Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women
Abstract
Simple Summary
Abstract
1. Introduction
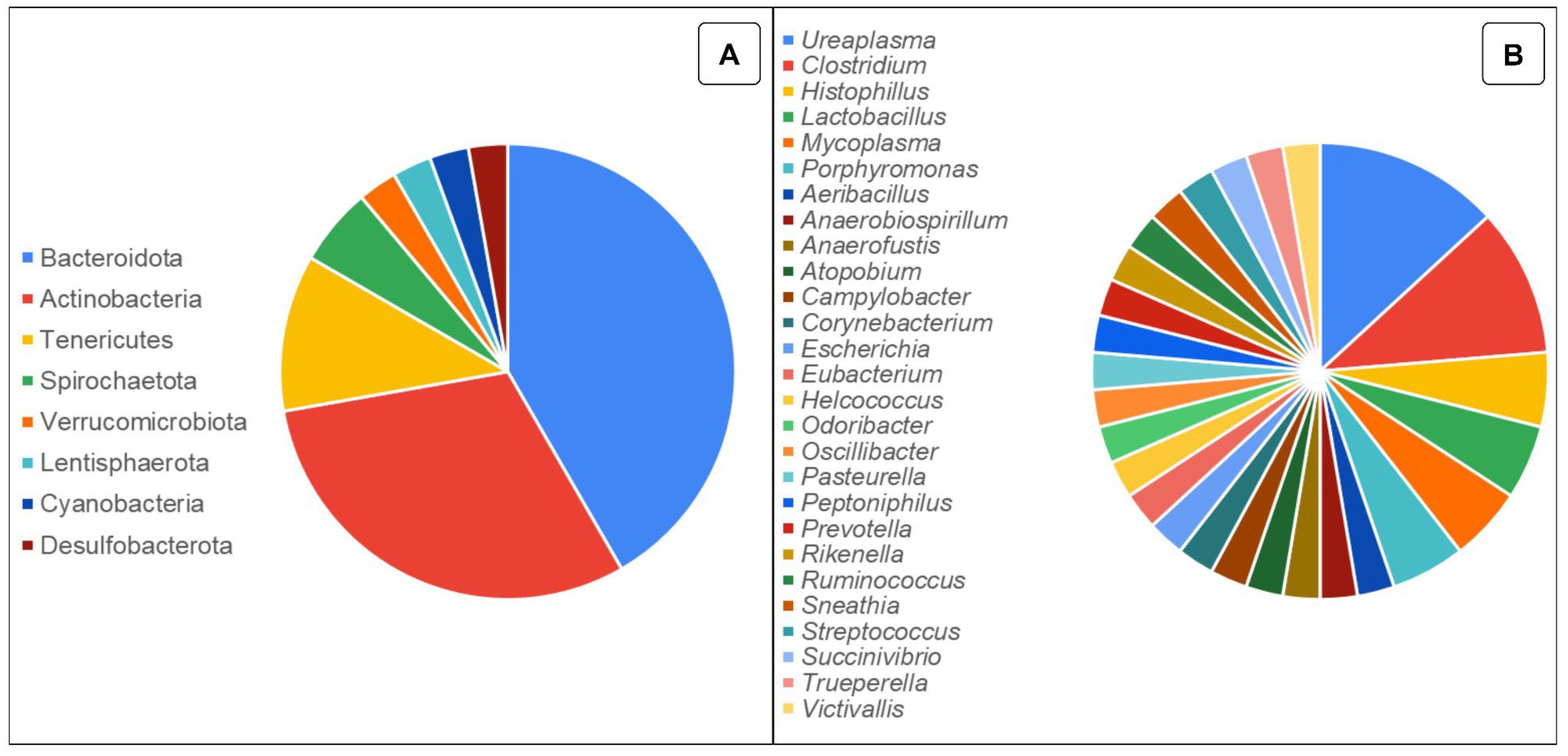
2. Understanding the Microbiota
3. Importance of the Microbiota
4. Reproductive Microbiome in Cows: Pathogenic Interactions
5. Reproductive Tract Microbiome in Cows: Non-Pathogenic Interactions and Association with Fertility
Microbiota and Hormones
6. Microbiota Manipulation: Strategies and Tools
6.1. Antibiotics
6.2. Prebiotics
6.3. Probiotics
6.4. Microbiota Transplantation
7. Challenges
8. Future Perspectives
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes Drive Evolution of Animals and Plants: The Hologenome Concept. mBio 2016, 7, e01395-15. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, L.W.; Moreau, C.S.; Russell, J.A. Introduction: The Host-Associated Microbiome: Pattern, Process and Function. Mol. Ecol. 2018, 27, 1749–1765. [Google Scholar] [CrossRef] [PubMed]
- Appiah, M.; Wang, J.; Lu, W. Microflora in the Reproductive Tract of Cattle: A Review. Agriculture 2020, 10, 232. [Google Scholar] [CrossRef]
- Mor, A.; Driggers, P.H.; Segars, J.H. Molecular Characterization of the Human Microbiome from a Reproductive Perspective. Fertil. Steril. 2015, 104, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.S.; Santin, T.; Rodrigues, M.X.; Marques, C.E.; Lima, S.F.; Bicalho, R.C. Dynamics of the Microbiota Found in the Vaginas of Dairy Cows during the Transition Period: Associations with Uterine Diseases and Reproductive Outcome. J. Dairy Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M. Increased Richness and Diversity of the Vaginal Microbiota and Spontaneous Preterm Birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediat. Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Borg, M.; Ouburg, S.; Morré, S.A. The Relation of the Vaginal Microbiota to Early Pregnancy Development during in Vitro Fertilization Treatment—A Meta-Analysis. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 223–229. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Uterine and Vaginal Bacterial Community Diversity Prior to Artificial Insemination between Pregnant and Nonpregnant Postpartum Cows1. J. Anim. Sci. 2019, 97, 4298–4304. [Google Scholar] [CrossRef]
- Hafez, E.S.E. Pregnancy, Pre-Physiology Natale Delivery. In Animal Reproduction; Hafez, E.S.E., Hafez, B., Eds.; Manole Saúde: São Paulo, Brazil, 2003; pp. 217–240. ISBN 85-204-1222-X. [Google Scholar]
- Genís, S.; Bach, À.; Arís, A. Effects of Intravaginal Lactic Acid Bacteria on Bovine Endometrium: Implications in Uterine Health. Vet. Microbiol. 2017, 204, 174–179. [Google Scholar] [CrossRef]
- Ni, J.; Wang, J.; Zhao, K.; Chen, Y.; Xia, S.; Lai, S. Vaginal Microbiome Dynamics of Cows in Different Parities. Animals 2023, 13, 2880. [Google Scholar] [CrossRef]
- Putignani, L.; Del Chierico, F.; Vernocchi, P.; Cicala, M.; Cucchiara, S.; Dallapiccola, B. Gut Microbiota Dysbiosis as Risk and Premorbid Factors of IBD and IBS Along the Childhood–Adulthood Transition. Inflamm. Bowel Dis. 2016, 22, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Huttenhower, C. Chapter 12: Human Microbiome Analysis. PLoS Comput. Biol. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Weinstock, G.M. Genomic Approaches to Studying the Human Microbiota. Nature 2012, 489, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, D. Microbiome Research Is Becoming the Key to Better Understanding Health and Nutrition. Front. Genet. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.F.; Kästle, J.; Coutinho, T.J.D.; Amorim, A.T.; Campos, G.B.; Santos, V.M.; Marques, L.M.; Timenetsky, J.; de Farias, S.T. Qualitative Analysis of the Vaginal Microbiota of Healthy Cattle and Cattle with Genital-Tract Disease. Genet. Mol. Res. 2015, 14, 6518–6528. [Google Scholar] [CrossRef]
- Fecteau, M.E.; Pitta, D.W.; Vecchiarelli, B.; Indugu, N.; Kumar, S.; Gallagher, S.C.; Fyock, T.L.; Sweeney, R.W. Dysbiosis of the Fecal Microbiota in Cattle Infected with Mycobacterium Avium Subsp. Paratuberculosis. PLoS ONE 2016, 11, e0160353. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.C. Microbial Ecology of the Gastrointestinal Tract. Annu. Rev. Microbiol. 1977, 31, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- American Society for Microbiology. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562894/ (accessed on 20 March 2024).
