Calculation of a Reference Interval for Rectal Temperature in Adult Dogs Presenting for Veterinary Care Using an Algorithm for Mixed Data
Abstract
Simple Summary
Abstract
1. Introduction
| Study | Number of Dogs | Health Status | Rectal Temperature | Aim of the Study |
|---|---|---|---|---|
| Veronesi et al., 2002 [17] | 7 | healthy, pregnant | at the onset of whelping: 37.6 ± 0.7 °C. After 12 h 38.3 ± 0.9 °C, 24 and 36 h after the onset of whelping (38.7 ± 0.3 and 38.5 ± 0.2 °C). | Correlation of temperature, progesterone, cortisol and prostaglandin of the periparturient bitch |
| Refinetti and Piccione, 2003 [18] | 7 | healthy | mean 39.1 °C, SD 0.01 °C | Daily rhythmicity of body temperature |
| Sousa et al., 2011 [19] | 88 | healthy | median 38.8 °C, SD 0.4 °C | Comparison between auricular and rectal temperature measurements |
| Lamb and McBrearty, 2013 [3] | 212 | unknown | median 38.0 °C, mean 37.9 °C, range 33.9–40.4 °C | Comparison of rectal, auricular and axillary temperature measurements |
| Goic et al., 2014 [20] | 94 | unknown | median 38.9 °C, range 36–40.8 °C | Comparison of rectal and axillary temperature measurements |
| Gomart et al., 2014 [21] | 250 | hospitalized | median 38.0 °C, range 35.0–40.4 °C, SD 0.85 °C | Comparison of rectal, auricular and axillary temperature measurement and associated stress response in hospitalized dogs |
| Konietschke et al., 2014 [22] | 238 | healthy and diseased | mean 38.1 °C, reference range 37.2–39.2 °C, 95% CI 38.0–38.3 °C | Comparison of auricular and rectal temperature measurement in normothermic, hypothermic and hyperthermic dogs |
| Osinchuk et al., 2014 [23] | 12 | healthy | mean 38.7 °C, range 37.6–39.5 °C, SD 0.37 °C | Comparison of ingestible temperature sensor and rectal temperature measurements |
| Kreissl and Neiger, 2015 [24] | 300 | healthy and diseased | median 38.3 °C, range 35.5–41.1 °C, 95% CI 38.2–38.4 °C | Comparison of ocular and rectal temperature measurement |
| Yanmaz et al., 2015 [25] | 30 | healthy | mean 38.0 °C, 95% CI, lower bound (°C): 37.8, upper bound (°C): 38.2, SD 0.12 °C | Comparison of rectal, ocular and auricular temperature measurements |
| Zanghi, 2016 [26] | 32 | healthy | median 38.0 °C ± 0.5 °C at rest, 39.7 °C ± 0.9 °C during exercise | Comparison of ocular, auricular and rectal temperature measurement at rest or with exercise |
| Cichocki et al., 2017 [27] | 50 | healthy | mean 38.2 °C (SD 0.88 °C) | Comparison of axillary, auricular and rectal temperature measurement in healthy dogs |
