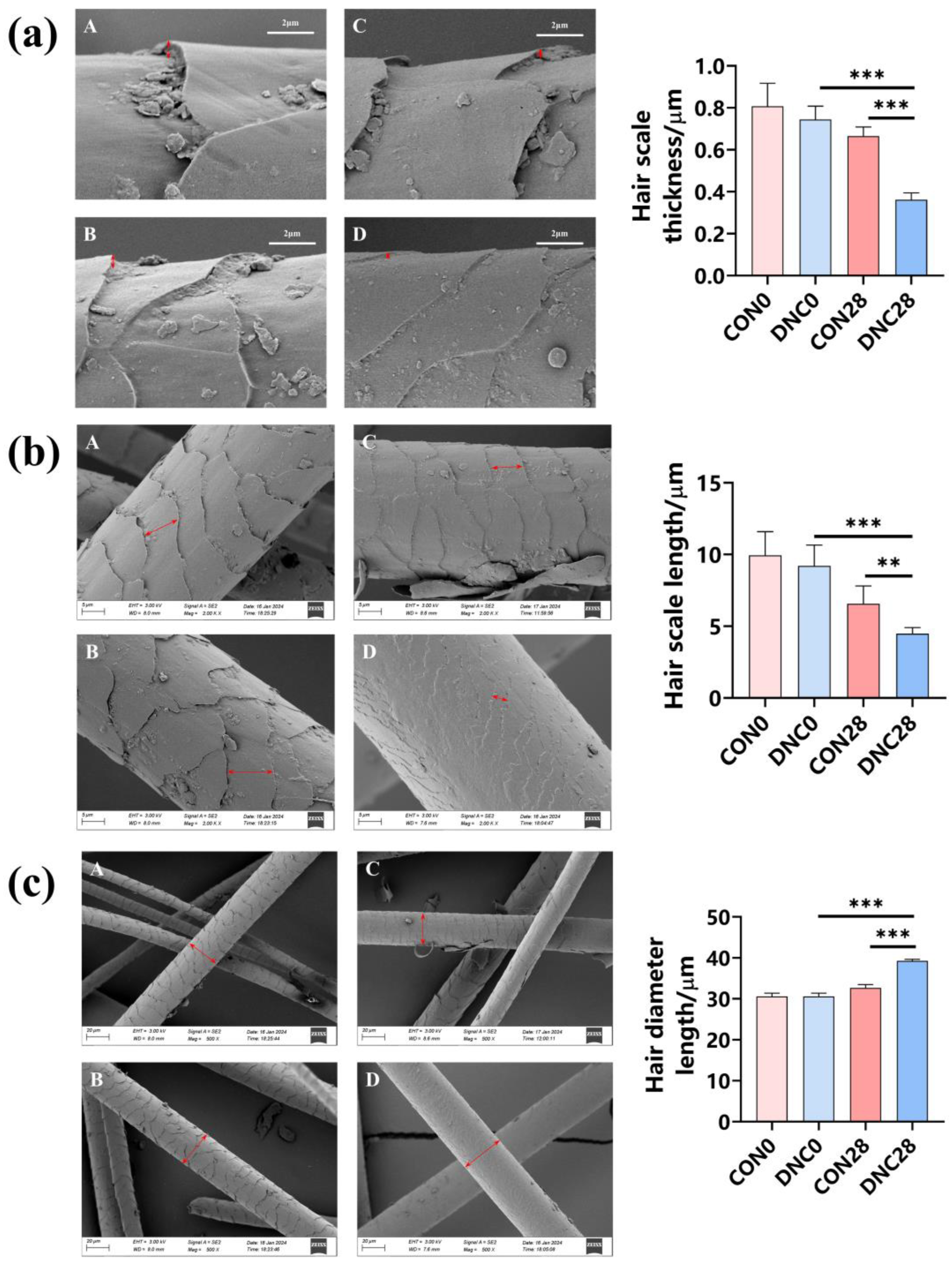
3.3. Hair Condition Analysis
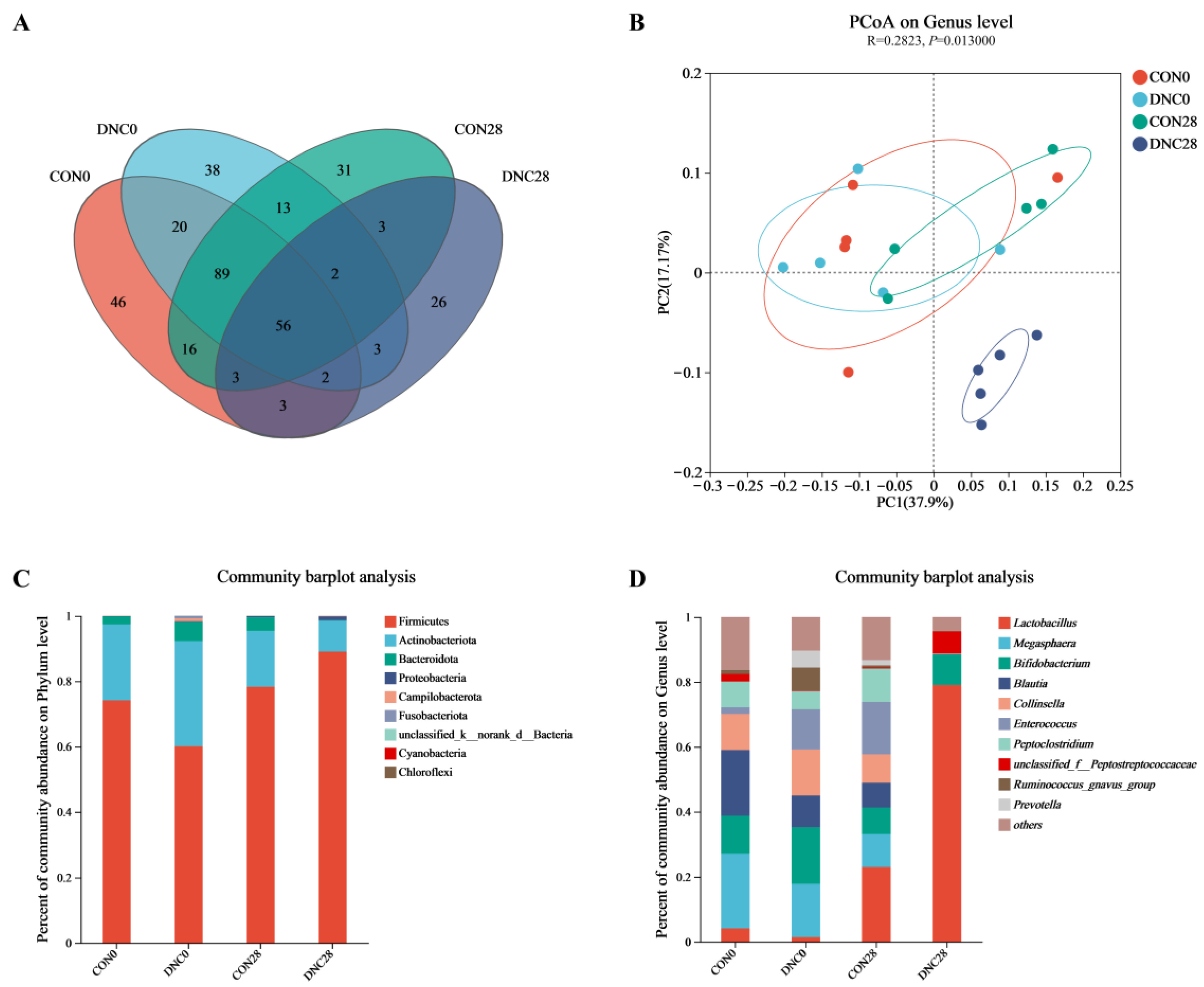
3.4. Fecal Microbiota Composition
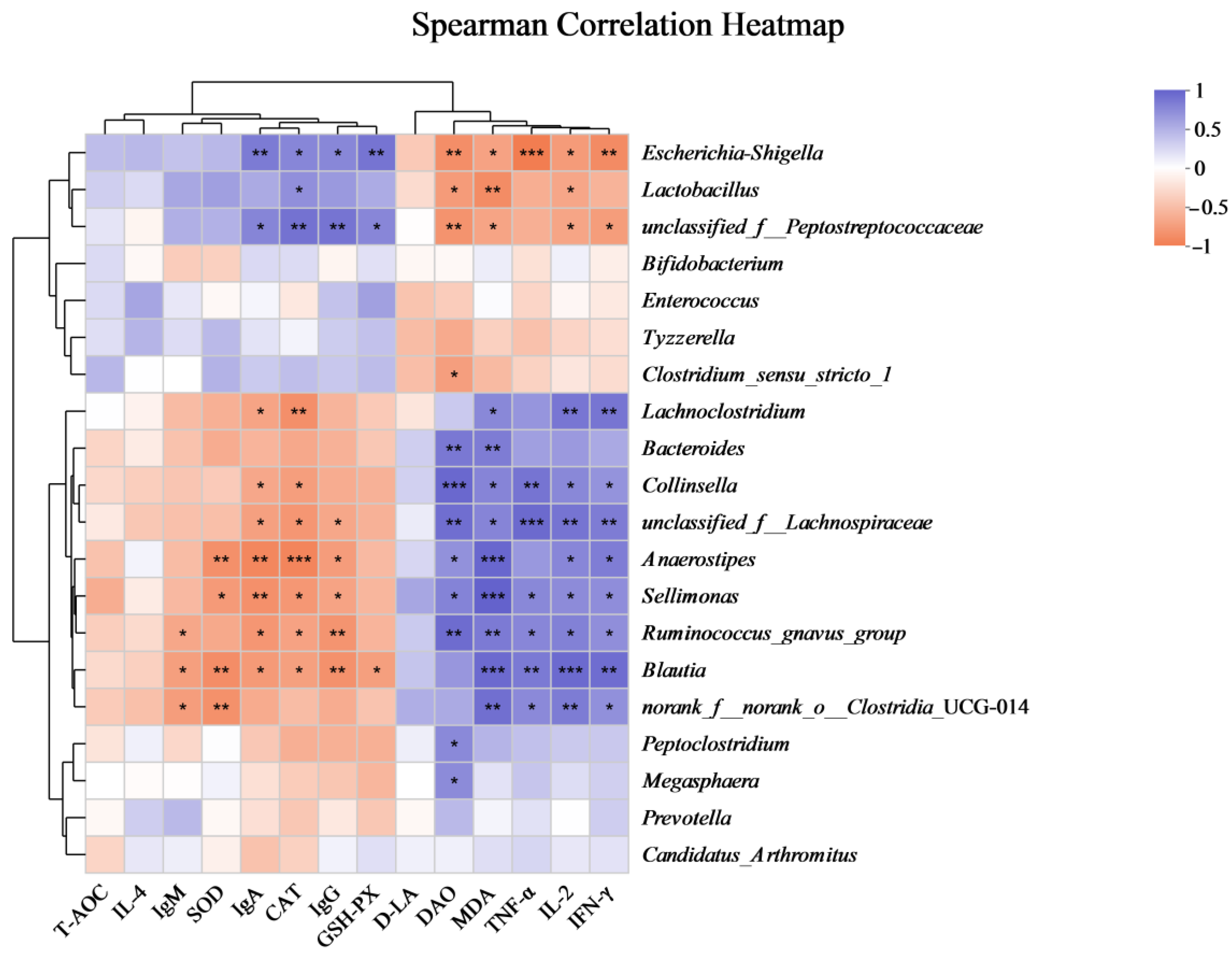
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Mouzon, C.; Gonthier, M.; Leboucher, G. Discrimination of cat-directed speech from human-directed speech in a population of indoor companion cats (Felis catus). Anim. Cogn. 2023, 26, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Estepa-Becerra, J.A.; Cajiao-Pachón, M.N.; Monsalve-Barrero, S. Canine and feline population management within the One Welfare framework: A retrospective look Bogotá 2004 to 2021. Rev. MVZ Cordoba 2023, 28, 27. [Google Scholar] [CrossRef]
- Peña-Corona, S.I.; Gomez-Vazquez, J.P.; López-Flores, E.A.; Vargas Estrada, D.; Arvizu-Tovar, L.O.; Pérez-Rivero, J.J.; Juárez Rodríguez, I.; Sierra Resendiz, A.; Soberanis-Ramos, O. Use of an extrapolation method to estimate the population of cats and dogs living at homes in Mexico in 2022. Vet. Mex. 2022, 9. [Google Scholar] [CrossRef]
- Biezus, G.; Machado, G.; Ferian, P.E.; da Costa, U.M.; da Silva Pereira, L.H.H.; Withoeft, J.A.; Nunes, I.A.C.; Muller, T.R.; de Cristo, T.G.; Casagrande, R.A. Prevalence of and factors associated with feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) in cats of the state of Santa Catarina, Brazil. Comp. Immunol. Microb. 2019, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.M.; Satyaraj, E.; Labuda, J.; Engler, R.; Sun, P.; Kerr, W.; Conboy-Schmidt, L. Supplementation of diets with bovine colostrum influences immune and gut function in kittens. Front. Vet. Sci. 2021, 8, 675712. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, N.A.; McKeever, P.J.; Eisenschenk, M.C. Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Vet. Dermatol. 2011, 22, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Möstl, K.; Lloret, A.; Thiry, E.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Hofmann-Lehmann, R. Vaccination of immunocompromised cats. Viruses 2022, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Lemos, F.; Almosny, N.P.; Soares, A.M.B.; Alencar, N.X. Cryptosporidium species screening using Kinyoun technique in domestic cats with diarrhea. J. Feline Med. Surg. 2012, 14, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Tourret, J.; Willing, B.P.; Dion, S.; MacPherson, J.; Denamur, E.; Finlay, B.B. Immunosuppressive treatment alters secretion of ileal antimicrobial peptides and gut microbiota, and favors subsequent colonization by uropathogenic Escherichia coli. Transplantation 2017, 101, 74–82. [Google Scholar] [CrossRef]
- Gaillard, V.; Chastant, S.; England, G.; Forman, O.; German, A.J.; Suchodolski, J.S.; Villaverde, C.; Chavatte-Palmer, P.; Péron, F. Environmental risk factors in puppies and kittens for developing chronic disorders in adulthood: A call for research on developmental programming. Front. Vet. Sci. 2022, 9, 944821. [Google Scholar] [CrossRef]
- Rossi, L.; Lumbreras, A.E.V.; Vagni, S.; Dell’Anno, M.; Bontempo, V. Nutritional and functional properties of colostrum in puppies and kittens. Animals 2021, 11, 3260. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Llopis, S.; Gonzalez, N.; Ramón, D.; Serrano, G.; Torrens, A.; Serrano, J.M.; Navarro, M.; Genovés, S. A nutritional supplement containing lactoferrin stimulates the immune system, extends lifespan, and reduces amyloid β peptide toxicity in Caenorhabditis elegans. Food