Molecular Detection of Viral and Bacterial Pathogens in Red Foxes (Vulpes vulpes) from Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Detection of Protoparvovirus carnivoran 1, Canine mastadenovirus, Circovirus canine, Canine Distemper Virus, and Leptospira spp. Nucleic Acids
2.3. Sequencing and Analysis of the Viruses Identified
2.4. Genotyping by Multi-Locus Sequence Typing of the Leptospira spp. Identified
2.5. Statistical Analysis
3. Results
3.1. Study Population and Sampling
3.2. Detection of Viral and Bacterial Infectious Agents
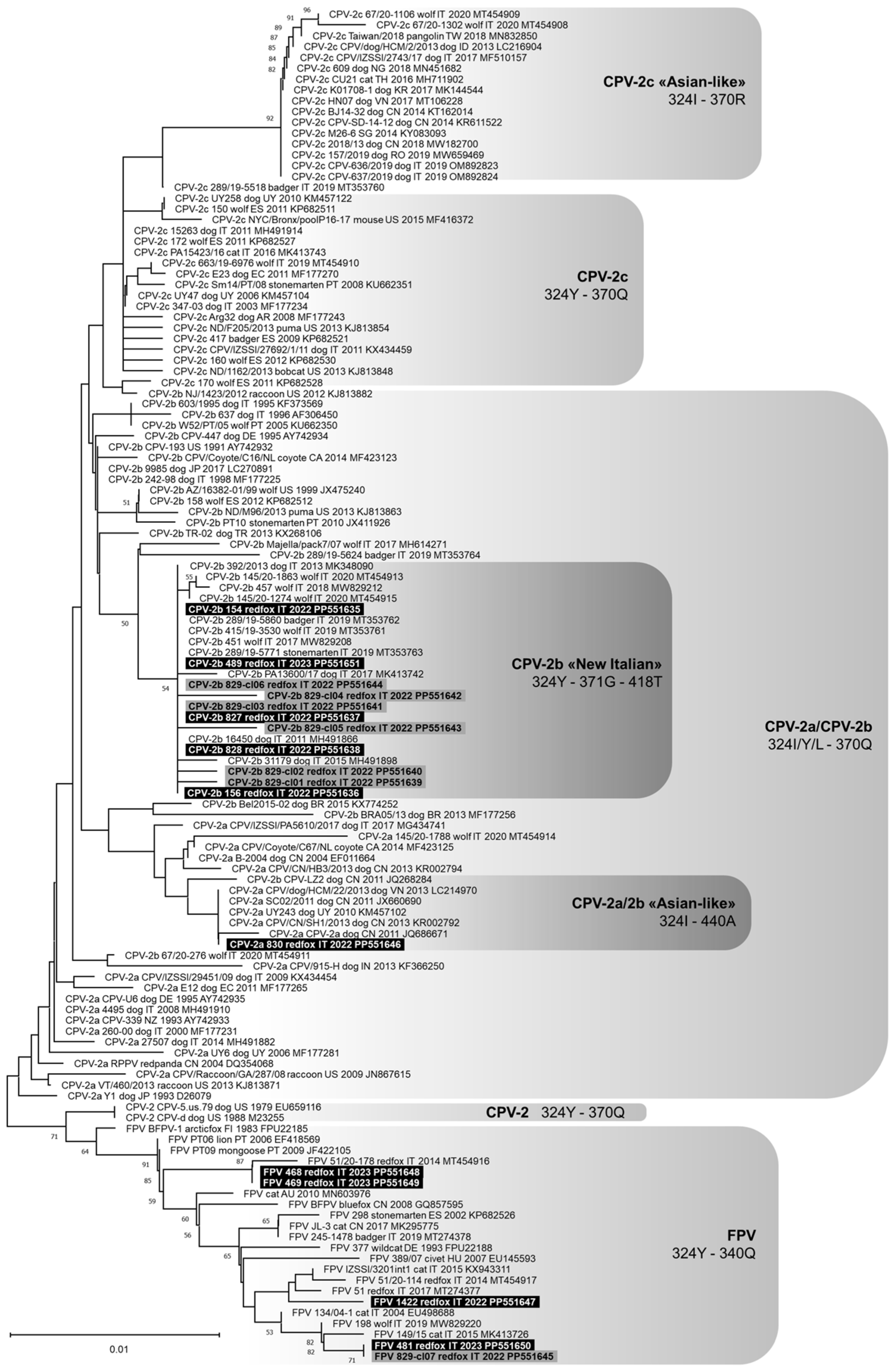
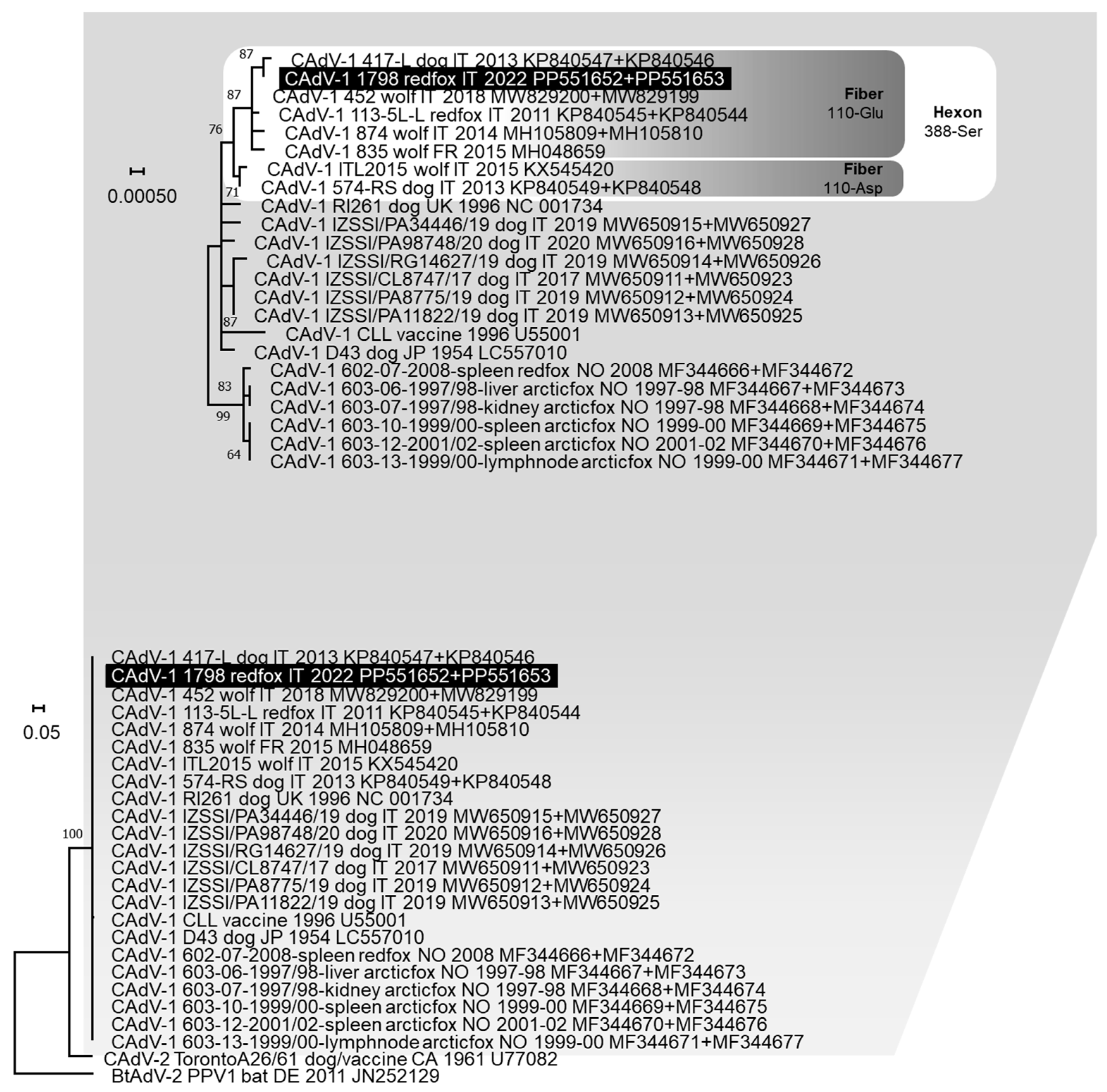
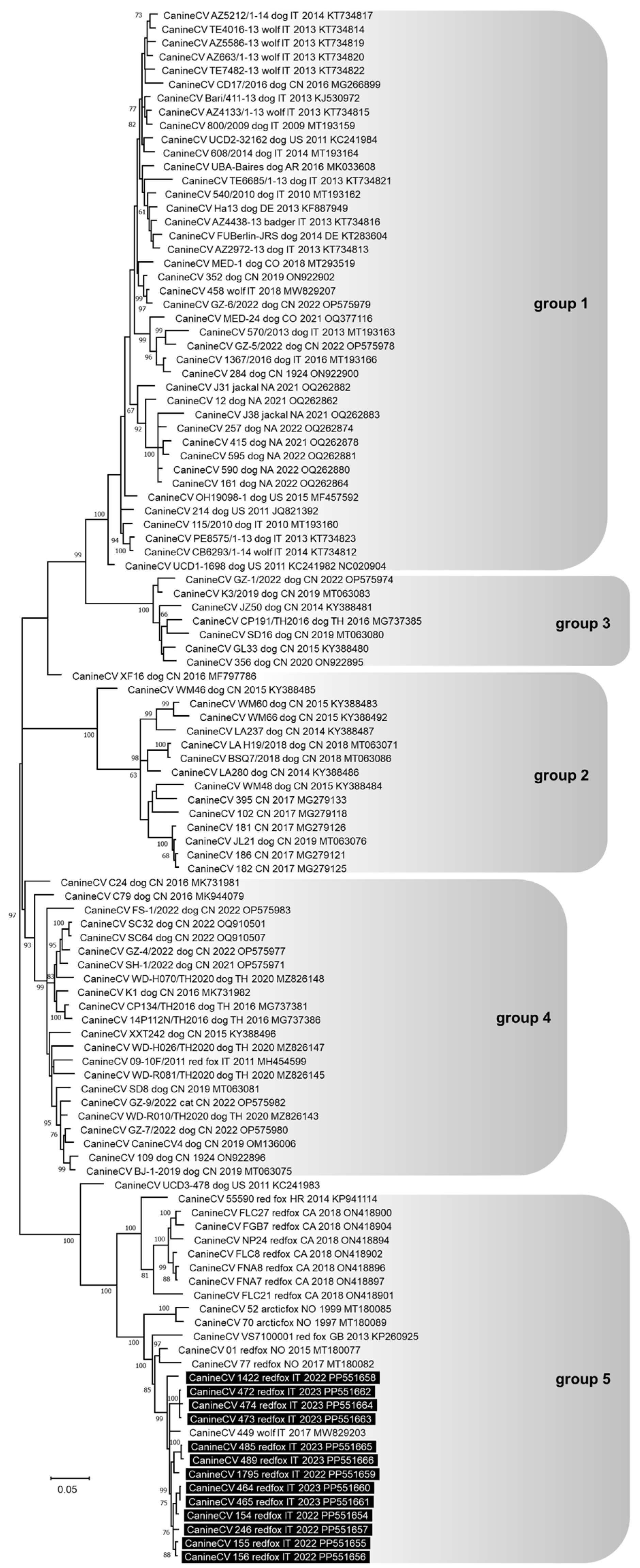
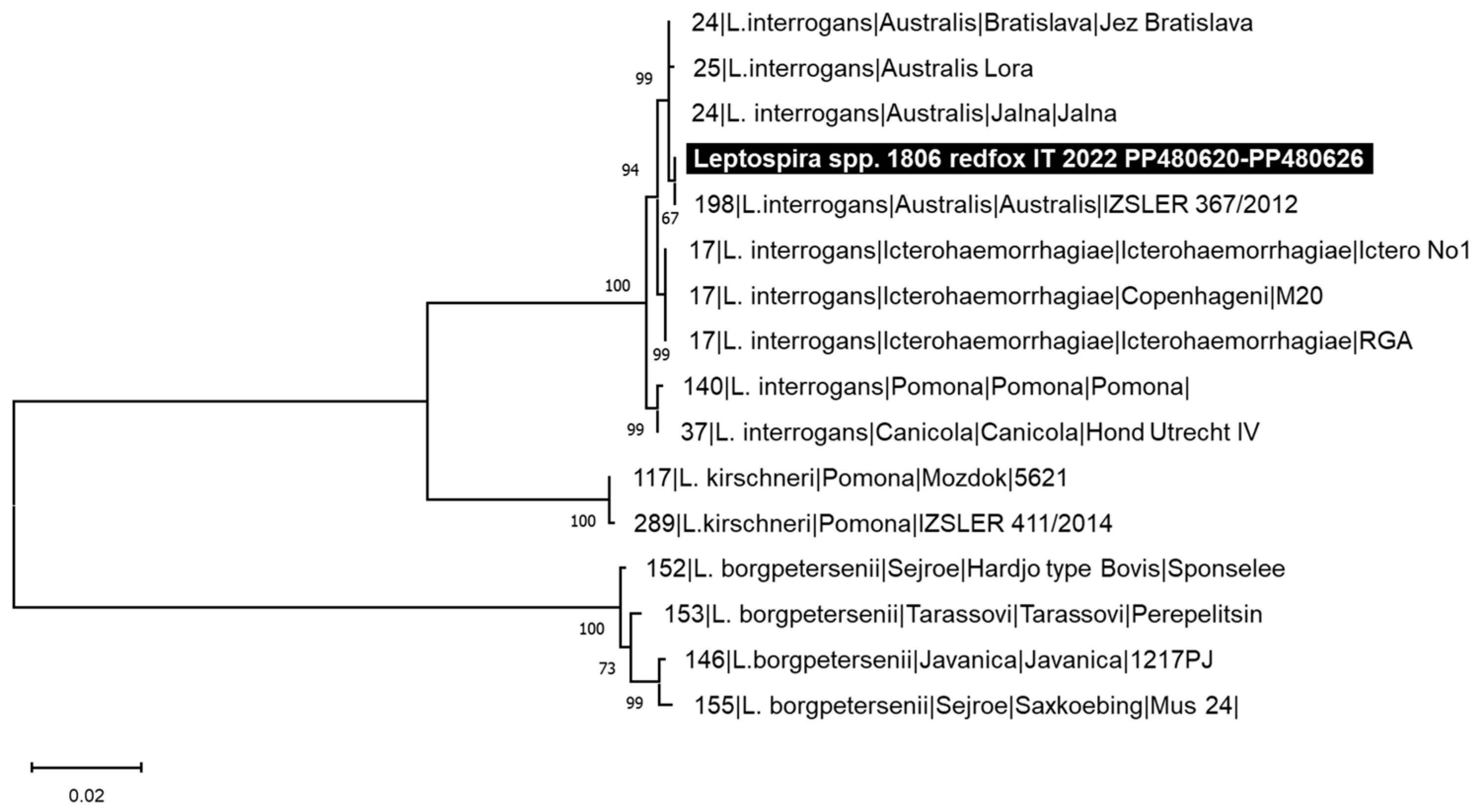
3.3. Genetic Analysis of the Pathogens Identified
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yon, L.; Duff, J.P.; Ågren, E.O.; Erdélyi, K.; Ferroglio, E.; Godfroid, J.; Hars, J.; Hestvik, G.; Horton, D.; Kuiken, T.; et al. Recent changes in infectious diseases in European wildlife. J. Wildl. Dis. 2019, 55, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.L.; Mazet, J.A.K.; Dubovi, E.J.; Garcelon, D.K.; Coonan, T.J.; Conrad, P.A.; Munson, L. Pathogen exposure in endangered island fox (Urocyon littoralis) populations: Implication for conservation management. Biol. Conserv. 2006, 131, 230–243. [Google Scholar] [CrossRef]
- Di Francesco, C.E.; Gentile, L.; Di Pirro, V.; Ladiana, L.; Tagliabue, S.; Marsilio, F. Serologic evidence for selected infectious diseases in Marsican brown bears (Ursus arctos marsicanus) in Italy (2004–09). J. Wildl. Dis. 2015, 51, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.P.D.; Foley, J.; Chomel, B. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a national park in California. J. Wildl. Dis. 2004, 40, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Pastoret, P.P.; Brochier, B.; Humblet, M.F.; Saegerman, C. A survey of the transmission of infectious diseases/infections between wild and domestic ungulates in Europe. Vet. Res. 2011, 42, 70. [Google Scholar] [CrossRef] [PubMed]
- Dellamaria, D.; Citterio, C.V.; Capelli, G.; Paternolli, S.; Turchetto, S.; Obber, F.; Cazzin, S.; Francione, E. Principali Patologie della Fauna: Conoscerle e Riconoscerle, 2014. Available online: https://issuu.com/izsvenezie/docs/patologie-fauna-selvatica (accessed on 10 March 2024).
- Brandell, E.E.; Cross, P.C.; Craft, M.E.; Smith, D.W.; Dubovi, E.J.; Gilbertson, M.L.J.; Wheeldon, T.; Stephenson, J.A.; Barber-Meyen, S.; Borg, B.L.; et al. Patterns and processes of pathogen exposure in gray wolves across North America. Sci. Rep. 2021, 11, 3722. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Pires, I. Secrets of the astute red fox (Vulpes vulpes, Linnaeus, 1758): An inside-ecosystem secret agent serving One Health. Environments 2021, 8, 103. [Google Scholar] [CrossRef]
- Pluemer, M.; Dubai, S.; Drake, D.; Crimmins, S.; Veverka, T.; Hovanec, H.; Torkelson, M.; Mueller, M. Red foxes (Vulpes vulpes) and coyotes (Canis latrans) in an urban landscape: Prevalence and risk factors for disease. J. Urban Ecol. 2019, 5, juz022. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruse (ICTV). Available online: https://ictv.global/taxonomy/ (accessed on 13 December 2023).