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal Microbiome Transplantation in Women with Intractable Bacterial Vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Chen, T.; Wang, W.; Liu, G.; Zhang, W.; Li, S.; Wang, M.; Zhao, C.; Zhou, H.; et al. Plant Phenotypic Traits Eventually Shape Its Microbiota: A Common Garden Test. Front. Microbiol. 2018, 9, 411694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Li, H.; Fu, K.; Pang, B.; Yang, Y.; Liu, Y.; Tian, W.; Cao, R. Characterization of the Cervical Bacterial Community in Dairy Cows with Metritis and during Different Physiological Phases. Theriogenology 2018, 108, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Rajendhran, J.; Gunasekaran, P. Microbial Phylogeny and Diversity: Small Subunit Ribosomal RNA Sequence Analysis and Beyond. Microbiol. Res. 2011, 166, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.C. Next-Generation Sequencing Transforms Today’s Biology. Nat. Methods 2008, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. A Framework for Human Microbiome Research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef]
- Kulski, J.K. Next-Generation Sequencing—An Overview of the History, Tools, and “Omic” Applications. In Next Generation Sequencing—Advances, Applications and Challenges; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S RRNA Gene Sequencing for Species and Strain-Level Microbiome Analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Debley, J.S.; Smith, J.M.; Redding, G.J.; Critchlow, C.W. Childhood Asthma Hospitalization Risk after Cesarean Delivery in Former Term and Premature Infants. Ann. Allergy Asthma Immunol. 2005, 94, 228–233. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Stene, L.C.; Joner, G.; Cinek, O.; Svensson, J.; Goldacre, M.J.; Parslow, R.C.; Pozzilli, P.; Brigis, G.; Stoyanov, D.; et al. Caesarean Section Is Associated with an Increased Risk of Childhood-Onset Type 1 Diabetes Mellitus: A Meta-Analysis of Observational Studies. Diabetologia 2008, 51, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Iizumi, T.; Battaglia, T.; Ruiz, V.; Perez Perez, G.I. Gut Microbiome and Antibiotics. Arch. Med. Res. 2017, 48, 727–734. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Blaser, M.J.; Ley, R.E.; Knight, R. Development of the Human Gastrointestinal Microbiota and Insights from High-Throughput Sequencing. Gastroenterology 2011, 140, 1713–1719. [Google Scholar] [CrossRef]
- Stewart, C.J.; Embleton, N.D.; Clements, E.; Luna, P.N.; Smith, D.P.; Fofanova, T.Y.; Nelson, A.; Taylor, G.; Orr, C.H.; Petrosino, J.F.; et al. Cesarean or Vaginal Birth Does Not Impact the Longitudinal Development of the Gut Microbiome in a Cohort of Exclusively Preterm Infants. Front. Microbiol. 2017, 8, 275668. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Blumberg, R.S. Life at the Beginning: Perturbation of the Microbiota by Antibiotics in Early Life and Its Role in Health and Disease. Nat. Immunol. 2014, 15, 307–310. [Google Scholar] [CrossRef]
- Agace, W.W.; McCoy, K.D. Regionalized Development and Maintenance of the Intestinal Adaptive Immune Landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Kostric, M.; Milger, K.; Krauss-Etschmann, S.; Engel, M.; Vestergaard, G.; Schloter, M.; Schöler, A. Development of a Stable Lung Microbiome in Healthy Neonatal Mice. Microb. Ecol. 2018, 75, 529–542. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the Bovine Rumen Bacterial Community from Birth to Adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The Impact of Human Activities and Lifestyles on the Interlinked Microbiota and Health of Humans and of Ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Seitz, J.; Trinh, S.; Herpertz-Dahlmann, B. The Microbiome and Eating Disorders. Psychiatr. Clin. N. Am. 2019, 42, 93–103. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Karlsson, E.K.; Kwiatkowski, D.P.; Sabeti, P.C. Natural Selection and Infectious Disease in Human Populations. Nat. Rev. Genet. 2014, 15, 379–393. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J.; et al. Reduced Genetic Potential for Butyrate Fermentation in the Gut Microbiome of Infants Who Develop Allergic Sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647. [Google Scholar] [CrossRef]
- Stephen-Victor, E.; Chatila, T.A. Regulation of Oral Immune Tolerance by the Microbiome in Food Allergy. Curr. Opin. Immunol. 2019, 60, 141–147. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; DiBaise, J.K.; Isern, N.G.; Hoyt, D.W.; Marcus, A.K.; Kang, D.-W.; Crowell, M.D.; Rittmann, B.E.; Krajmalnik-Brown, R. Distinctive Microbiomes and Metabolites Linked with Weight Loss after Gastric Bypass, but Not Gastric Banding. ISME J. 2017, 11, 2047–2058. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Dreyer, J.L.; Liebl, A.L. Early Colonization of the Gut Microbiome and Its Relationship with Obesity. Hum. Microb. J. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the Bacterial Microbiota across the Gastrointestinal Tracts of Dairy Cattle: Membership and Potential Function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef] [PubMed]
