Evaluation of Gamma Aminobutyric Acid and Sodium Butyrate in Juvenile Red Seabream (Pagrus major) Diets Containing Graded Levels of Fish Meal and Soy Protein Concentrate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish Feeding Trial and Conditions
2.3. Sample Collection and Analyses
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Feed Utilization and Survival
3.3. Non-Specific Immune Responses
3.4. Plasma Biochemical Parameters
3.5. Biometric Parameters
3.6. Muscle Proximate Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varghese, T.; Rejish Kumar, V.J.; Anand, G.; Dasgupta, S.; Pal, A.K. Dietary GABA enhances hypoxia tolerance of a bottom-dwelling carp, Cirrhinus mrigala by modulating HIF-1α, thyroid hormones and metabolic responses. Fish Physiol. Biochem. 2020, 46, 199–212. [Google Scholar] [CrossRef]
- Farris, N.W.; Hamidoghli, A.; Bae, J.; Won, S.; Choi, W.; Biró, J.; Lee, S.; Bai, S.C. Dietary supplementation with γ-aminobutyric acid improves growth, digestive enzyme activity, non-specific immunity and disease resistance against Streptococcus iniae in juvenile olive flounder, Paralichthys olivaceus. Animals 2022, 12, 248. [Google Scholar] [CrossRef]
- Ruenkoed, S.; Nontasan, S.; Phudkliang, J.; Phudinsai, P.; Pongtanalert, P.; Panprommin, D.; Mongkolwit, K.; Wangkahart, E. Effect of dietary gamma aminobutyric acid (GABA) modulated the growth performance, immune and antioxidant capacity, digestive enzymes, intestinal histology and gene expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2023, 141, 109056. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Moniruzzaman, M.; Farris, N.; Min, T.; Bai, S.C. Interactive Effect of Dietary Gamma-Aminobutyric Acid (GABA) and Water Temperature on Growth Performance, Blood Plasma Indices, Heat Shock Proteins and GABAergic Gene Expression in Juvenile Olive Flounder Paralichthys olivaceus. Metabolites 2023, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Daeman, N.H.; Chong, C.M.; Karami, A.; Kumar, V.; Hoseinifar, S.H.; Romano, N. Comparing the effects of different dietary organic acids on the growth, intestinal short-chain fatty acids, and liver histopathology of red hybrid tilapia (Oreochromis sp.) and potential use of these as preservatives. Fish Physiol. Biochem. 2017, 43, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, B.E.; Veille, A.; Tharaka, K.; Shin, J.; Shin, J.; Jeong, J.B.; Meallet, V.; Lee, K. Evaluation of marine algal (Ulva spp./Solieria spp.) extracts combined with organic acids in diets for olive flounder (Paralichthys olivaceus). Aquac. Res. 2021, 52, 3270–3279. [Google Scholar] [CrossRef]
- Volatiana, J.A.; Sagada, G.; Xu, B.; Zhang, J.; Ng, W.K.; Shao, Q. Effects of butyrate glycerides supplementation in high soybean meal diet on growth performance, intestinal morphology and antioxidative status of juvenile black sea bream, Acanthopagrus schlegelii. Aquac. Nutr. 2020, 26, 15–25. [Google Scholar] [CrossRef]