Sci. Nutr. 2017, 5, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Williams, L.M.; Williams, E.J.; Wood, L.G. Effect of lactoferrin supplementation on inflammation, immune function, and prevention of respiratory tract infections in humans: A systematic review and meta-analysis. Adv. Nutr. 2022, 13, 1799–1819. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Sortino, O.; Hullsiek, K.H.; Richards, E.; Rupert, A.; Schminke, A.; Tetekpor, N.; Quinones, M.; Prosser, R.; Schacker, T.; Sereti, I. The effects of recombinant human lactoferrin on immune activation and the intestinal microbiome among persons living with human immunodeficiency virus and receiving antiretroviral therapy. J. Infect. Dis. 2019, 219, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, Y.; Kim, E.H.; Jang, H.; Cho, H.; Han, G.; Song, H.K.; Kim, S.H.; Yang, Y. Potential of colostrum-derived exosomes for promoting hair regeneration through the transition from telogen to anagen phase. Front. Cell Dev. Biol. 2022, 10, 815205. [Google Scholar] [CrossRef]
- Lubbs, D.; Vester, B.; Fastinger, N.; Swanson, K. Dietary protein concentration affects intestinal microbiota of adult cats: A study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J. Anim. Physiol. Anim. Nutr. 2009, 93, 113–121. [Google Scholar] [CrossRef]
- Bybee, S.; Scorza, A.; Lappin, M. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J. Vet. Intern. Med. 2011, 25, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Nguyen, T.S.; Le, T.H.H.; Nguyen, Q.U.; Le Bui, N.; Chu, D.T.; Van Vinh, H. Evaluation of the safety and immune stimulatory effects of multi-strain Lab Mix product on laboratory animals. Heliyon 2024, 10, e24691. [Google Scholar] [CrossRef]