- Bessegatto, J.A.; Paulino, L.R.; Lisbôa, J.A.N.; Alfieri, A.A.; Montemor, C.H.; Medeiros, L.P.; Kobayashi, R.K.T.; Weese, J.S.; Costa, M.C. Changes in the Fecal Microbiota of Beef Cattle Caused by Change in Management and the Use of Virginiamycin as a Growth Promoter. Res. Vet. Sci. 2017, 114, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, F.P.; Woolums, A.R.; Francoz, D.; Rosenbusch, R.F.; Step, D.L.; Wilson, D.J.; Janzen, E.D. Mycoplasma bovis Infections in Cattle. J. Vet. Intern. Med. 2011, 25, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Earley, B.; Cormican, P.; Murray, G.; Kenny, D.A.; Waters, S.M.; McGee, M.; Kelly, A.K.; McCabe, M.S. Illumina MiSeq 16S Amplicon Sequence Analysis of Bovine Respiratory Disease Associated Bacteria in Lung and Mediastinal Lymph Node Tissue. BMC Vet. Res. 2017, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Quadros, D.L.; Zanella, R.; Bondan, C.; Zanella, G.C.; Facioli, F.L.; da Silva, A.N.; Zanella, E.L. Study of Vaginal Microbiota of Holstein Cows Submitted to an Estrus Synchronization Protocol with the Use of Intravaginal Progesterone Device. Res. Vet. Sci. 2020, 131, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. The Evolving Role of Genetic Tests in Reproductive Medicine. J. Transl. Med. 2019, 17, 267. [Google Scholar] [CrossRef] [PubMed]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A Retrospective Pilot Study to Determine Whether the Reproductive Tract Microbiota Differs between Women with a History of Infertility and Fertile Women. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Relevance of Assessing the Uterine Microbiota in Infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations with Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Verhelst, R.; Claeys, G.; De Backer, E.; Temmerman, M.; Vaneechoutte, M. Longitudinal Analysis of the Vaginal Microflora in Pregnancy Suggests That L. crispatus Promotes the Stability of the Normal Vaginal Microflora and That L. gasseri and/or L. iners Are More Conducive to the Occurrence of Abnormal Vaginal Microflora. BMC Microbiol. 2009, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McCormick, J.; Bocking, A.; Reid, G. Importance of Vaginal Microbes in Reproductive Health. Reprod. Sci. 2012, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Salah, R.M.; Allam, A.M.; Magdy, A.M.; Mohamed, A.S. Bacterial Vaginosis and Infertility: Cause or Association? Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kindinger, L.; MacIntyre, D.; Lee, Y.; Teoh, T.; Bennett, P. Identification of Vaginal Microbial Communities Associated with Specific Etiologies of Preterm Birth, In: Scientific Abstracts. Reprod. Sci. 2016, 23, 51A–344A. [Google Scholar] [CrossRef]
- Eastment, M.C.; McClelland, R.S. Vaginal Microbiota and Susceptibility to HIV. AIDS 2018, 32, 687–698. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal PH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different from That of Non-Pregnant Women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the Vaginal Microbiota of Ewes and Cows Reveals a Unique Microbiota with Low Levels of Lactobacilli and Near-Neutral PH. Front. Vet. Sci. 2014, 1, 116383. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Yeoman, C.J.; Janga, S.C.; Thomas, S.M.; Ho, M.; Leigh, S.R.; White, B.A.; Wilson, B.A.; Stumpf, R.M. Primate Vaginal Microbiomes Exhibit Species Specificity without Universal Lactobacillus Dominance. ISME J. 2014, 8, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Tatum, F. Bovine Brucellosis. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 15–27. [Google Scholar] [CrossRef]
- Pritchard, G.C.; Smith, R.P.; Errington, J.; Hannon, S.; Jones, R.M.; Mearns, R. Prevalence of Coxiella burnetii in Livestock Abortion Material Using PCR. Vet. Rec. 2011, 169, 391. [Google Scholar] [CrossRef]
- Galvão, K.N.; Higgins, C.H.; Zinicola, M.; Jeon, S.J.; Korzec, H.; Bicalho, R.C. Effect of Pegbovigrastim Administration on the Microbiome Found in the Vagina of Cows Postpartum. J. Dairy Sci. 2019, 102, 3439–3451. [Google Scholar] [CrossRef]