| Hall and Carter, 2017 [28] | 24 | healthy | median 38.3 °C, mean 38.3 °C, range 37.4–39.1 °C, SD 0.39 °C | Comparison of rectal and auricular temperature measurement in healthy exercising dogs |
| Cugmas et al., 2020 [29] | 204 | unknown | mean 38.1 °C, SD 1.0 °C; | Comparison between rectal and body surface temperature in dogs |
2. Materials and Methods
2.1. Selection Criteria
2.2. Statistical Analysis
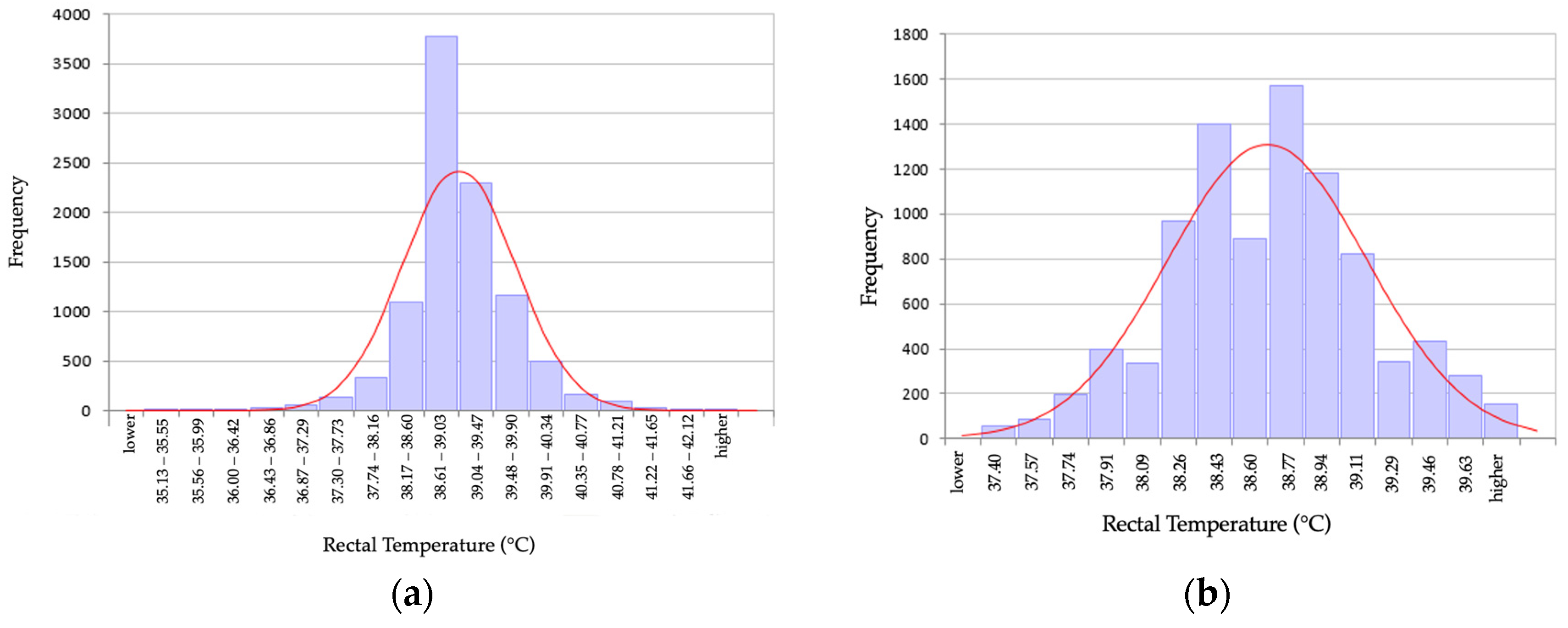
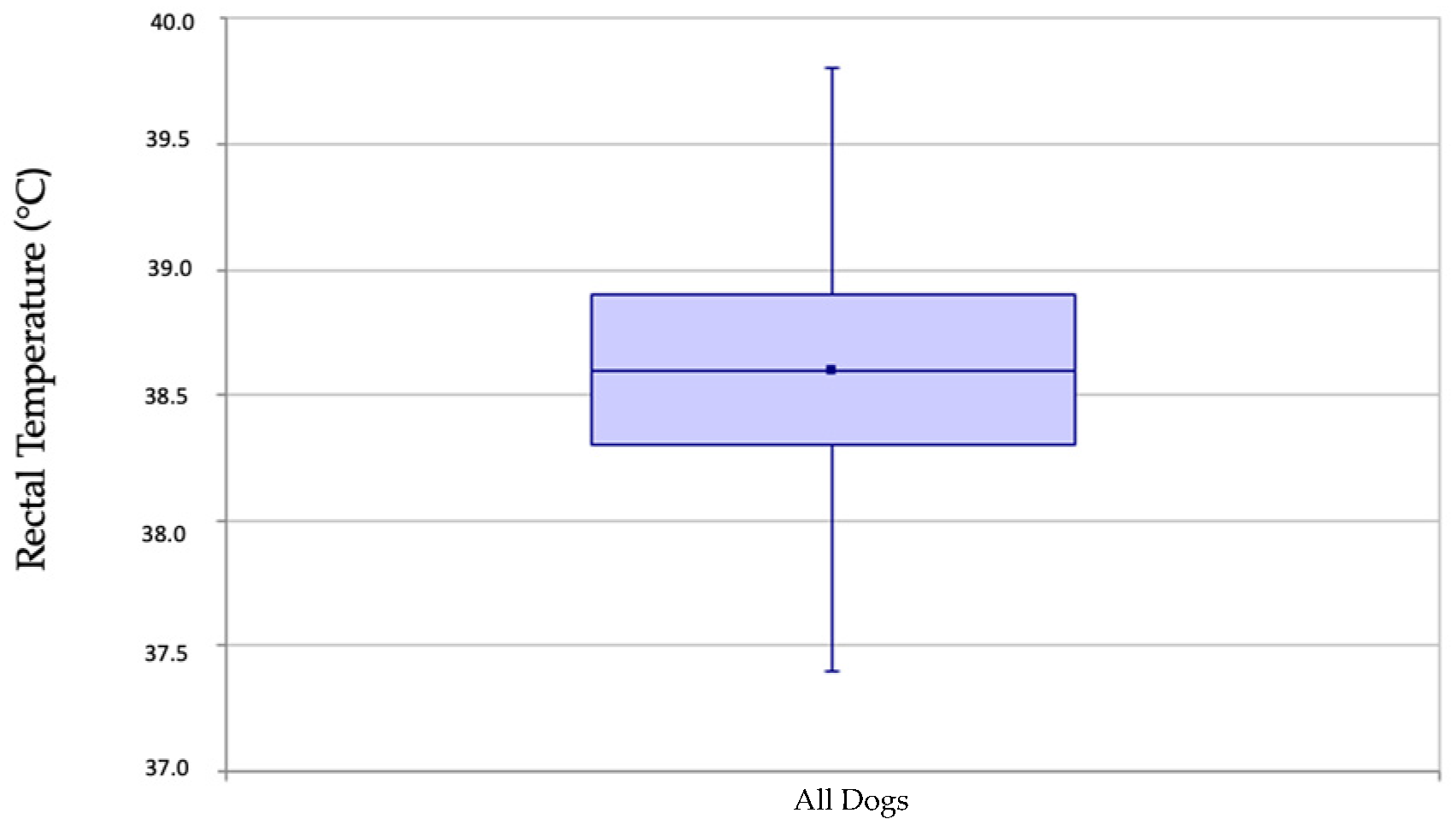
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramsey, I.; Tasker, S. Section III: Differential diagnosis for physical examination abnormalities, Chapter 48: Fever. In Textbook of Veterinary Internal Medicine; Ettinger, S., Feldman, E., Cote, E., Eds.; Elsevier: St. Louis, MO, USA, 2017. [Google Scholar]
- Lee, J. Section III: Differential diagnosis for physical examination abnormalities, Chapter 49: Hypothermia. In Textbook of Veterinary Internal Medicine; Ettinger, S., Feldman, E., Cote, E., Eds.; Elsevier: St. Loius, MO, USA, 2017. [Google Scholar]
- Lamb, V.; McBrearty, A.R. Comparison of rectal, tympanic membrane and axillary temperature measurement methods in dogs. Vet. Rec. 2013, 173, 524. [Google Scholar] [CrossRef]
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef]
- Reece, W.O. Body temperature and its regulation. In Dukes’ Physiology of Domestic Animals; Reece, W.O., Erickson, H.H., Goff, J.P., Uemura, E.E., Eds.; Cornell University Press: Ithaca, NY, USA, 2015; pp. 149–155. [Google Scholar]
- Gerber, B. Hyperthermie, Fieber, Fieber unbekannter Ursache. In Praktikum der Hundeklinik; Kohn, B., Schwarz, G., Niemand, H.H., Eds.; Enke: Stuttgart, Germany, 2017; pp. 110–114. [Google Scholar]
- Friedrichs, K.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP Reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef]
- Grasbeck, R.; Fellman, J. Normal values and statistics. Scand. J. Clin. Lab. Investig. 1968, 21, 193–195. [Google Scholar] [CrossRef]
- Lumsden, J.H.; Mullen, K. On establishing reference values. Can. J. Comp. Med. 1978, 42, 293–301. [Google Scholar]
- Solberg, H. Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin. Chim. Acta 1987, 170, S13–S32. [Google Scholar] [CrossRef]
- Bhattacharya, C. A simple method of resolution of a distribution into gaussian components. Biom. J. 1967, 23, 115–135. [Google Scholar] [CrossRef]