- Lennon, S.; Lackie, T.; Miltko, A.; Kearns, Z.C.; Paquette, M.R.; Bloomer, R.J.; Wang, A.; van der Merwe, M. Safety and efficacy of a probiotic cocktail containing P. acidilactici and L. plantarum for gastrointestinal discomfort in endurance runners: Randomized double-blinded crossover clinical trial. Appl. Physiol. Nutr. Metab. 2024. [Google Scholar] [CrossRef]
- Sharma, N.; Navik, U.; Tikoo, K. Unveiling the presence of epigenetic mark by Lactobacillus supplementation in high-fat diet-induced metabolic disorder in Sprague-Dawley rats. J. Nutr. Biochem. 2020, 84, 108442. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Li, W.; Zhao, Y.; Long, H.; Muhindo, E.M.; Liu, R.; Sui, W.; Li, Q.; Zhang, M. Lactobacillus rhamnosus LRa05 ameliorate hyperglycemia through a regulating glucagon-mediated signaling pathway and gut microbiota in type 2 diabetic mice. J. Agric. Food Chem. 2021, 69, 8797–8806. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, D.; Park, G.-S.; Ko, S.-H.; Park, J.; Lee, Y.-K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, 12, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, Z.; Zhang, W.; Sun, T. Lactobacillus rhamnosus Probio-M9 improves the quality of life in stressed adults by gut microbiota. Foods 2021, 10, 2384. [Google Scholar] [CrossRef]
- Young, W.; Moon, C.D.; Thomas, D.G.; Cave, N.J.; Bermingham, E.N. Pre-and post-weaning diet alters the faecal metagenome in the cat with differences in vitamin and carbohydrate metabolism gene abundances. Sci. Rep. 2016, 6, 34668. [Google Scholar] [CrossRef] [PubMed]
- Association of American feed control officials [AAFCO]. In Proceedings of the AAFCO Annual Meeting Agenda and Committee Reports, Pittsburgh, PA, USA, 31 July–3 August 2016; p. 112.