- Dohmen, M.J.W.; Lohuis, J.A.C.M.; Huszenicza, G.; Nagy, P.; Gacs, M. The Relationship between Bacteriological and Clinical Findings in Cows with Subacute/Chronic Endometritis. Theriogenology 1995, 43, 1379–1388. [Google Scholar] [CrossRef]
- Quereda, J.J.; Barba, M.; Mocé, M.L.; Gomis, J.; Jiménez-Trigos, E.; García-Muñoz, Á.; Gómez-Martín, Á.; González-Torres, P.; Carbonetto, B.; García-Roselló, E. Vaginal Microbiota Changes During Estrous Cycle in Dairy Heifers. Front. Vet. Sci. 2020, 7, 552090. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.; Nakao, T. Prevalence of Urovagina and Its Effects on Reproductive Performance in Holstein Cows. Theriogenology 2009, 71, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Laguardia-Nascimento, M.; Branco, K.M.G.R.; Gasparini, M.R.; Giannattasio-Ferraz, S.; Leite, L.R.; Araujo, F.M.G.; De Matos Salim, A.C.; Nicoli, J.R.; De Oliveira, G.C.; Barbosa-Stancioli, E.F. Vaginal Microbiome Characterization of Nellore Cattle Using Metagenomic Analysis. PLoS ONE 2015, 10, e0143294. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The Vaginal Microbiota, Host Defence and Reproductive Physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Deng, F.; McClure, M.; Rorie, R.; Wang, X.; Chai, J.; Wei, X.; Lai, S.; Zhao, J. The Vaginal and Fecal Microbiomes Are Related to Pregnancy Status in Beef Heifers. J. Anim. Sci. Biotechnol. 2019, 10, 92. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Sobel, J.D. Current Treatment of Bacterial Vaginosis-Limitations and Need for Innovation. J. Infect. Dis. 2016, 214 (Suppl. 1), S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial Microbiome at the Time of Embryo Transfer: Next-Generation Sequencing of the 16S Ribosomal Subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, C.; Liu, C.; Yang, Y.; Lu, W. Comparison of Vaginal Microbial Community Structure in Healthy and Endometritis Dairy Cows by PCR-DGGE and Real-Time PCR. Anaerobe 2016, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.O.; Meijer, W.G. Delayed Differentiation of Vaginal and Uterine Microbiomes in Dairy Cows Developing Postpartum Endometritis. PLoS ONE 2019, 14, e0200974. [Google Scholar] [CrossRef] [PubMed]
- Otero, C.; Saavedra, L.; Silva de Ruiz, C.; Wilde, O.; Holgado, A.R.; Nader-Macias, M.E. Vaginal Bacterial Microflora Modifications during the Growth of Healthy Cows. Lett. Appl. Microbiol. 2000, 31, 251–254. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.W.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical Evaluation of Postpartum Vaginal Mucus Reflects Uterine Bacterial Infection and the Immune Response in Cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Osawa, T.; Dubuc, J. Reproductive Tract Defense and Disease in Postpartum Dairy Cows. Theriogenology 2011, 76, 1610–1618. [Google Scholar] [CrossRef]
- Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Adeyosoye, O.I.; Pohler, K.G.; Myer, P.R. Vaginal and Uterine Bacterial Communities in Postpartum Lactating Cows. Front. Microbiol. 2017, 8, 1047. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Lima, S.; Higgins, C.H.; Machado, V.S.; Lima, F.S.; Bicalho, R.C. Genetic and Functional Analysis of the Bovine Uterine Microbiota. Part II: Purulent Vaginal Discharge versus Healthy Cows. J. Dairy Sci. 2017, 100, 3863–3874. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.R.V.; Karstrup, C.C.; Pedersen, H.G.; Angen, Ø.; Agerholm, J.S.; Rasmussen, E.L.; Jensen, T.K.; Klitgaard, K. An Investigation of the Microbiota in Uterine Flush Samples and Endometrial Biopsies from Dairy Cows during the First 7 Weeks Postpartum. Theriogenology 2016, 86, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.B.; de Freitas, S.L.; da Costa, A.N.L.; Bicalho, R.C.; Lima, S.; et al. Uterine Microbiota Progression from Calving until Establishment of Metritis in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Lima, F.S.; Vieira-Neto, A.; Machado, V.S.; Lima, S.F.; Bicalho, R.C.; Santos, J.E.P.; Galvão, K.N. Shift of Uterine Microbiota Associated with Antibiotic Treatment and Cure of Metritis in Dairy Cows. Vet. Microbiol. 2018, 214, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Adnane, M.; Archunan, G. Significance of Cervico-Vaginal Microbes in Bovine Reproduction and Pheromone Production—A Hypothetical Review. Res. Vet. Sci. 2021, 135, 66–71. [Google Scholar] [CrossRef]
- Eva, S.; Radomra, N.; Soňa, G.; Igor, V.; Andrea, L. Bovine Vaginal Lactobacilli and Their Adherence to Mucus in Different Phases of the Estrous Cycle. Afr. J. Microbiol. Res. 2014, 8, 3017–3024. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Lamont, R.; Sobel, J.; Akins, R.; Hassan, S.; Chaiworapongsa, T.; Kusanovic, J.; Romero, R. The Vaginal Microbiome: New Information about Genital Tract Flora Using Molecular Based Techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef]