- Mallioris, P.; Kotzamanis, Y.; Vardali, S.; Roussos, E.; Ilia, V.; Paschali, E.; Kampantais, D.; Bouziotis, D.; Khadem, A.; Vatsos, I.N. Modulation of intestinal health and hepatic vacuolation in gilthead sea bream (Sparus aurata) juveniles by a mixture of dietary esterified butyrins, emulsifiers from plants and yeast extracts at low and high fish meal inclusion. Anim. Feed. Sci. Technol. 2022, 284, 115194. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringø, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Guo, X.; Pan, X.; Li, X. Effects of dietary sodium butyrate on growth performance, antioxidant capacity, intestinal histomorphology and immune response in juvenile Pengze crucian carp (Carassius auratus Pengze). Aquac. Rep. 2021, 21, 100828. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Shukry, M.; Farrag, F.A.; El-Shafai, N.M.; Dawood, M.A.; Abdel-Latif, H.M. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 2021, 536, 736467. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Niu, B.; Chang, K.; Chen, W. Dietary Sodium Butyrate Supplementation Attenuates the Detrimental Effects of High-Fat Diets on Growth Performance, Liver Health, and Disease Resistance in Grass Carp. N. Am. J. Aquac. 2022, 84, 392–401. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S.; Chang, K.; Zhao, X.; Niu, B. Dietary sodium butyrate supplementation improves fish growth, intestinal microbiota composition, and liver health in largemouth bass (Micropterus salmoides) fed high-fat diets. Aquaculture 2023, 564, 739040. [Google Scholar] [CrossRef]
- Dawood, M.A.; Eweedah, N.M.; Elbialy, Z.I.; Abdelhamid, A.I. Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile tilapia (Oreochromis niloticus) exposed to heat stress. J. Therm. Biol. 2020, 88, 102500. [Google Scholar] [CrossRef] [PubMed]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Ma, Q.; Zhang, B. Dietary gamma-aminobutyric acid ameliorates growth impairment and intestinal dysfunction in turbot (Scophthalmus maximus L.) fed a high soybean meal diet. Food Funct. 2022, 13, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Xu, W.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 2019, 88, 65–75. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Du, X.; Xu, H.; Wang, G.; Zhang, D. Effects of dietary γ-aminobutyric acid levels on the growth, serum biochemical indexes, immune-related signalling molecules of Jian carp. Aquac. Res. 2021, 52, 1096–1105. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Xie, Q.; Tan, P. Effects of dietary sodium butyrate on growth, diet conversion, body chemical compositions and distal intestinal health in yellow drum (Nibea albiflora, Richardson). Aquac. Res. 2020, 51, 69–79. [Google Scholar] [CrossRef]
- Robles, R.; Lozano, A.B.; Sevilla, A.; Márquez, L.; Nuez-Ortín, W.; Moyano, F.J. Effect of partially protected butyrate used as feed additive on growth and intestinal metabolism in sea bream (Sparus aurata). Fish Physiol. Biochem. 2013, 39, 1567–1580. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Ballester-Lozano, G.F.; Simó, P.; Karalazos, V.; Ortiz, A.; Calduch-Giner, J.; Pérez-Sánchez, J. Lasting effects of butyrate and low FM/FO diets on growth performance, blood haematology/biochemistry and molecular growth-related markers in gilthead sea bream (Sparus aurata). Aquaculture 2016, 454, 8–18. [Google Scholar] [CrossRef]
- Estensoro, I.; Ballester-Lozano, G.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.-T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, A.; et al. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Zhang, G.; Zhang, J.; Shengli, T.; Wang, L.; Kalhoro, H.; Chen, Y.; Volatiana, J.A.; Sagada, G.; Zheng, L.; et al. Effects of microencapsulated sodium butyrate supplementation on growth performance, intestinal development and antioxidative capacity of juvenile black sea bream (Acanthopagrus schlegelii). Aquac. Res. 2020, 51, 4893–4904. [Google Scholar] [CrossRef]