- Wyrostek, A.; Roman, K.; Czyż, K.; Janczak, M.; Patkowska-Sokoła, B. Analysis of the hair coat of domestic cats with special focus on histological structure. Anim. Sci. Genet. 2017, 13, 47–58. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Shah, N.; Depasquale, J.; Baddoura, W.; Spira, R.; Rector, T. Use of Bristol Stool Form Scale to predict the adequacy of bowel preparation–a prospective study. Color. Dis. 2016, 18, 200–204. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef]
- Sarkar, V.; De, U.; Kala, A.; Chauhan, A.; Verma, A.; Paul, B.; Soni, S.; Chaudhuri, P.; Patra, M.; Gaur, G. Effects of oral probiotic and lactoferrin interventions on iron-zinc homeostasis, oxidant/antioxidant equilibrium and diarrhoea incidence of neonatal piglets. Benef. Microbes 2023, 14, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Marelli, S.P.; Fusi, E.; Giardini, A.; Martino, P.A.; Polli, M.; Bruni, N.; Rizzi, R. Effects of probiotic Lactobacillus acidophilus D2/CSL (CECT 4529) on the nutritional and health status of boxer dogs. Vet. Rec. 2020, 187, e28. [Google Scholar] [CrossRef]
- Yang, Y.; Park, J.; Kim, I. Effects of probiotics containing (Lactobacillus planetarium) and chlortetracycline on growth performance, nutrient digestibility, fecal microflora, diarrhea score and fecal gas emission in weanling pigs. Livest. Sci. 2020, 241, 104186. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Romero, M.; Yang, T.; Izquierdo-Garcia, J.L.; Jiménez, R.; Ruiz-Cabello, J. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol. Nutr. Food Res. 2020, 64, 1900616. [Google Scholar] [CrossRef]
- Fong, F.L.Y.; El-Nezami, H.; Mykkänen, O.; Kirjavainen, P.V. The effects of single strains and mixtures of probiotic bacteria on immune profile in liver, spleen, and peripheral blood. Front. Nutr. 2022, 9, 773298. [Google Scholar] [CrossRef] [PubMed]
- Yazhini, P.; Visha, P.; Selvaraj, P.; Vasanthakumar, P.; Chandran, V. Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet. World 2018, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.A.; Sheikh Veisi, R.; Hosseini Shekarabi, S.P.; Shahbazi Naserabad, S.; Bagheri, D.; Ghafarifarsani, H. Effect of dietary Lactobacillus casei on physiometabolic responses and liver histopathology in common carp (Cyprinus carpio) after exposure to iron oxide nanoparticles. Biol. Trace. Elem. Res. 2022, 200, 3346–3354. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2008, 65, 2535–2547. [Google Scholar] [CrossRef]
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A dietary factor to modulate the gut microbiome, host immune system, and gut–brain interaction. Microorganisms 2020, 8, 1401. [Google Scholar] [CrossRef]
- Colas, L.; Magnan, A.; Brouard, S. Immunoglobulin E response in health and disease beyond allergic disorders. Allergy 2022, 77, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernández, T.R.; Gómez-Jiménez, D.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. Analysis of the intestinal IgA response in mice exposed to chronic stress and treated with bovine lactoferrin. Mol. Med. Rep. 2021, 23, 11765. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Iwabuchi, N.; Takahashi, S.; Xiao, J.-z.; Abe, F.; Hachimura, S. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS ONE 2018, 13, e0199018. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fukudome, H.; Ueno, H.M.; Watanabe-Matsuhashi, S.; Nakano, T.; Kobayashi, T.; Ishimaru, K.; Nakao, A. Effects of probiotic supplementation on TGF-β1, TGF-β2, and IgA levels in the milk of Japanese women: An open-label pilot study. Front. Nutr. 2019, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V.; Mathijssen, G.B.; DaPra, C.; Medo, E. The effects of probiotic supplementation on the gene expressions of immune cell surface markers and levels of antibodies and pro-inflammatory cytokines in human milk. J. Perinatol. 2021, 41, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.-S.; Kim, Y.; Jeong, Y.; Kim, J.-E.; Paek, N.