- Martinez-Sanchez, L.; Marques-Garcia, F.; Ozarda, Y.; Blanco, A.; Brouwer, N.; Canalias, F.; Cobbaert, C.; Thelen, M.; den Elzen, W. Big data and reference intervals: Rationale, current practices, harmonization and standardization prerequisites and future perspectives of indirect determination of reference intervals using routine data. Adv. Lab. Med. 2020, 2, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lottati, M.; Aucoin, D.; Bruyette, D.S. Expected total thyroxine (TT4) concentrations and outlier values in 531,765 cats in the United States (2014–2015). PLoS ONE 2019, 14, e0213259. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J. Exploratory Data Analysis; Addison-Wesley: Boston, MA, USA, 1977. [Google Scholar]
- Geffré, A.; Friedrichs, K.; Harr, K.; Concordet, D.; Trumel, C.; Braun, J.P. Reference values: A review. Vet. Clin. Pathol. 2009, 38, 288–298. [Google Scholar] [CrossRef]
- Zierk, J.; Arzideh, F.; Kapsner, L.A.; Prokosch, H.U.; Metzler, M.; Rauh, M. Reference interval estimation from mixed distributions using truncation points and the kolmogorov-smirnov distance (kosmic). Sci. Rep. 2020, 10, 1704. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Battocchio, M.; Marinelli, L.; Faustini, M.; Kindahl, H.; Cairoli, F. Correlations among body temperature, plasma progesterone, cortisol and prostaglandin F2alpha of the periparturient bitch. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R.; Piccione, G. Daily rhythmicity of body temperature in the dog. J. Vet. Med. Sci. 2003, 65, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.G.; Carareto, R.; Pereira-Junior, V.A.; Aquino, M.C. Comparison between auricular and standard rectal thermometers for the measurement of body temperature in dogs. Can. Vet. J. 2011, 52, 403–406. [Google Scholar] [PubMed]
- Goic, J.B.; Reineke, E.L.; Drobatz, K.J. Comparison of rectal and axillary temperatures in dogs and cats. J. Am. Vet. Med. Assoc. 2014, 244, 1170–1175. [Google Scholar] [CrossRef]
- Gomart, S.B.; Allerton, F.J.; Gommeren, K. Accuracy of different temperature reading techniques and associated stress response in hospitalized dogs. J. Vet. Emerg. Crit. Care 2014, 24, 279–285. [Google Scholar] [CrossRef]
- Konietschke, U.; Kruse, B.D.; Müller, R.; Stockhaus, C.; Hartmann, K.; Wehner, A. Comparison of auricular and rectal temperature measurement in normothermic, hypothermic, and hyperthermic dogs. Tierarztl Prax Ausg K Kleintiere Heimtiere 2014, 42, 13–19. [Google Scholar]
- Osinchuk, S.; Taylor, S.M.; Shmon, C.L.; Pharr, J.; Campbell, J. Comparison between core temperatures measured telemetrically using the CorTemp(R) ingestible temperature sensor and rectal temperature in healthy labrador retrievers. Can. Vet. J. 2014, 55, 939–945. [Google Scholar] [PubMed]