- Amin, J.D.; Zarial, T.; Malgwir, M. Vaginal Aerobic Bacterial Flora of Apparently Healthy Cattle in Various Stages of the Reproductive Cycle in the Sahel Region of Nigeria. Bull. Anim. Health Prod. Afr. 1996, 44, 8–15. [Google Scholar]
- Otero, C.; Silva De Ruiz, C.; Ibañez, R.; Wilde, O.R.; De Ruiz Holgado, A.A.P.; Nader-Macias, M.E. Lactobacilli and Enterococci Isolated from the Bovine Vagina During the Estrous Cycle. Anaerobe 1999, 5, 305–307. [Google Scholar] [CrossRef]
- Zambrano-Nava, S.; Boscán-Ocando, J.; Nava, J. Normal Bacterial Flora from Vaginas of Criollo Limonero Cows. Trop. Anim. Health Prod. 2011, 43, 291–294. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, G.; Li, C.; Yang, J.; Li, J.; Chen, D.; Zou, W.; Jin, S.; Zhang, H.; Li, D.; et al. The Normal Vaginal and Uterine Bacterial Microbiome in Giant Pandas (Ailuropoda melanoleuca). Microbiol. Res. 2017, 199, 1–9. [Google Scholar] [CrossRef]
- Chen, D.; Li, C.; Feng, L.; Zhang, Z.; Zhang, H.; Cheng, G.; Li, D.; Zhang, G.; Wang, H.; Chen, Y.; et al. Analysis of the Influence of Living Environment and Age on Vaginal Fungal Microbiome in Giant Pandas (Ailuropoda melanoleuca) by High Throughput Sequencing. Microb. Pathog. 2018, 115, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio-Ferraz, S.; Laguardia-Nascimento, M.; Gasparini, M.R.; Leite, L.R.; Araujo, F.M.G.; de Matos Salim, A.C.; de Oliveira, A.P.; Nicoli, J.R.; de Oliveira, G.C.; da Fonseca, F.G.; et al. A Common Vaginal Microbiota Composition among Breeds of Bos Taurus Indicus (Gyr and Nellore). Braz. J. Microbiol. 2019, 50, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Messman, R.D.; Contreras-Correa, Z.E.; Paz, H.A.; Perry, G.; Lemley, C.O. Vaginal Bacterial Community Composition and Concentrations of Estradiol at the Time of Artificial Insemination in Brangus Heifers. J. Anim. Sci. 2020, 98, skaa178. [Google Scholar] [CrossRef]
- Chen, S.Y.; Deng, F.; Zhang, M.; Jia, X.; Lai, S.J. Characterization of Vaginal Microbiota Associated with Pregnancy Outcomes of Artificial Insemination in Dairy Cows. J. Microbiol. Biotechnol. 2020, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Holman, D.B.; Schmidt, K.; Menezes, A.C.B.; Baumgaertner, F.; Winders, T.; Kirsch, J.D.; Liu, T.; Schwinghamer, T.D.; Sedivec, K.K.; et al. The Nasopharyngeal, Ruminal, and Vaginal Microbiota and the Core Taxa Shared across These Microbiomes in Virgin Yearling Heifers Exposed to Divergent in Utero Nutrition during Their First Trimester of Gestation and in Pregnant Beef Heifers in Response to Mineral Supplementation. Microorganisms 2021, 9, 2011. [Google Scholar] [CrossRef]
- Nesengani, L.T.; Wang, J.; Yang, Y.; Yang, L.; Lu, W. Unravelling Vaginal Microbial Genetic Diversity and Abundance between Holstein and Fleckvieh Cattle. RSC Adv. 2017, 7, 56137–56143. [Google Scholar] [CrossRef]
- Manes, J.; Fiorentino, M.A.; Martino, S.S.; Ungerfeld, R. Changes in the Vaginal Microbiota in Ewes after Insertion of Intravaginal Sponges at Different Stages of the Oestrous Cycle. Livest. Sci. 2018, 208, 55–59. [Google Scholar] [CrossRef]
- Rodríguez, C.; Cofré, J.V.; Sánchez, M.; Fernández, P.; Boggiano, G.; Castro, E. Lactobacilli Isolated from Vaginal Vault of Dairy and Meat Cows during Progesteronic Stage of Estrous Cycle. Anaerobe 2011, 17, 15–18. [Google Scholar] [CrossRef]
- Olson, J.D.; Bretzlaff, K.N.; Mortimer, R.G.; Ball, L. The Metritis and Pyometra Complex. In Current Therapy in Theriogenology; Morrow, D.A., Ed.; Saunders: Philadelphia, PA, USA, 1986; pp. 227–236. ISBN 9780721665801. [Google Scholar]
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-Amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028. [Google Scholar] [CrossRef]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of Vaginal Acidity: High d/l Lactate Ratio Is Consistent with Bacteria Being the Primary Source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ametaj, B.N.; Ambrose, D.J.; Gänzle, M.G. Characterisation of the Bacterial Microbiota of the Vagina of Dairy Cows and Isolation of Pediocin-Producing Pediococcus acidilactici. BMC Microbiol. 2013, 13, 19. [Google Scholar] [CrossRef]
- Leclaire, S.; Strandh, M.; Mardon, J.; Westerdahl, H.; Bonadonna, F. Odour-Based Discrimination of Similarity at the Major Histocompatibility Complex in Birds. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162466. [Google Scholar] [CrossRef] [PubMed]