- Sykes, J.E. Infectious Diseases of the Dog and Cat, 5th ed.; Elsevier: St. Louis, MO, USA, 2023; pp. 341–357. [Google Scholar]
- Duarte, M.D.; Henriques, A.M.; Barros, S.C.; Fagulha, T.; Mendonca, P.; Carvalho, P.; Monteiro, M.; Fevereiro, M.; Basto, M.P.; Rosalino, L.M.; et al. Snapshot of viral infections in wild carnivores reveals ubiquity of parvovirus and susceptibility of egyptian mongoose to feline panleukopenia virus. PLoS ONE 2013, 8, e59399. [Google Scholar] [CrossRef]
- Ndiana, L.A.; Lanave, G.; Desario, C.; Berjaoui, S.; Alfano, F.; Puglia, I.; Fusco, G.; Colaianni, M.L.; Vincifori, G.; Camarda, A.; et al. Circulation of diverse protoparvoviruses in wild carnivores, Italy. Transbound. Emerg. Dis. 2020, 68, 2489–2502. [Google Scholar] [CrossRef]
- Calatayud, O.; Esperón, F.; Velarde, R.; Oleaga, Á.; Llaneza, L.; Ribas, A.; Negre, N.; de la Torre, A.; Rodríguez, A.; Millán, J. Genetic characterization of Carnivore Parvoviruses in Spanish wildlife reveals domestic dog and cat-related sequences. Transbound. Emerg. Dis. 2019, 67, 626–634. [Google Scholar] [CrossRef]
- Leopardi, S.; Milani, A.; Cocchi, M.; Bregoli, M.; Schivo, A.; Leardini, S.; Festa, F.; Pastori, A.; de Zan, G.; Gobbo, F.; et al. Carnivore protoparvovirus 1 (CPV-2 and FPV) circulating in wild carnivores and in puppies illegally imported into North-Eastern Italy. Viruses 2022, 14, 2612. [Google Scholar] [CrossRef]
- Battilani, M.; Scagliarini, A.; Tisato, E.; Turilli, C.; Jacoboni, I.; Casadio, R.; Prosperi, S. Analysis of canine parvovirus sequences from wolves and dogs isolated in Italy. J. Gen. Virol. 2001, 82 Pt 7, 1555–1560. [Google Scholar] [CrossRef]
- Sobrino, R.; Arnal, M.C.; Luco, D.F.; Gortazar, C. Prevalence of antibodies against canine distemper virus and canine parvovirus among foxes and wolves from Spain. Vet. Microbiol. 2008, 126, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Urbani, L.; Delogu, M.; Musto, C.; Fontana, M.C.; Merialdi, G.; Lucifora, G.; Terrusi, A.; Dondi, F.; Battilani, M. Integrated use of molecular techniques to detect and genetically characterise DNA viruses in Italian wolves (Canis lupus italicus). Animals 2021, 11, 2198. [Google Scholar] [CrossRef]
- Ferrara, G.; Brocherel, G.; Falorni, B.; Gori, R.; Pagnini, U.; Montagnaro, S. A retrospective serosurvey of selected pathogens in red foxes (Vulpes vulpes) in the Tuscany region, Italy. Acta Vet. Scand. 2023, 65, 35. [Google Scholar] [CrossRef]
- Frölich, K.; Streich, W.J.; Fickel, J.; Jung, S.; Truyen, U.; Hentschke, J.; Dedek, J.; Prager, D.; Latz, N. Epizootiologic investigations of parvovirus infections in free-ranging carnivores from Germany. J. Wildl. Dis. 2005, 41, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus infections in wild carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Kurucay, H.M.; Tamer, C.; Muftuoglu, B.; Elhag, A.E.; Gozel, S.; Cicek-Yildiz, Y.; Demirtas, S.; Ozan, E.; Albayrak, H.; Okur-Gumusova, S.; et al. First isolation and molecular characterization of canine parvovirus-type 2b (CPV-2b) from red foxes (Vulpes vulpes) living in the wild habitat of Turkey. Virol. J. 2023, 20, 27. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Buonavoglia, C. Canine adenovirus and herpesvirus. Veterinary Clinics of North America. J. Small Anim. Pract. 2008, 38, 799–814. [Google Scholar] [CrossRef]
- Pizzurro, F.; Marcacci, M.; Zaccaria, G.; Orsini, M.; Cito, F.; Rosamilia, A.; Di Renzo, L.; Malatesta, D.; Di Sabatino, D.; Lorusso, A. Genome sequence of canine Adenovirus type 1 isolated from a wolf (Canis lupus) in southern Italy. Genome Announc. 2017, 5, e00225-17. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Balseiro, A.; Espi, A.; Royo, L.J. Wolf (Canis lupus) as canine adenovirus type 1 (CAdV-1) sentinel for the endangered cantabrian brown bear (Ursus arctos arctos). Transbound. Emerg. Dis. 2022, 69, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Caiaffa, M.G.; da Costa, A.L.M.; Teixeira, R.H.F.; Ervedosa, T.B.; Machado, E.F.; Suárez, P.E.N.; Réssio, R.A.; Borges, C.C.; de Jesus, I.P.; et al. Canine distemper virus and canine adenovirus type 1 co-infection in a free-living hoary fox (Lycalopex vetulus) from Brazil. Braz. J. Microbiol. 2023, 54, 587–595. [Google Scholar] [PubMed]
- Junge, E.R.; Bauman, K.; King, M.; Gompper, M.E. A serologic assessment of exposure to viral pathogens and Leptospira in an urban raccoon (Procyon lotor) population inhabiting a large zoological park. J. Zoo Wildl. Med. 2007, 38, 18–26. [Google Scholar] [PubMed]
- Hou, J.; Xu, J.; Wang, B.; Zhang, H.; Yin, B.; Li, G.; Lei, F.; Cai, X.; Zhu, Y.; Wang, L. First identification of canine adenovirus 1 in mink and bioinformatics analysis of its 100 K protein. Front. Microbiol. 2023, 14, 1245581. [Google Scholar]
- García Marín, J.F.; Royo, L.J.; Oleaga, A.; Gayo, E.; Alarcia, O.; Pinto, D.; Martínez, I.Z.; González, P.; Balsera, R.; Marcos, J.L.; et al. Canine adenovirus type 1 (CAdV-1) in free-ranging European brown bear (Ursus arctos arctos): A threat for Cantabrian population? Transbound. Emerg. Dis. 2018, 65, 2049–2056. [Google Scholar] [PubMed]