-S.; Kang, C.-H. Antioxidant and probiotic properties of Lactobacilli and Bifidobacteria of human origins. Biotechnol. Bioproc. Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, C.-H. Probiotics alleviate oxidative stress in H2O2-exposed hepatocytes and t-BHP-induced C57BL/6 mice. Microorganisms 2022, 10, 234. [Google Scholar] [CrossRef]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal lactoferrin administration decreases oxidative stress in the amniotic fluid of pregnant women: An open-label randomized pilot study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, J.-W.; Kim, S.-I.; Kim, S.; Nguyen, T.H.; Kang, C.-H. Antioxidant and anti-inflammatory effect and probiotic properties of lactic acid bacteria isolated from canine and feline feces. Microorganisms 2021, 9, 1971. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L.; Xiao, Y.; Xie, S.; Long, Y.; Wei, Y.; Meng, Q.; Li, X.; Luo, H.; Zhu, H. The expression of cytokine profiles and related receptors in idiopathic inflammatory myopathies. Front. Pharmacol. 2022, 13, 852055. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, J.Y.; Kim, H.; Moon, E.C.; Heo, K.; Shim, J.-J.; Lee, J.-L. Immunostimulatory effects of dairy probiotic strains Bifidobacterium animalis ssp. lactis HY8002 and Lactobacillus plantarum HY7717. J. Anim. Sci. Technol. 2022, 64, 1117. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-L.; Xu, J.-Y.; Zhang, R.; Zhang, Z.; Zhao, L.; Qin, L.-Q. Effects of lactoferrin on X-ray-induced intestinal injury in Balb/C mice. Appl. Radiat. Isot. 2019, 146, 72–77. [Google Scholar] [CrossRef]
- Kobayashi-Sakamoto, M.; Maeda, T.; Kimura, M.; Yusa, J.; Ito, H.; Tani, H.; Kato, Y.; Hirose, K. Bovine lactoferrin increases the poly (I: C)-induced antiviral response in vitro. Biochem. Cell Biol. 2022, 100, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-κB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T. 136 Intestinal Barrier Function: Physiological Regulation, Injury, and Repair in Horses and Swine. J. Anim. Sci. 2022, 100, 63–64. [Google Scholar] [CrossRef]
- Bellés, A.; Aguirre-Ramírez, D.; Abad, I.; Parras-Moltó, M.; Sánchez, L.; Grasa, L. Lactoferrin modulates gut microbiota and Toll-like receptors (TLRs) in mice with dysbiosis induced by antibiotics. Food Funct. 2022, 13, 5854–5869. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.-H.; Song, Y.; Jeong, S.; Kim, H.-J. Induction of autophagy improves skin and hair conditions in dogs with underlying diseases. Front. Vet. Sci. 2023, 10, 1078259. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Lin, H.; Huang, M.-C. Lactoferrin promotes hair growth in mice and increases dermal papilla cell proliferation through Erk/Akt and Wnt signaling pathways. Arch. Dermatol. Res. 2019, 311, 411–420. [Google Scholar] [CrossRef]
- Yu, P.; Teng, X.; Liu, T.; Li, Y.; Ni, J.; Xue, S.; Wang, J. Effect of an oral probiotic formula on scalp and facial skin condition, glucose, and lipid metabolism. Funct. Foods Health Dis. 2022, 12, 394–409. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Linninge, C.; Xu, J.; Bahl, M.I.; Ahrné, S.; Molin, G. Lactobacillus fermentum and Lactobacillus plantarum increased gut microbiota diversity and functionality, and mitigated Enterobacteriaceae, in a mouse model. Benef. Microbes 2019, 10, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, B.; Gao, H.; Zang, J.; Tao, S.; Zhang, S.; Huang, S.; He, B.; Wang, J. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet. Res. 2019, 15, 239. [Google Scholar] [CrossRef]
- Shinohara, M.; Kiyosue, M.; Tochio, T.; Kimura, S.; Koga, Y. Activation of butyrate-producing bacteria as well as bifidobacteria in the cat intestinal microbiota by the administration of 1-kestose, the smallest component of fructo-oligosaccharide. J. Vet. Med. Sci. 2020, 82, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, F.; Hou, Q.; Huang, W.; Liu, Y.; Zhang, H.; Sun, Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019, 10, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; He, F.; Su, S.; Sun, K.; Zhao, L.; Zhao, Y.; Bao, N.; Pan, L.; Sun, H. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA, DHA, and succinic and palmitic acids, and enhance immunity of weaned piglets. Res. Vet. Sci. 2021, 137, 56–67. [Google Scholar] [CrossRef] [PubMed]