- Archie, E.A.; Theis, K.R. Animal Behaviour Meets Microbial Ecology. Anim. Behav. 2011, 82, 425–436. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Gerardo, N.M.; Inouye, D.W.; Medina, M.; Xavier, J.B. Animal Behavior and the Microbiome. Science 2012, 338, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.C.; Morelli, L.; Nader-Macías, M.E. Probiotic Properties of Vaginal Lactic Acid Bacteria to Prevent Metritis in Cattle. Lett. Appl. Microbiol. 2006, 43, 91–97. [Google Scholar] [CrossRef]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of Antibiotic Prescribing in Primary Care on Antimicrobial Resistance in Individual Patients: Systematic Review and Meta-Analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S RRNA Sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef]
- Costa, M.C.; Bessegatto, J.A.; Alfieri, A.A.; Weese, J.S.; Filho, J.A.B.; Oba, A. Different Antibiotic Growth Promoters Induce Specific Changes in the Cecal Microbiota Membership of Broiler Chicken. PLoS ONE 2017, 12, e0171642. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 164577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, D.-C. Facing a New Challenge. Chin. Med. J. 2019, 132, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Stewardson, A.J.; Huttner, B.; Harbarth, S. At Least It Won’t Hurt: The Personal Risks of Antibiotic Exposure. Curr. Opin. Pharmacol. 2011, 11, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ocal, H.; Yuksel, M.; Ayar, A. Effects of Gentamicin Sulfate on the Contractility of Myometrium Isolated from Non-Pregnant Cows. Anim. Reprod. Sci. 2004, 84, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.S. Uterine Health and Disorders. J. Dairy Sci. 1997, 80, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Lotthammer, K.; Wittkowski, G. Scheinden Und Gebarmuttererkrankugen. In Fruchtbarbeit und Gesundheit der Rinder; Lotthammer, K.H., Wittkowski, G., Eds.; Ulmer: Stuttgart, Germany, 1994; pp. 60–66. [Google Scholar]
- Taggart, H.; Bergstrom, L. An Overview of the Microbiome and the Effects of Antibiotics. J. Nurse Pract. 2014, 10, 445–450. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary Prebiotics: Current Status and New Definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Abbasi, A.; Aghebati-Maleki, A.; Yousefi, M.; Aghebati-Maleki, L. Probiotic Intervention as a Potential Therapeutic for Managing Gestational Disorders and Improving Pregnancy Outcomes. J. Reprod. Immunol. 2021, 143, 103244. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic Bacteria: Safety, Functional and Technological Properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Vitagliano, A.; Pellegrini, L.; Chiurazzi, M.; Andriasani, A.; Ambrosini, G.; Garrido, N. Therapy with Probiotics and Synbiotics for Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2020, 59, 2841–2856. [Google Scholar] [CrossRef]
- de Brito Alves, J.L.; de Oliveira, Y.; Carvalho, N.N.C.; Cavalcante, R.G.S.; Pereira Lira, M.M.; do Nascimento, L.C.P.; Magnani, M.; Vidal, H.; Braga, V.d.A.; de Souza, E.L. Gut Microbiota and Probiotic Intervention as a Promising Therapeutic for Pregnant Women with Cardiometabolic Disorders: Present and Future Directions. Pharmacol. Res. 2019, 145, 104252. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Schwebke, J.R. Secnidazole: Next-Generation Antimicrobial Agent for Bacterial Vaginosis Treatment. Future Microbiol. 2018, 13, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.J.; Masztal, A.; Aldridge, K.E.; Fortenberry, J.D.; Fidel, P.L.; Martin, D.H. Association of Atopobium vaginae, a Recently Described Metronidazole Resistant Anaerobe, with Bacterial Vaginosis. BMC Infect. Dis. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.L.; Vodstrcil, L.A.; Danielewski, J.A.; Murray, G.L.; Fairley, C.K.; Garland, S.M.; Hocking, J.S.; Tabrizi, S.N.; Bradshaw, C.S. Combined Oral and Topical Antimicrobial Therapy for Male Partners of Women with Bacterial Vaginosis: Acceptability, Tolerability and Impact on the Genital Microbiota of Couples—A Pilot Study. PLoS ONE 2018, 13, e0190199. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of Endometrial Microbiota by 16S Ribosomal RNA Gene Sequencing among Infertile Patients: A Single-center Pilot Study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic Effects of Lactobacillus reuteri DSM 17938 in People with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Zarrati Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic Supplementation in Diabetic Hemodialysis Patients Has Beneficial Metabolic Effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine Infection and Prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef]