- Green, R.G.; Ziegler, N.R.; Green, B.B.; Dewey, E.T. Epizootic fox encephalitis. Am. J. Trop. Med. Hyg. 1930, 12, 109–129. [Google Scholar]
- Balboni, A.; Verin, R.; Morandi, F.; Poli, A.; Prosperi, S.; Battilani, M. Molecular epidemiology of canine adenovirus type 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Vet. Microbiol. 2013, 162, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Fee, S.A.; Hartley, G.; Learmount, J.; O’Hagan, M.J.H.; Meredith, A.L.; de C. Bronsvoort, B.M.; Porphyre, T.; Sharp, C.P.; Philbey, A.W. Serological and molecular epidemiology of canine adenovirus type 1 in red foxes (Vulpes vulpes) in the United Kingdom. Sci. Rep. 2016, 6, 36051. [Google Scholar] [CrossRef]
- Di Francesco, C.E.; Smoglica, C.; Paoletti, B.; Angelucci, S.; Innocenti, M.; Antonucci, A.; Di Domenico, G.; Marsilio, F. Detection of selected pathogens in Appennine wolf (Canis lupus italicus) by a non-invasive GPS-based telemetry sampling of two packs from Majella National Park, Italy. J. Wildl. Res. 2019, 65, 84. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.Y.; Kim, Y.S.; Na, E.J.; Park, J.S.; Oem, J.K. Genetic characteristics of canine adenovirus type 2 detected in wild raccoon dogs (Nyctereutes procyonoides) in Korea. Vet. Sci. 2022, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Dubovi, E.J.; Henriquez-Rivera, J.A.; Lipkin, W.I. Complete genome sequence of the first Canine circovirus. J. Virol. 2012, 86, 7018. [Google Scholar] [CrossRef] [PubMed]
- Urbani, L.; Tryland, M.; Ehrich, D.; Fuglei, E.; Battilani, M.; Balboni, A. Ancient origin and genetic segregation of canine circovirus infecting arctic foxes (Vulpes lagopus) in Svalbard and red foxes (Vulpes vulpes) in Northern Norway. Transbound. Emerg. Dis. 2021, 68, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Zaccaria, G.; Malatesta, D.; Scipioni, G.; Di Felice, E.; Campolo, M.; Casaccia, C.; Savini, G.; Di Sabatino, D.; Lorusso, A. Circovirus in domestic and wild carnivores: An important opportunistic agent? Virology 2016, 490, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Martella, V.; Desario, C.; Lanave, G.; Circella, E.; Cavalli, A.; Elia, G.; Camero, M.; Buonavoglia, C. Genomic characterization of a circovirus associated with fatal hemorrhagic enteritis in dog, Italy. PLoS ONE 2014, 9, e105909. [Google Scholar] [CrossRef] [PubMed]
- Bexton, S.; Wiersma, L.C.; Getu, S.; Van Run, P.R.; Verjans, G.M.G.M.; Schipper, D.; Schapendonk, C.M.E.; Bodewes, R.; Oldroyd, L.; Haagmans, B.L.; et al. Detection of circovirus in foxes with meningoencephalitis, United Kingdom, 2009–2013. Emerg. Infect. Dis. 2015, 21, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Origgi, F.C.; Plattet, P.; Sattler, U.; Robert, N.; Casaubon, J.; Mavrot, F.; Pewsner, M.; Wu, N.; Giovannini, S.; Oevermann, A.; et al. Emergence of Canine Distemper Virus strains with modified molecular signature and enhanced neuronal tropism leading to high mortality in wild carnivores. Vet. Pathol. 2012, 49, 0300985812436743. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Baumgärtner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus-an update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, N.; Sun, Y.; Martella, V.; Nikolin, V.; Zhu, C.; Zhang, H.; Hu, B.; Bai, X.; Yan, X. Pathogenesis of canine distemper virus in experimentally infected raccoon dogs, foxes, and minks. Antivir. Res. 2015, 122, 1–11. [Google Scholar] [CrossRef]
- Martella, V.; Lucente, M.S.; Cirone, F.; Lorusso, E.; Elia, G.; Camero, M.; Buonavoglia, C. Canine distemper virus (CDV): From the arctic ecosystem to Italy. Veterinaria 2007, 21, 31–38. [Google Scholar]
- Di Sabatino, D.; Lorusso, A.; Di Francesco, C.E.; Gentile, L.; Di Pirro, V.; Bellacicco, A.L.; Giovannini, A.; Di Francesco, G.; Marruchella, G.; Marsilio, F.; et al. Arctic lineage-Canine Distemper Virus as a cause of death in Appenine wolves (Canis lupus) in Italy. PLoS ONE 2014, 9, e82356. [Google Scholar] [CrossRef]
- Bianco, A.; Zecchin, B.; Fusaro, A.; Schivo, A.; Ormelli, S.; Bregoli, M.; Citterio, C.V.; Obber, F.; Dellamaria, D.; Trevisiol, K.; et al. Two waves of canine distemper virus showing different spatio-temporal dynamics in Alpine wildlife (2006–2018). Infect. Genet. Evol. 2020, 84, 104359. [Google Scholar] [CrossRef]
- Kličková, E.; Černíková, L.; Dumondin, A.; Bártová, E.; Budíková, M.; Sedlák, K. Canine distemper virus in wild carnivore populations from the Czech Republic (2012–2020): Occurrence, geographical distribution, and phylogenetic analysis. Life 2022, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Monne, I.; Fusaro, A.; Valastro, V.; Citterio, C.; Dalla Pozza, M.; Obber, F.; Trevisiol, K.; Cova, M.; De Benedictis, P.; Bregoli, M.; et al. A distinct CDV genotype causing a major epidemic in Alpine wildlife. Vet. Microbiol. 2011, 150, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, D.; Di Francesco, G.; Zaccaria, G.; Malatesta, D.; Brugnola, L.; Marcacci, M.; Portanti, O.; De Massis, F.; Savini, G.; Teodori, L.; et al. Lethal distemper in badgers (Meles meles) following epidemic in dogs and wolves. Infect. Genet. Evol. 2016, 46, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Savini, F.; Scagliarini, A.; Berti, E.; Naldi, M.; Urbani, L.; Fontana, M.C.; Carra, E.; Gibelli, L.R.M.; Gobbo, F.; et al. Natural distemper infection in stone martens (Martes foina): From infection to neutralizing antibodies. Res. Vet. Sci. 2021, 138, 196–200. [Google Scholar] [CrossRef]