- Zarei, S.; Badzohreh, G.; Davoodi, R.; Nafisi Bahabadi, M.; Salehi, F. Effects of dietary butyric acid glycerides on growth performance, haemato-immunological and antioxidant status of yellowfin seabream (Acanthopagrus latus) fingerlings. Aquac. Res. 2021, 52, 5840–5848. [Google Scholar] [CrossRef]
- Biswas, A.; Araki, H.; Sakata, T.; Nakamori, T.; Takii, K. Optimum fish meal replacement by soy protein concentrate from soymilk and phytase supplementation in diet of red sea bream, Pagrus major. Aquaculture 2019, 506, 51–59. [Google Scholar] [CrossRef]
- Takagi, S.; Shimeno, S.; Hosokawa, H.; Ukawa, M. Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream Pagrus major. Fish. Sci. 2001, 67, 1088–1096. [Google Scholar] [CrossRef]
- Hossain, M.S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M.; Islam, M.J. Fishmeal replacement by soya protein concentrate with inosine monophosphate supplementation influences growth, digestibility, immunity, blood health, and stress resistance of red sea bream, Pagrus major. Fish Physiol. Biochem. 2019, 45, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, B.E.; Khosravi, S.; Herault, M.; Fournier, V.; Lee, C.; Jeong, J.B.; Lee, K.J. Evaluation of shrimp or tilapia protein hydrolysate at graded dosages in low fish meal diet for olive flounder (Paralichthys olivaceus). Aquac. Nutr. 2020, 26, 1592–1603. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Khosravi, S.; Bui, H.T.D.; Rahimnejad, S.; Herault, M.; Fournier, V.; Kim, S.-S.; Jeong, J.-B.; Lee, K.-J. Dietary supplementation of marine protein hydrolysates in fish-meal based diets for red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus). Aquaculture 2015, 435, 371–376. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple-Range and Multiple F-Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, G.; Varghese, T. Effect of dietary gamma-aminobutyric acid on growth performance, haemato-immunological responses, antioxidant enzymes activity, ghrelin and IGF-I expression of Labeo rohita (Hamilton, 1822) fingerlings. Comp. Clin. Pathol. 2023, 32, 53–65. [Google Scholar] [CrossRef]
- Wu, F.; Liu, M.; Chen, C.; Chen, J.; Tan, Q. Effects of dietary gamma aminobutyric acid on growth performance, antioxidant status, and feeding-related gene expression of juvenile grass carp, Ctenopharyngodon idellus. J. World Aquac. Soc. 2016, 47, 820–829. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, G.; Wang, H.; Mo, W.; Huang, Y.; Cao, J.; Li, P. Effects of dietary sodium butyrate on growth, digestive enzymes, body composition and nutrient retention-related gene expression of juvenile yellow catfish (Pelteobagrus fulvidraco). Anim. Nutr. 2021, 7, 539–547. [Google Scholar] [CrossRef]
- El-Sharkawy, E.A.; Abd El-Razek, I.M.; Amer, A.A.; Soliman, A.A.; Shukry, M.; Gewaily, M.S.; Téllez-Isaías, G.; Kari, Z.A.; Dawood, M.A. Effects of sodium butyrate on the growth performance, digestive enzyme activity, intestinal health, and immune responses of Thinlip Grey Mullet (Liza ramada) juveniles. Aquac. Rep. 2023, 30, 101530. [Google Scholar] [CrossRef]