- de Sá Almeida, J.S.; de Oliveira Marre, A.T.; Teixeira, F.L.; Boente, R.F.; Domingues, R.M.; de Paula, G.R.; Lobo, L.A. Lactoferrin and lactoferricin B reduce adhesion and biofilm formation in the intestinal symbionts Bacteroides fragilis and Bacteroides thetaiotaomicron. Anaerobe 2020, 64, 102232. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Lu, W.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Chen, W. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: A pilot study. Eur. J. Nutr. 2020, 59, 2119–2130. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Xing, X.; Ji, P.; Li, M.; Pan, H.; Guo, R.; An, Q. Effect of maternal lactoferrin supplementation on iron contents and anti-oxidant capacity in Dahe black Pig neonates. Front. Vet. Sci. 2022, 9, 1034084. [Google Scholar] [CrossRef]


| Items | Content (%) |
|---|---|
| Ingredients | |
| Fresh chicken | 28.00 |
| Chicken meat meal | 25.00 |
| Corn | 12.00 |
| Brown rice | 10.00 |
| Wholemeal | 8.00 |
| Chicken oil | 7.40 |
| Beetroot meal | 3.00 |
| Alfalfa pellets | 2.00 |
| Vitamins and minerals premix | 1.70 |
| Butter | 1.20 |
| Whole egg powder | 1.00 |
| Soya lecithin | 0.30 |
| Spirulina powder | 0.10 |
| Plantago | 0.10 |
| Yucca extract | 0.10 |
| Beer yeast | 0.10 |
| Nutrient content | |
| DM | 89.74 |
| CP | 31.54 |
| EE | 19.81 |
| Ash | 9.16 |
| WSC | 0.65 |
| Items | 2 Points | 1 Point | 0 Points |
|---|---|---|---|
| Eyes | Bright, clear, no discharge | Dull, cloudy, little discharge | Closed, swollen, heavy discharge |
| Ears | Clean, no odor, no mites or otitis | Dirty, slight odor, some mites or mild otitis | Very dirty, strong odor, many mites or severe otitis |
| Nose | Moist, no discharge, no congestion or sneezing | Dry, little discharge, slight congestion or occasional sneezing | Very dry, heavy discharge, severe congestion or frequent sneezing |
| Oral Cavity | Clean, no odor, no gum swelling or oral ulcers | Unclean, slight odor, some gum swelling or mild oral ulcers | Very unclean, strong odor, severe gum swelling or severe oral ulcers |
| Fur | Smooth, no matting, no fleas or skin issues | Dry, some matting, some fleas or minor skin issues | Very dry, heavy matting, many fleas or severe skin issues |
| Body Shape | Well-proportioned, no obesity or emaciation | Unbalanced, slight obesity or slight emaciation | Very unbalanced, severe obesity or severe emaciation |
| Activity | Frequent, curious, playful | Infrequent, lethargic, little play | Almost no activity, drowsy or comatose |
| Appetite | Keen to grab food, moderate quantity, not fussy or fed up | Occasionally poor appetite, or eats too much or too little | Long-term refusal of food or vomiting |
| Social | Likes to be close to people or other animals, shows friendliness and trust | Occasionally lazy or overly active | Long-term fear or aggression |
| Items | CON | DNC | Normal Reference Range | p-Value |
|---|---|---|---|---|
| Total White Blood Cells (109/L) | 21.50 ± 3.81 a | 16.33 ± 4.10 b | 5.5–19.5 | <0.05 |
| Lymphocyte Ratio (%) | 56.50 ± 7.12 | 56.02 ± 8.68 | 20.0–56.0 | 0.92 |
| Intermediate Cell Ratio (%) | 12.02 ± 4.41 | 16.77 ± 7.76 | 2.0–19.0 | 0.26 |
| Granulocyte Ratio (%) | 31.48 ± 8.22 | 27.27 ± 8.90 | 25.0–85.0 | 0.44 |
| Lymphocytes (109/L) | 12.28 ± 6.36 | 9.00 ± 3.37 | 0.8–9.0 | 0.30 |
| Intermediate Cells (109/L) | 2.44 ± 1.08 | 2.73 ± 1.63 | 0.1–2.8 | 0.74 |
| Granulocytes (109/L) | 6.78 ± 3.95 | 4.60 ± 2.73 | 2.1–15.0 | 0.31 |
| Total Red Blood Cell s (1012/L) | 6.44 ± 0.75 | 7.10 ± 1.30 | 4.60–10.00 | 0.35 |
| Hemoglobin (g/L) | 104.60 ± 10.78 | 106.33 ± 19.15 | 93–153 | 0.86 |