- Swartwout, B.; Luo, X.M. Implications of Probiotics on the Maternal-Neonatal Interface: Gut Microbiota, Immunomodulation, and Autoimmunity. Front. Immunol. 2018, 9, 2840. [Google Scholar] [CrossRef] [PubMed]
- Dawe, J.P.; McCowan, L.M.E.; Wilson, J.; Okesene-Gafa, K.A.M.; Serlachius, A.S. Probiotics and Maternal Mental Health: A Randomised Controlled Trial among Pregnant Women with Obesity. Sci. Rep. 2020, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Campero, C.M.; Conosciuto, G.; Odriozola, E.; Moreira, A.R.; Lodeiro, R.; Garcia Boissou, R.; Hernaiz, R. Hallazgos Clínicos, Bacteriológicos e Histopatológicos En Vacas Lecheras Asociados Con Problemas Reproductivos. Rev. Med. Vet. 1992, 73, 264–272. [Google Scholar]
- Otero, M.C.; Nader-Macías, M.E. Inhibition of Staphylococcus Aureus by H2O2-Producing Lactobacillus Gasseri Isolated from the Vaginal Tract of Cattle. Anim. Reprod. Sci. 2006, 96, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.A.; Larson, J.E.; Vann, R.C. The Estrous Cycle of Cattle; Agricultural Communications: St. Paul, MN, USA, 2016. [Google Scholar]
- Zawistowska-Rojek, A.; Tyski, S. Are Probiotic Really Safe for Humans? Pol. J. Microbiol. 2018, 67, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, C.; Liu, Y.; Yao, J.; Yang, X.; Wu, S.; Du, J.; Yang, X. Beyond Faecal Microbiota Transplantation, the Non-Negligible Role of Faecal Virome or Bacteriophage Transplantation. J. Microbiol. Immunol. Infect. 2023, 56, 893–908. [Google Scholar] [CrossRef]
- Saha, S.; Tariq, R.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Faecal Microbiota Transplantation for Eradicating Carriage of Multidrug-Resistant Organisms: A Systematic Review. Clin. Microbiol. Infect. 2019, 25, 958–963. [Google Scholar] [CrossRef]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and Cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut Microbiota Influence Immunotherapy Responses: Mechanisms and Therapeutic Strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Hensley-McBain, T.; Zevin, A.S.; Manuzak, J.; Smith, E.; Gile, J.; Miller, C.; Agricola, B.; Katze, M.; Reeves, R.K.; Kraft, C.S.; et al. Effects of Fecal Microbial Transplantation on Microbiome and Immunity in Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2016, 90, 4981–4989. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Rutishauser, R.L.; Pao, M.; Hunt, P.W.; Lynch, S.V.; McCune, J.M.; Somsouk, M. Limited Engraftment of Donor Microbiome via One-Time Fecal Microbial Transplantation in Treated HIV-Infected Individuals. Gut Microbes 2017, 8, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, G.; Zhao, Z.; Liu, Y.; Shen, Q.; Li, P.; Chen, Y.; Yin, H.; Wang, H.; Marcella, C.; et al. Washed Microbiota Transplantation vs. Manual Fecal Microbiota Transplantation: Clinical Findings, Animal Studies and in Vitro Screening. Protein Cell 2020, 11, 251–266. [Google Scholar] [CrossRef]
- Terveer, E.M.; van Beurden, Y.H.; Goorhuis, A.; Seegers, J.F.M.L.; Bauer, M.P.; van Nood, E.; Dijkgraaf, M.G.W.; Mulder, C.J.J.; Vandenbroucke-Grauls, C.M.J.E.; Verspaget, H.W.; et al. How to: Establish and Run a Stool Bank. Clin. Microbiol. Infect. 2017, 23, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, J.; Zhang, G. Vaginal Microbiota Transplantation Is a Truly Opulent and Promising Edge: Fully Grasp Its Potential. Front. Cell. Infect. Microbiol. 2024, 14, 1280636. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Jiang, Y.; Lessing, D.J.; Chu, W. Synthetic Bacterial Consortia Transplantation for the Treatment of Gardnerella vaginalis-Induced Bacterial Vaginosis in Mice. Microbiome 2023, 11, 54. [Google Scholar] [CrossRef]
- Wrønding, T.; Vomstein, K.; Bosma, E.F.; Mortensen, B.; Westh, H.; Heintz, J.E.; Mollerup, S.; Petersen, A.M.; Ensign, L.M.; DeLong, K.; et al. Antibiotic-Free Vaginal Microbiota Transplant with Donor Engraftment, Dysbiosis Resolution and Live Birth after Recurrent Pregnancy Loss: A Proof of Concept Case Study. EClinicalMedicine 2023, 61, 102070. [Google Scholar] [CrossRef] [PubMed]
- Tuniyazi, M.; Zhang, N. Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation. Microorganisms 2023, 11, 1427. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Lager, S.; Sovio, U.; Gaccioli, F.; Cook, E.; Peacock, S.J.; Parkhill, J.; Charnock-Jones, D.S.; Smith, G.C.S. Human Placenta Has No Microbiome but Can Contain Potential Pathogens. Nature 2019, 572, 329–334. [Google Scholar] [CrossRef]