- Tola, S.; Fukada, H.; Masumoto, T. Effects of natural feeding stimulants and glutamic acid supplementation on the feed intake, growth performance and digestive enzyme activities of red sea bream (Pagrus major) fed fish meal-free soy protein concentrate (SPC)-based diet. Aquac. Res. 2019, 50, 1912–1920. [Google Scholar] [CrossRef]
- Herault, M.; Gunathilaka, B.E.; Fournier, V.; Le Bris, H.; Lee, K.-J.; Sadoul, B. Aquatic product hydrolysates increase rearing performance in red seabream (Pagrus major), fed a low fish meal diet, in both controlled and stressed conditions: From growth to stress responses. Aquaculture 2023, 576, 739830. [Google Scholar] [CrossRef]
- Temu, V.; Kim, H.; Hamidoghli, A.; Park, M.; Won, S.; Oh, M.; Han, J.K.; Bai, S.C. Effects of dietary gamma-aminobutyric acid in juvenile Nile tilapia, Orechromis niloticus. Aquaculture 2019, 507, 475–480. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Wang, Y.; Lu, S.; Han, S.; Liu, Y.; Jiang, H.; Wang, C.A.; Liu, H. Dietary Sodium Butyrate Improves Intestinal Health of Triploid Oncorhynchus mykiss Fed a Low Fish Meal Diet. Biology 2023, 12, 145. [Google Scholar] [CrossRef]
- Chen, W.; Chang, K.; Chen, J.; Zhao, X.; Gao, S. Dietary sodium butyrate supplementation attenuates intestinal inflammatory response and improves gut microbiota composition in largemouth bass (Micropterus salmoides) fed with a high soybean meal diet. Fish Physiol. Biochem. 2021, 47, 1805–1819. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Yu, P.; Xi, Z.; Xu, L.; Li, X.; He, N. Comparison of taurine, GABA, Glu, and Asp as scavengers of malondialdehyde in vitro and in vivo. Nanoscale Res. Lett. 2013, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.S.; Lee, H.-M.; Jin, H.S.; Neupane, C.; Kim, S.; Lee, S.-H.; Min, J.-J.; Sasai, M.; Jeong, J.-H.; et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat. Commun. 2018, 9, 4184. [Google Scholar] [CrossRef] [PubMed]
- Bhandage, A.K.; Barragan, A. GABAergic signaling by cells of the immune system: More the rule than the exception. Cell. Mol. Life Sci. 2021, 78, 5667–5679. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, B.E.; Jeong, S.-M.; Cho, M.-U.; Kim, K.-W.; Hur, S.-W.; Lee, S.; You, S.-G.; Lee, S.-M. Effects of Dietary Fish Meal Replacement with Alternative Protein Ingredients and Their Combinations on Growth Performance, Feed Utilization, Fillet Composition, and Biochemical Parameters of Red Seabream (Pagrus major). Aquac. Nutr. 2023, 2023, 8883739. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Araki, H.; Sakata, T.; Nakamori, T.; Kato, K.; Takii, K. Fish meal replacement by soy protein from soymilk in the diets of red sea bream (Pagrus major). Aquac. Nutr. 2017, 23, 1379–1389. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.; El Basuini, M.F.; El-Hais, A.M.; Olivier, A. Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 2018, 490, 228–235. [Google Scholar] [CrossRef]
- Kader, M.A.; Bulbul, M.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Nguyen, B.T.; Komilus, C.F. Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream, Pagrus major. Aquaculture 2012, 350, 109–116. [Google Scholar] [CrossRef]