- Kreissl, H.; Neiger, R. Measurement of body temperature in 300 dogs with a novel noncontact infrared thermometer on the cornea in comparison to a standard rectal digital thermometer. J. Vet. Emerg. Crit. Care 2015, 25, 372–378. [Google Scholar] [CrossRef]
- Yanmaz, L.; Doğan, E.; Okumuș, Z.; Șenocak, M.G.; Yıldırım, F. Comparison of rectal, eye and ear temperatures in kangal breed dogs. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 615–617. [Google Scholar]
- Zanghi, B.M. Eye and ear temperature using infrared thermography are related to rectal temperature in dogs at rest or with exercise. Front. Vet. Sci. 2016, 3, 111. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, B.; Dugat, D.; Payton, M. Agreement of axillary and auricular temperature with rectal temperature in systemically healthy dogs undergoing surgery. J. Am. Anim. Hosp. Assoc. 2017, 53, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Carter, A.J. Comparison of rectal and tympanic membrane temperature in healthy exercising dogs. Comp. Exerc. Physiol. 2017, 13, 37–44. [Google Scholar] [CrossRef]
- Cugmas, B.; Šušterič, P.; Gorenjec, N.R.; Plavec, T. Comparison between rectal and body surface temperature in dogs by the calibrated infrared thermometer. Vet. Anim. Sci. 2020, 9, 100120. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.; Senthamarai, K. Comparison of methods for detecting outliers. Int. J. Sci. Eng. Res. 2013, 4, 709–714. [Google Scholar]
- Arzideh, F.; Brandhorst, G.; Gurr, E.; Hinsch, W.; Hoff, T.; Roggenbuck, L.; Rothe, G.; Schumann, G.; Wolters, B.; Wosniok, W.; et al. An improved indirect approach for determining reference limits from intra-laboratory data bases exemplified by concentrations of electrolytes/Ein verbesserter indirekter Ansatz zur Bestimmung von Referenzgrenzen mittels intra-laboratorieller Datensätze am Beispiel von Elektrolyt-Konzentrationen. J. Lab. Med. 2009, 33, 52–66. [Google Scholar]
- Arzideh, F.; Wosniok, W.; Haeckel, R. Reference limits of plasma and serum creatinine concentrations from intra-laboratory data bases of several German and Italian medical centres: Comparison between direct and indirect procedures. Clin. Chim. Acta 2010, 411, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Arzideh, F.; Wosniok, W.; Haeckel, R. Indirect reference intervals of plasma and serum thyrotropin (TSH) concentrations from intra-laboratory data bases from several German and Italian medical centres. Clin. Chem. Lab. Med. 2011, 49, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Wittek, T. Innere Körpertemperatur. In Klinische Propädeutik der Haus-und Heimtiere; Baumgartner, W., Ed.; Enke: Stuttgart, Germany, 2017; pp. 68–72. [Google Scholar]