- Kadam, R.G.; Karikalan, M.; Siddappa, C.M.; Mahendran, K.; Srivastava, G.; Rajak, K.K.; Bhardwaj, Y.; Varshney, R.; War, Z.A.; Singh, R.; et al. Molecular and pathological screening of canine distemper virus in Asiatic lions, tigers, leopards, snow leopards, clouded leopards, leopard cats, jungle cats, civet cats, fishing cat, and jaguar of different states, India. Infect. Genet. Evol. 2022, 98, 105211. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Cilia, G.; Bertelloni, F.; Fratini, F. Leptospira infections in domestic and wild animals. Pathogens 2020, 9, 573. [Google Scholar] [CrossRef]
- Tan, C.G.; Dharmarajan, G.; Beasley, J.; Rhodes, O., Jr.; Moore, G.; Wu, C.C.; Lin, T.L. Neglected leptospirosis in raccoons (Procyon lotor) in Indiana, USA. Vet. Quart. 2014, 34, 1–10. [Google Scholar] [CrossRef]
- López, M.C.; Vila, A.; Rodón, J.; Roura, X. Leptospira seroprevalence in owned dogs from Spain. Heliyon 2019, 5, e02373. [Google Scholar] [CrossRef] [PubMed]
- Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Bellinati, L.; Mazzucato, M.; Furlanello, T.; D’Incau, M.; Natale, A. Detection of new Leptospira genotypes infecting symptomatic dogs: Is a new vaccine formulation needed? Pathogens 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Roquelo, C.; Kodjo, A.; Marié, J.L.; Davoust, B. Serological and molecular survey of Leptospira spp. infections in wild boars and red foxes from Southeastern France. Vet. World 2021, 14, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.I.; Goarant, C.; Picardeau, M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 2009, 7, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Zamagni, S.; Bertasio, C.; Boniotti, M.B.; Troìa, R.; Battilani, M.; Dondi, F. Identification of serogroups Australis and icterohaemorrhagiae in two dogs with a severe form of acute Leptospirosis in Italy. Pathogens 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, J.; Lillehaug, A.; Larsen, I.L.; Eide, N.E.; Arnemo, J.M.; Handeland, K. Serosurvey for Canine Distemper Virus, Canine Adenovirus, Leptospira interrogans and Toxoplasma gondii in free-ranging canids in Scandinavia and Svalbard. J. Wildl. Dis. 2010, 46, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Bellinati, L.; Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Martignago, F.; Leopardi, S.; Natale, A. Synanthropic and wild animals as sentinels of zoonotic agents: A study of Leptospira genotypes circulating in northeastern Italy. Int. J. Environ. Res. Public Health 2023, 20, 3783. [Google Scholar] [CrossRef]
- Regione Emilia Romagna Sorveglianza e Monitoraggio della Fauna Selvatica. Available online: https://servizissiir.regione.emilia-romagna.it/deliberegiunta/servlet/AdapterHTTP?action_name=ACTIONRICERCADELIBERE&operation=dettaglioByDatiAdozione&ENTE=1&TIPO_ATTO=DL&ANNO_ADOZIONE=2017&NUM_ADOZIONE=1763 (accessed on 20 November 2023).
- Troìa, R.; Balboni, A.; Zamagni, S.; Frigo, S.; Magna, L.; Perissinotto, L.; Battilani, M.; Dondi, F. Prospective evaluation of rapid point-of-care tests for the diagnosis of acute leptospirosis in dogs. Vet. J. 2018, 237, 37–42. [Google Scholar] [CrossRef]
- Scagliarini, A.; Dal Pozzo, F.; Gallina, L.; Vaccari, F.; Morganti, L. TaqMan based real time PCR for the quantification of canine distemper virus. Vet. Res. Commun. 2007, 31, 261–263. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A single multilocus sequence typing (MLST) scheme for seven pathogenic Leptospira species. PLoS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Menezes, A.; Woods, K.; Chanthongthip, A.; Dittrich, S.; Opoku-Boateng, A.; Kimuli, M.; Chalker, V. An extended multilocus sequence typing (MLST) scheme for rapid direct typing of Leptospira from clinical samples. PLoS Negl. Trop. Dis. 2016, 10, e0004996. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 24, 124. [Google Scholar] [CrossRef]
- Niu, L.; Wang, Z.; Zhao, L.; Wang, Y.; Cui, X.; Shi, Y.; Chen, H.; Ge, J. Detection and molecular characterization of canine circovirus circulating in northeastern China during 2014–2016. Arch. Virol. 2020, 165, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Thaiwong, T.; Wise, A.G.; Maes, R.K.; Mullaney, T.; Kiupel, M. Canine circovirus 1 (CaCV-1) and canine parvovirus 2 (CPV-2): Recurrent dual infections in a papillon breeding colony. Vet. Pathol. 2016, 53, 1204–1209. [Google Scholar] [CrossRef]
- Faraji, R.; Sadeghi, M.; Mozhgani, S.H.; Vasinioti, V.; Ndiana, L.A.; Desario, C.; Beikpour, F.; Decaro, N. Detection of canine circovirus in dogs infected with canine parvovirus. Acta Tropica 2022, 235, 106646. [Google Scholar] [CrossRef]
- da Rocha Gizzi, A.B.; Oliveira, S.T.; Leutenegger, C.M.; Estrada, M.; Kozemjakin, D.A.; Stedile, R.; Marcondes, M.; Biondo, A.W. Presence of infectious agents and co-infections in diarrheic dogs determined with a real-time polymerase chain reaction-based panel. BMC Vet. Res. 2014, 10, 23. [Google Scholar]