- Souza, A.K.; Zangirolamo, A.F.; Droher, R.G.; Bonato, F.G.C.; Alfieri, A.A.; da Costa, M.C.; Seneda, M.M. Investigation of the Vaginal Microbiota of Dairy Cows through Genetic Sequencing of Short (Illumina) and Long (PacBio) Reads and Associations with Gestational Status. PLoS ONE 2023, 18, e0290026. [Google Scholar] [CrossRef] [PubMed]
- Ziemer, C.J. Newly Cultured Bacteria with Broad Diversity Isolated from Eight-Week Continuous Culture Enrichments of Cow Feces on Complex Polysaccharides. Appl. Environ. Microbiol. 2014, 80, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, A.; Au, K.F. PacBio Sequencing and Its Applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Colston, S.M.; Fullmer, M.S.; Beka, L.; Lamy, B.; Peter Gogarten, J.; Graf, J. Bioinformatic Genome Comparisons for Taxonomic and Phylogenetic Assignments Using Aeromonas as a Test Case. mBio 2014, 5, e02136-14. [Google Scholar] [CrossRef]
- Graf, J.; Ledala, N.; Caimano, M.J.; Jackson, E.; Gratalo, D.; Fasulo, D.; Driscoll, M.D.; Coleman, S.; Matson, A.P. High-Resolution Differentiation of Enteric Bacteria in Premature Infant Fecal Microbiomes Using a Novel RRNA Amplicon. mBio 2021, 12, e03656-20. [Google Scholar] [CrossRef]
- Callahan, B.J.; Wong, J.; Heiner, C.; Oh, S.; Theriot, C.M.; Gulati, A.S.; McGill, S.K.; Dougherty, M.K. High-Throughput Amplicon Sequencing of the Full-Length 16S RRNA Gene with Single-Nucleotide Resolution. Nucleic Acids Res. 2019, 47, e103. [Google Scholar] [CrossRef]
- Costa, M.; Weese, J.S. Methods and Basic Concepts for Microbiota Assessment. Vet. J. 2019, 249, 10–15. [Google Scholar] [CrossRef]
- Benjamino, J.; Lincoln, S.; Srivastava, R.; Graf, J. Low-Abundant Bacteria Drive Compositional Changes in the Gut Microbiota after Dietary Alteration. Microbiome 2018, 6, 86. [Google Scholar] [CrossRef]
- Webb, E.M.; Holman, D.B.; Schmidt, K.N.; Pun, B.; Sedivec, K.K.; Hurlbert, J.L.; Bochantin, K.A.; Ward, A.K.; Dahlen, C.R.; Amat, S. Sequencing and Culture-Based Characterization of the Vaginal and Uterine Microbiota in Beef Cattle That Became Pregnant or Remained Open Following Artificial Insemination. Microbiol. Spectr. 2023, 11, e02732-23. [Google Scholar] [CrossRef]
- Machado, V.S.; Oikonomou, G.; Bicalho, M.L.S.; Knauer, W.A.; Gilbert, R.; Bicalho, R.C. Investigation of Postpartum Dairy Cows’ Uterine Microbial Diversity Using Metagenomic Pyrosequencing of the 16S RRNA Gene. Vet. Microbiol. 2012, 159, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Wagener, K.; Prunner, I.; Pothmann, H.; Drillich, M.; Ehling-Schulz, M. Diversity and Health Status Specific Fluctuations of Intrauterine Microbial Communities in Postpartum Dairy Cows. Vet. Microbiol. 2015, 175, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Prunner, I.; Pothmann, H.; Wagener, K.; Giuliodori, M.; Huber, J.; Ehling-Schulz, M.; Drillich, M. Dynamics of Bacteriologic and Cytologic Changes in the Uterus of Postpartum Dairy Cows. Theriogenology 2014, 82, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.A.; Gilbert, R.O.; Bicalho, R.C. Metagenomic Analysis of the Uterine Bacterial Microbiota in Healthy and Metritic Postpartum Dairy Cows. J. Dairy Sci. 2011, 94, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lundahl, S.; Nørstebø, S.F.; Klem, T.B.; Gilfillan, G.D.; Dalland, M.; Gillund, P.; Krogenæs, A. The Microbiota of Uterine Biopsies, Cytobrush and Vaginal Swabs at Artificial Insemination in Norwegian Red Cows. Theriogenology 2023, 209, 115–125. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangirolamo, A.F.; Souza, A.K.; Yokomizo, D.N.; Miguel, A.K.A.; Costa, M.C.d.; Alfieri, A.A.; Seneda, M.M. Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women. Animals 2024, 14, 1971. https://doi.org/10.3390/ani14131971
Zangirolamo AF, Souza AK, Yokomizo DN, Miguel AKA, Costa MCd, Alfieri AA, Seneda MM. Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women. Animals. 2024; 14(13):1971. https://doi.org/10.3390/ani14131971
Chicago/Turabian StyleZangirolamo, Amanda Fonseca, Anne Kemmer Souza, Deborah Nakayama Yokomizo, Ana Karolyne Alves Miguel, Márcio Carvalho da Costa, Amauri Alcindo Alfieri, and Marcelo Marcondes Seneda. 2024. "Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women" Animals 14, no. 13: 1971. https://doi.org/10.3390/ani14131971
APA StyleZangirolamo, A. F., Souza, A. K., Yokomizo, D. N., Miguel, A. K. A., Costa, M. C. d., Alfieri, A. A., & Seneda, M. M. (2024). Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women. Animals, 14(13), 1971. https://doi.org/10.3390/ani14131971