| Hematocrit (%) | 29.46 ± 2.03 | 31.35 ± 3.43 | 28.0–49.0 | 0.31 |
| Mean Corpuscular Volume (fL) | 46.26 ± 5.71 | 44.70 ± 3.76 | 39.0–52.0 | 0.60 |
| Hemoglobin Content (pg) | 16.48 ± 3.29 | 14.92 ± 0.61 | 13.0–21.0 | 0.28 |
| Hemoglobin Concentration (g/L) | 355.00 ± 33.75 | 336.67 ± 22.71 | 300–380 | 0.31 |
| Red Cell Distribution Width SD (fL) | 38.66 ± 5.65 | 37.80 ± 3.84 | 47.0–62.7 | 0.77 |
| Red Cell Distribution Width CV (%) | 18.04 ± 1.33 | 18.32 ± 1.18 | 14.0–18.0 | 0.92 |
| Total Platelet Count (109/L) | 320.20 ± 165.94 | 497.17 ± 391.27 | 100–514 | 0.37 |
| Mean Platelet Volume (fL) | 11.08 ± 1.75 | 12.08 ± 2.84 | 5.0–12.8 | 0.51 |
| Platelet Distribution Width (%) | 11.20 ± 2.50 | 11.92 ± 3.49 | 0.1–30.0 | 0.71 |
| Plateletcrit (%) | 0.36 ± 0.23 | 0.66 ± 0.60 | 0.01–9.99 | 0.32 |
| Platelet/large cell ratio (%) | 21.54 ± 8.26 | 28.88 ± 13.49 | 0.1–99.9 | 0.32 |
| Items | CON | DNC | Normal Reference Range | p-Value |
|---|---|---|---|---|
| Albumin (g/L) | 35.70 ± 3.36 | 31.97 ± 2.58 | 22.0–43.0 | 0.06 |
| Total Protein (g/L) | 64.45 ± 8.36 | 55.97 ± 7.41 | 53.0–82.0 | 0.09 |
| Globulin (g/L) | 28.85 ± 5.14 | 24.03 ± 3.65 | 23.0–48.0 | 0.09 |
| Albumin/Globulin Ratio | 1.24 ± 0.21 | 1.34 ± 0.34 | - | 0.96 |
| Aspartate Aminotransferase (U/L) | 22.14 ± 3.86 a | 12.51 ± 5.20 b | 0.0–48.0 | <0.01 |
| Alanine Aminotransferase (U/L) | 65.17 ± 12.68 | 58.33 ± 11.59 | 5–115 | 0.36 |
| Amylase (U/L) | 449.50 ± 52.50 | 478.46 ± 36.45 | 450–1500 | 0.30 |
| Creatine Kinase (CK) (U/L) | 502.42 ± 95.31 a | 373.31 ± 65.22 b | 0–467 | <0.05 |
| Creatinine (umol/L) | 45.90 ± 7.35 | 50.03 ± 6.74 | 25.0–141.0 | 0.33 |
| Blood Urea Nitrogen (mmol/L) | 10.28 ± 4.87 | 12.12 ± 3.85 | 4.00–12.80 | 0.48 |
| BUN/Creatinine Ratio | 308.81 ± 66.37 | 242.97 ± 42.34 | - | 0.07 |
| Glucose (mmol/L) | 6.35 ± 0.56 | 5.83 ± 0.21 | 4.28–8.50 | 0.06 |
| Triglycerides (mmol/L) | 1.54 ± 0.42 | 1.98 ± 0.54 | 0.00–2.20 | 0.14 |
| Calcium (mmol/L) | 2.80 ± 0.44 | 2.66 ± 0.36 | 1.98–2.83 | 0.57 |
| Inorganic Phosphorus (umol/L) | 3.21 ± 0.03 | 3.23 ± 0.09 | 1.45–3.35 | 0.67 |
| Total Bilirubin (mmol/L) | 1.70 ± 0.24 | 1.30 ± 0.47 | 0.0–15.0 | 0.09 |
| Items | CON 0 | CON 28 | DNC 0 | DNC 28 | p-Value |
|---|---|---|---|---|---|
| Ace | 100.50 ± 35.18 a | 106.80 ± 8.95 a | 109.00 ± 8.10 a | 56.67 ± 10.55 b | <0.01 |
| Chao1 | 101.60 ± 35.35 a | 103.90 ± 9.63 a | 110.8 ± 10.97 a | 54.27 ± 8.12 b | <0.01 |
| Coverage | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 0.77 |
| Shannon | 2.12 ± 0.80 a | 2.19 ± 0.28 a | 2.16 ± 0.37 a | 1.67 ± 0.22 b | 0.06 |
| Simpson | 0.26 ± 0.23 a | 0.22 ± 0.11 a | 0.23 ± 0.09 a | 0.28 ± 0.07 a | 0.71 |
| Sobs | 93.60 ± 33.02 a | 94.60 ± 10.60 a | 96.20 ± 7.59 a | 50.80 ± 8.67 b | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Wang, W.; Chen, Q.; Chang, X.; Wang, L.; Chen, S.; Chen, L.; Wang, R.; Ge, S.; Xiong, W. Effects of Lactoferrin and Lactobacillus Supplementation on Immune Function, Oxidative Stress, and Gut Microbiota in Kittens. Animals 2024, 14, 1949. https://doi.org/10.3390/ani14131949
Dong H, Wang W, Chen Q, Chang X, Wang L, Chen S, Chen L, Wang R, Ge S, Xiong W. Effects of Lactoferrin and Lactobacillus Supplementation on Immune Function, Oxidative Stress, and Gut Microbiota in Kittens. Animals. 2024; 14(13):1949. https://doi.org/10.3390/ani14131949
Chicago/Turabian StyleDong, Hao, Weiwei Wang, Qianqian Chen, Xiaohan Chang, Longjiao Wang, Shuxing Chen, Lishui Chen, Ran Wang, Shaoyang Ge, and Wei Xiong. 2024. "Effects of Lactoferrin and Lactobacillus Supplementation on Immune Function, Oxidative Stress, and Gut Microbiota in Kittens" Animals 14, no. 13: 1949. https://doi.org/10.3390/ani14131949
APA StyleDong, H., Wang, W., Chen, Q., Chang, X., Wang, L., Chen, S., Chen, L., Wang, R., Ge, S., & Xiong, W. (2024). Effects of Lactoferrin and Lactobacillus Supplementation on Immune Function, Oxidative Stress, and Gut Microbiota in Kittens. Animals, 14(13), 1949. https://doi.org/10.3390/ani14131949