- Anderson, A.; Hartmann, K.; Leutenegger, C.M.; Proksch, A.L.; Mueller, R.S.; Unterer, S. Role of canine circovirus in dogs with acute haemorrhagic diarrhoea. Vet. Rec. 2017, 180, 542. [Google Scholar] [CrossRef]
- Grassi, L.; Menandro, M.L.; Obber, F.; Drigo, M.; Legnardi, M.; Pasotto, D.; Tucciarone, C.M.; Faustini, G.; Citterio, C.; Cecchinato, M.; et al. Investigation of Carnivore protoparvovirus 1 and Amdoparvovirus infections in red fox populations of the Italian Dolomites. Vet. Res. Commun. 2022, 46, 1291–1295. [Google Scholar] [CrossRef]
- Han, S.C.; Guo, H.C.; Sun, S.Q.; Shu, L.; Wei, Y.Q.; Sun, D.H.; Cao, S.Z.; Peng, G.N.; Liu, X.T. Full-length genomic characterizations of two canine parvoviruses prevalent in Northwest China. Arch. Microbiol. 2015, 197, 621–626. [Google Scholar] [CrossRef]
- Battilani, M.; Balboni, A.; Ustulin, M.; Giunti, M.; Scagliarini, A.; Prosperi, S. Genetic complexity and multiple infections with more Parvovirus species in naturally infected cats. Vet. Res. 2011, 42, 43. [Google Scholar] [CrossRef]
- Domingo, E. Rapid evolution of viral RNA genomes. J. Nutr. 1997, 127 (Suppl. 5), 958S–961S. [Google Scholar] [CrossRef]
- Truyen, U.; Müller, T.; Heidrich, R.; Tackmann, K.; Carmichael, L.E. Survey on viral pathogens in wild red foxes (Vulpes vulpes) in Germany with emphasis on parvoviruses and analysis of a DNA sequence from a red fox parvovirus. Epidemiol. Infect. 1998, 121, 433–440. [Google Scholar] [CrossRef]
- Allison, A.B.; Harbison, C.E.; Pagan, I.; Stucker, K.M.; Kaelber, J.T.; Brown, J.D.; Ruder, M.G.; Keel, M.K.; Dubovi, E.J.; Holmes, E.C.; et al. Role of multiple hosts in the cross-species transmission and emergence of a pandemic parvovirus. J. Virol. 2012, 86, 865–872. [Google Scholar] [CrossRef]
- Franzo, G.; Menandro, M.L.; Grassi, L. Canine circovirus in foxes from northern Italy: Where did it all begin? Pathogens 2021, 10, 1002. [Google Scholar] [CrossRef]
- Ndiana, L.A.; Lanave, G.; Vasinioti, V.; Desario, C.; Martino, C.; Colaianni, M.L.; Pellegrini, F.; Camarda, A.; Berjaoui, S.; Sgroi, G.; et al. Detection and genetic characterization of canine adenoviruses, circoviruses, and novel cycloviruses from wild carnivores in Italy. Front. Vet. Sci. 2022, 9, 851987. [Google Scholar] [CrossRef]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European consensus statement on leptospirosis in dogs and cats. J. Small Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef]
- Clark, L.G.; Kresse, J.I.; Marshak, R.R.; Hollister, C.J. Leptospira pomona infection in an Eastern red fox (Vulpes fulva fulva). Nature 1960, 188, 1040–1041. [Google Scholar] [CrossRef]
- Alić, A.; Šupić, J.; Goletić, T.; Rešidbegović, E.; Lutvikadić, I.; Hodžić, A. A unique case of fatal coinfection caused by Leptospira spp. and Hepatozoon canis in a red fox cub (Vulpes vulpes). Pathogens 2021, 11, 11. [Google Scholar] [CrossRef]
- Huber, D.; Habuš, J.; Turk, N.; Vinicki, K.; Šoštarić-Zuckermann, I.C. Acute lethal leptospirosis in a red fox (Vulpes vulpes). J. Comp. Pathol. 2023, 201, 77–80. [Google Scholar] [CrossRef]
- Müller, H.; Winkler, P. Ergebnisse serologischer untersuchungen auf Leptospira-antikörper bei Füchsen [Results of serological studies of Leptospira antibodies in foxes]. Berl. Münch. Tierärztl. Wochenschr. 1994, 107, 90–93. [Google Scholar]
- Milas, Z.; Turk, N.; Janicki, Z.; Slavica, A.; Starešina, V.; Barbić, L.J.; Lojkić, M.; Modrić, Z. Leptospiral antibodies in red foxes (Vulpes vulpes) in northwest Croatia. Vet. Arhiv. 2006, 76, 51–57. [Google Scholar]
- Millán, J.; Candela, M.G.; López-Bao, J.V.; Pereira, M.; Jiménez, M.A.; León-Vizcaíno, L. Leptospirosis in wild and domestic carnivores in natural areas in Andalusia, Spain. Vector-Borne Zoonotic Dis. 2009, 9, 549–554. [Google Scholar] [CrossRef]
- Slavica, A.; Dezdek, D.; Konjevic, D.; Cvetnic, Z.; Sindicic, M.; Stanin, D.; Habus, J.; Turk, N. Prevalence of leptospiral antibodies in the red fox (Vulpes vulpes) population of Croatia. Vet. Med. 2011, 56, 209–213. [Google Scholar] [CrossRef]
- Tagliabue, S.; Figarolli, B.M.; D’Incau, M.; Foschi, G.; Gennero, M.S.; Giordani, R.; Natale, A.; Papa, P.; Ponti, N.; Scaltrito, D.; et al. Serological surveillance of Leptospirosis in Italy: Two-year national data (2010–2011). Vet. Ital. 2016, 52, 129–138. [Google Scholar]
- Żmudzki, J.; Arent, Z.; Jabłoński, A.; Nowak, A.; Zębek, S.; Stolarek, A.; Bocian, Ł.; Brzana, A.; Pejsak, Z. Seroprevalence of 12 serovars of pathogenic Leptospira in red foxes (Vulpes vulpes) in Poland. Acta Vet. Scand. 2018, 60, 34. [Google Scholar] [CrossRef]
- Žele-Vengušt, D.; Lindtner-Knific, R.; Mlakar-Hrženjak, N.; Jerina, K.; Vengušt, G. Exposure of free-ranging wild animals to zoonotic Leptospira interrogans sensu stricto in Slovenia. Animals 2021, 11, 2722. [Google Scholar] [CrossRef]
- Kuhnert, P.; Brodard, I.; Ackermann, S.; Schierack, P.; Jores, J. Serological and molecular detection as well as typing of Leptospira spp. in foxes, raccoons, and other wild carnivores in North-Eastern Germany, 2021–2022. Heliyon 2023, 10, e23268. [Google Scholar] [CrossRef]