- Steinlechner, S.; Arnold, W. Thermoregulation. In Physiologie der Haustiere; Breves, G., Diener, M., Gäbel, G., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2022; pp. 516–532. [Google Scholar]
- CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline, 3rd ed.; CLSI document EP28-A3c; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; pp. 1–59. [Google Scholar]
- Ozarda, Y. Reference intervals: Current status, recent developments and future considerations. Biochem. Med. 2016, 26, 5–16. [Google Scholar] [CrossRef]
- Jones, G.R.D.; Haeckel, R.; Loh, T.P.; Sikaris, K.; Streichert, T.; Katayev, A.; Barth, J.H.; Ozarda, Y.; IFCC Committee on Reference Intervals and Decision Limits. Indirect methods for reference interval determination—Review and recommendations. Clin. Chem. Lab. Med. 2018, 57, 20–29. [Google Scholar] [CrossRef]
- Horn, P.S.; Feng, L.; Li, Y.; Pesce, A.J. Effect of outliers and nonhealthy individuals on reference interval estimation. Clin. Chem. 2001, 47, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Matwichuk, C.L.; Taylor, S.M.; Shmon, C.L.; Kass, P.H.; Shelton, G.D. Changes in rectal temperature and hematologic, biochemical, blood gas, and acid-base values in healthy labrador retrievers before and after strenuous exercise. Am. J. Vet. Res. 1999, 60, 88–92. [Google Scholar] [CrossRef]
- Jensen, C.; Ederstrom, H.E. Development of temperature regulation in the dog. Am. J. Physiol. 1955, 183, 340–344. [Google Scholar] [CrossRef]
- Karnezi, G.; Tzimtzimis, E.; Rafailidis, V.; Kostakis, C.; Savvas, I.; Ververidis, H. Body temperature fluctuation after ovariohysterectomy in dogs in luteal phase, inactive phase and pyometra: A clinical study of 77 cases. Top. Companion Anim. Med. 2020, 40, 100440. [Google Scholar] [CrossRef] [PubMed]
- Olğaç, K.T.; Akçay, E.; Çil, B.; Uçar, B.M.; Daşkın, A. The use of infrared thermography to detect the stages of estrus cycle and ovulation time in anatolian shepherd dogs. J. Anim. Sci. Technol. 2017, 59, 21. [Google Scholar] [CrossRef]
- Schulze, L.; Heuwieser, W.; Arlt, S. Body temperature of bitches in the first week after parturition measured by ingestible loggers. Reprod. Domest. Anim. 2018, 53, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.C.; Campbell, V.L. Comparison of axillary and rectal temperatures for healthy Beagles in a temperature- and humidity-controlled environment. Am. J. Vet. Res. 2015, 76, 632–636. [Google Scholar] [CrossRef]