- Straub, M.H.; Foley, J.E. Cross-sectional evaluation of multiple epidemiological cycles of Leptospira species in peri-urban wildlife in California. J. Am. Vet. Med. Assoc. 2020, 257, 840–848. [Google Scholar] [CrossRef]
- Piredda, I.; Scarpa, F.; Sanna, D.; Casu, M.; Ponti, M.N.; Chisu, V. Draft genome sequences of four different strains belonging to Leptospira interrogans serovar Pomona isolated from mammals in the island of Sardinia, Italy. Microbiol. Resour. Announc. 2021, 10, e0069821. [Google Scholar] [CrossRef]
- Ebani, V.V.; Trebino, C.; Guardone, L.; Bertelloni, F.; Cagnoli, G.; Nardoni, S.; Sel, E.; Wilde, E.; Poli, A.; Mancianti, F. Occurrence of bacterial and protozoan pathogens in red foxes (Vulpes vulpes) in central Italy. Animals 2022, 12, 2891. [Google Scholar] [CrossRef]
- Helman, S.K.; Tokuyama, A.F.N.; Mummah, R.O.; Stone, N.E.; Gamble, M.W.; Snedden, C.E.; Borremans, B.; Gomez, A.C.R.; Cox, C.; Nussbaum, J.; et al. Pathogenic Leptospira are widespread in the urban wildlife of southern California. Sci. Rep. 2023, 13, 14368. [Google Scholar] [CrossRef]
- Boniotti, M.B.; Gelmini, L.; Carra, E.; Figarolli, B.M.; D’incau, M.; Tagliabue, S. Leptospira Interrogans serogroup Australis in hedgehog in Northern Italy. In Proceedings of the International Leptospirosis Society of the Conference, Fukuoka, Japan, 7–11 October 2013. [Google Scholar]
- Trogu, T.; Canziani, S.; Salvato, S.; Bianchi, A.; Bertoletti, I.; Gibelli, L.R.; Alborali, G.L.; Barbieri, I.; Gaffuri, A.; Sala, G.; et al. Canine distemper outbreaks in wild carnivores in Northern Italy. Viruses 2021, 13, 99. [Google Scholar] [CrossRef]
- Ricci, I.; Cersini, A.; Manna, G.; Marcario, G.A.; Conti, R.; Brocherel, G.; Grifoni, G.; Eleni, C.; Scicluna, M.T. A canine distemper virus retrospective study conducted from 2011 to 2019 in Central Italy (Latium and Tuscany regions). Viruses 2021, 13, 272. [Google Scholar] [CrossRef]
- Trogu, T.; Castelli, A.; Canziani, S.; Tolini, C.; Carrera, M.; Sozzi, E.; Lelli, D.; Tosi, G.; Fiorentini, L.; Di Donato, A.; et al. Detection and molecular characterization of canine distemper virus in wildlife from Northern Italy. Pathogens 2022, 11, 1557. [Google Scholar] [CrossRef]
| Total | PPVC-1 | CAdV | CanineCV | CDV | Leptospira spp. | Total of Positive Red Foxes | p Value | |
|---|---|---|---|---|---|---|---|---|
| Number of foxes | 126 | 20/126 (15.9; 10.5–23.3) | 3/126 (2.4; 0.8–6.7) | 20/126 (15.9; 10.5–23.3) | 0/126 (0) | 2/126 (1.6; 0.4–5.6) | 39/126 (30.9; 23.5–39.5) | |
| Sex | ||||||||
| Male | 62/126 (49.3) | 9/62 (14.5; 7.8–25.3) | 1/62 (1.6; 0.3–8.6) | 8/62 (12.9; 6.7–23.5) | 0/62 (0) | 2/62 (3.2; 0.9–11) | 18/62 (29; 19.2–41.3) | 0.56 |
| Female | 56/126 (44.4) | 10/56 (17.9; 10–29.8) | 2/56 (3.6; 0.9–12.1) | 12/56 (21.4; 12.7–33.8) | 0/56 (0) | 0/56 (0) | 20/56 (35.7; 24.5–48.8) | |
| NA | 8/126 (6.3) | 1/8 (12.5; 2.2–47.1) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 1/8 (12.5; 2.2–47.1) | |
| Age | ||||||||
| Young (<1 year old) | 28/126 (22.2) | 7/28 (25; 12.7–43.4) | 0/28 (0) | 4/28 (14.3; 5.7–31.5) | 0/28 (0) | 0/28 (0) | 10/28 (35.7; 20.7–54.2) | 0.89 |
| Adult (≥1 year old) | 57/126 (45.2) | 11/57 (19.3; 11.1–31.3) | 2/57 (3.5; 0.9–11.9) | 13/57 (22.8; 13.8–35.2) | 0/57 (0) | 0/57 (0) | 21/57 (36.8; 25.5–49.8) | |
| NA | 41/126 (32.6) | 2/41 (4.9; 1.4–16.1) | 1/41 (2.4; 0.4–12.6) | 3/41 (7.3; 2.5–19.4) | 0/41 (0) | 2/41 (4.9; 1.4–16.1) | 8/41 (19.5; 10.2–34) | |
| Geographical origin | ||||||||
| Bologna | 55/126 (43.6) | 12/55 (21.8; 12.9–34.4) | 0/55 (0) | 3/55 (5.5; 1.9–14.9) | 0/55 (0) | 0/55 (0) | 15/55 (27.3; 17.3–40.2) | 0.17 |
| Modena | 66/126 (52.4) | 8/66 (12.1; 6.3–22.1) | 3/66 (4.5; 1.6–12.5) | 17/66 (25.8; 16.8–37.4) | 0/66 (0) | 2/66 (3; 1.4–16.1) | 24/66 (36.4; 25.8–48.4) | |
| Ferrara | 5/126 (4) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 0/5 (0) |
| Red Fox | ST | glmU | pntA | pfkB | caiB | mreA | sucA | tpiA |
|---|---|---|---|---|---|---|---|---|
| 1805/2022 | 198 | 1 | 66 | 5 | 4 | ND | 2 | 1 |
| 1806/2022 | 198 | 1 | 66 | 5 | 4 | 3 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magliocca, M.; Taddei, R.; Urbani, L.; Bertasio, C.; Facile, V.; Gallina, L.; Sampieri, M.; Rugna, G.; Rubini, S.; Maioli, G.; et al. Molecular Detection of Viral and Bacterial Pathogens in Red Foxes (Vulpes vulpes) from Italy. Animals 2024, 14, 1969. https://doi.org/10.3390/ani14131969
Magliocca M, Taddei R, Urbani L, Bertasio C, Facile V, Gallina L, Sampieri M, Rugna G, Rubini S, Maioli G, et al. Molecular Detection of Viral and Bacterial Pathogens in Red Foxes (Vulpes vulpes) from Italy. Animals. 2024; 14(13):1969. https://doi.org/10.3390/ani14131969
Chicago/Turabian StyleMagliocca, Martina, Roberta Taddei, Lorenza Urbani, Cristina Bertasio, Veronica Facile, Laura Gallina, Maria Sampieri, Gianluca Rugna, Silva Rubini, Giulia Maioli, and et al. 2024. "Molecular Detection of Viral and Bacterial Pathogens in Red Foxes (Vulpes vulpes) from Italy" Animals 14, no. 13: 1969. https://doi.org/10.3390/ani14131969