- Sousa, M.G. Measuring body temperature: How do different sites compare? Vet. Rec. 2016, 178, 190–191. [Google Scholar] [CrossRef]
- González, A.M.; Mann, F.A.; Preziosi, D.E.; Meadows, R.L.; Wagner-Mann, C.C. Measurement of body temperature by use of auricular thermometers versus rectal thermometers in dogs with otitis externa. J. Am. Vet. Med. Assoc. 2002, 221, 378–380. [Google Scholar] [CrossRef]
- Vickers, L.A.; Burfeind, O.; Von Keyserlingk, M.A.; Veira, D.M.; Weary, D.M.; Heuwieser, W. Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 2010, 93, 5246–5251. [Google Scholar] [CrossRef]
- Rijnberk, A.; Stokhof, A.A. Allgemeinuntersuchung. In Die Richtige Diagnose in der Kleintierpraxis: Untersuchung und Befunderhebung; Rijnberk, A., Van Sluijs, F.J., Eds.; Schlütersche: Hannover, Germany, 2011; pp. 49–65. [Google Scholar]
- Posner, L. Perioperative hypothermia in veterinary patients. NAVC Clin. Brief 2007, 5, 19–23. [Google Scholar]
- Phillips, C.J.; Coppinger, R.P.; Schimel, D.S. Hyperthermia in running sled dogs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S. Comparison of body temperatures of goats, horses, and sheep measured with a tympanic infrared thermometer, an implantable microchip transponder, and a rectal thermometer. Contemp. Top. Lab. Anim. Sci. 1998, 37, 51–55. [Google Scholar]
- Kiley, J.P.; Eldridge, F.L.; Millhorn, D.E. Brain, blood and rectal temperature during whole body cooling. Comp. Biochem. Physiol. A Comp. Physiol. 1984, 79, 631–634. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorn, E.; Bogedale, K.; Pankraz, A.; Neiger, R. Calculation of a Reference Interval for Rectal Temperature in Adult Dogs Presenting for Veterinary Care Using an Algorithm for Mixed Data. Animals 2024, 14, 1970. https://doi.org/10.3390/ani14131970
Dorn E, Bogedale K, Pankraz A, Neiger R. Calculation of a Reference Interval for Rectal Temperature in Adult Dogs Presenting for Veterinary Care Using an Algorithm for Mixed Data. Animals. 2024; 14(13):1970. https://doi.org/10.3390/ani14131970
Chicago/Turabian StyleDorn, Elisabeth, Kirsten Bogedale, Alexander Pankraz, and Reto Neiger. 2024. "Calculation of a Reference Interval for Rectal Temperature in Adult Dogs Presenting for Veterinary Care Using an Algorithm for Mixed Data" Animals 14, no. 13: 1970. https://doi.org/10.3390/ani14131970
APA StyleDorn, E., Bogedale, K., Pankraz, A., & Neiger, R. (2024). Calculation of a Reference Interval for Rectal Temperature in Adult Dogs Presenting for Veterinary Care Using an Algorithm for Mixed Data. Animals, 14(13), 1970. https://doi.org/10.3390/ani14131970