- Biswas, A.; Takahashi, Y.; Isaka, K.; Takakuwa, F.; Tanaka, H.; Takii, K. Total Replacement of Fish Meal by the Combination of Fish Residue Meal and Soy Protein from Soymilk in the Diet of Red Sea Bream (Pagrus major). Animals 2022, 12, 3351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-C. The selenium accumulation and its effect on growth, and haematological parameters in red sea bream, Pagrus major, exposed to waterborne selenium. Ecotoxicol. Environ. Saf. 2014, 104, 96–102. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Bakr, A.; El-Keredy, A.M.; Dawood, M.A.; Dossou, S.; Wang, W.; Yukun, Z. Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish. Immunol. 2018, 79, 303–312. [Google Scholar] [CrossRef]
- Storebakken; Shearer; Roem. Growth, uptake and retention of nitrogen and phosphorus, and absorption of other minerals in Atlantic salmon Salmo salar fed diets with fish meal and soy-protein concentrate as the main sources of protein. Aquac. Nutr. 2000, 6, 103–108. [Google Scholar] [CrossRef]
- Medagoda, N.; Kim, M.G.; Gunathilaka, B.E.; Park, S.H.; Lee, K.J. Effect of total replacement of fish oil with tallow and emulsifier in diet on growth, feed utilization, and immunity of olive flounder (Paralichthys olivaceus). J. World Aquac. Soc. 2022, 53, 558–571. [Google Scholar] [CrossRef]
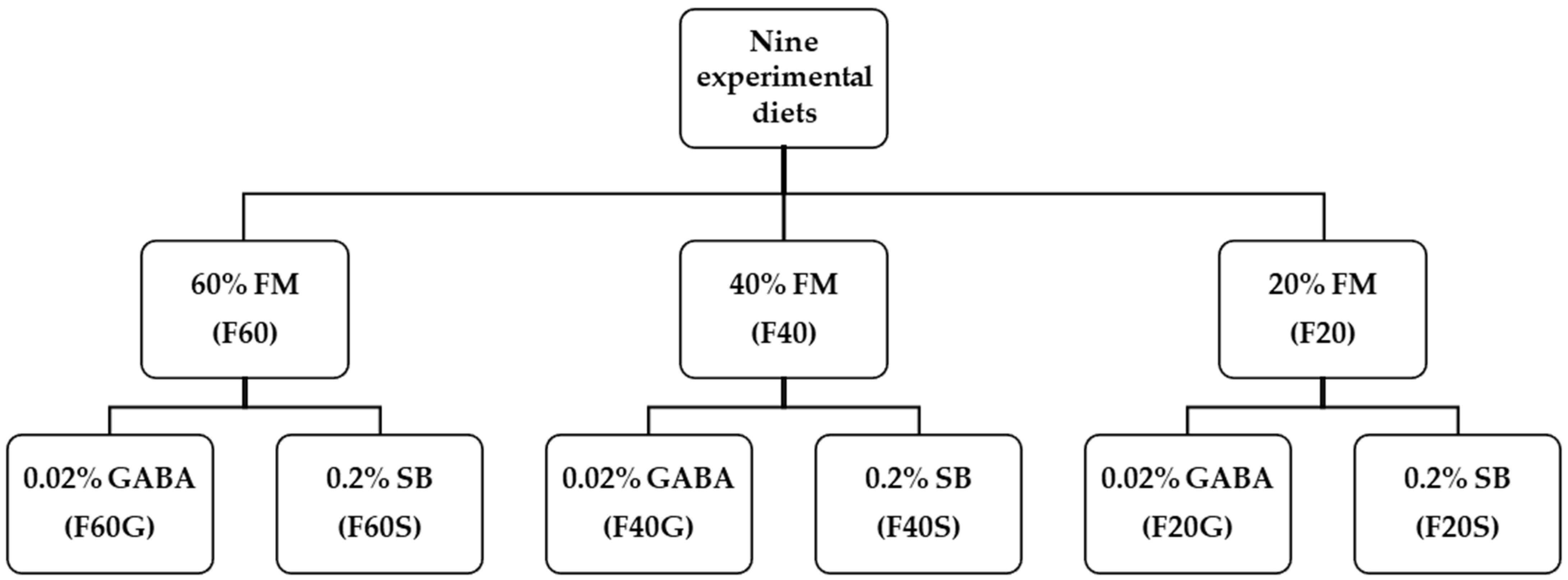

| Ingredients | F60 | F60G | F60S | F40 | F40G | F40S | F20 | F20G | F20S |
|---|---|---|---|---|---|---|---|---|---|
| Fish meal (FAQ 65) 1 | 60.0 | 60.0 | 60.0 | 40.0 | 40.0 | 40.0 | 20.0 | 20.0 | 20.0 |
| GABA 2 | 0.02 | 0.02 | 0.02 | ||||||
| Sodium butyrate 3 | 0.20 | 0.20 | 0.20 | ||||||
| Soy protein concentrate | 3.75 | 3.75 | 3.75 | 20.10 | 20.10 | 20.10 | 38.45 | 38.45 | 38.45 |
| Corn gluten meal | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |||
| Wheat flour | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Fish oil | 1.00 | 1.00 | 1.00 | 3.00 | 3.00 | 3.00 | 4.95 | 4.95 | 4.95 |
| Soybean oil | 5.78 | 5.78 | 5.78 | 5.49 | 5.49 | 5.49 | 5.24 | 5.24 | 5.24 |
| Mineral mix 4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin mix 5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Starch | 6.97 | 6.95 | 6.77 | 4.51 | 4.49 | 4.31 | 2.60 | 2.58 | 2.40 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Lysine | 0.34 | 0.34 | 0.34 | 0.63 | 0.63 | 0.63 | |||
| Methionine | 0.49 | 0.49 | 0.49 | 1.00 | 1.00 | 1.00 | |||
| Taurine | 0.07 | 0.07 | 0.07 | 0.13 | 0.13 | 0.13 | |||
| Monocalcium phosphate | 1.50 | 1.50 | 1.50 | 2.50 | 2.50 | 2.50 | |||
| Proximate composition | |||||||||
| Dry matter | 93.6 | 93.3 | 94.2 | 93.3 | 93.6 | 94.3 | 93.8 | 93.7 | 95.1 |
| Protein | 46.4 | 47.1 | 47.9 | 46.1 | 46.3 | 46.0 | 47.6 | 47.2 | 46.5 |
| Lipid | 13.3 | 14.3 | 14.0 | 13.8 | 13.7 | 14.3 | 14.6 | 14.7 | 14.2 |
| Ash | 11.1 | 11.2 | 11.3 | 10.0 | 9.83 | 10.0 | 8.73 | 8.34 | 8.53 |
| FBW (g) 1 | WG (%) 2 | SGR (%) 3 | FI (g/fish) 4 | FE (%) 5 | PER 6 | SUR (%) 7 | |
|---|---|---|---|---|---|---|---|
| F60 | 19.5 ± 1.20 b | 255 ± 19.9 bc | 2.26 ± 0.10 bc | 22.3 ± 1.52 | 59.4 ± 0.46 ab | 1.28 ± 0.01 ab | 88.0 ± 4.00 |
| F60G | 21.0 ± 0.51 a | 281 ± 18.3 ab | 2.39 ± 0.09 ab | 23.5 ± 1.81 | 61.9 ± 8.42 ab | 1.31 ± 0.18 ab | 84.0 ± 4.00 |
| F60S | 21.4 ± 0.29 a | 287 ± 9.56 a | 2.42 ± 0.04 a | 24.1 ± 0.43 | 61.4 ± 2.50 ab | 1.28 ± 0.05 ab | 84.0 ± 4.00 |
| F40 | 19.1 ± 0.23 b | 247 ± 0.67 c | 2.22 ± 0.00 c | 20.8 ± 0.50 | 59.2 ± 2.75 ab | 1.28 ± 0.06 ab | 81.3 ± 2.31 |
| F40G | 20.1 ± 0.53 ab | 265 ± 6.49 abc | 2.31 ± 0.03 abc | 23.1 ± 1.82 | 59.0 ± 5.26 ab | 1.27 ± 0.11 ab | 84.0 ± 4.00 |
| F40S | 21.1 ± 0.62 a | 285 ± 21.2 a | 2.40 ± 0.10 ab | 22.5 ± 1.39 | 66.0 ± 5.26 a | 1.44 ± 0.11 a | 88.0 ± 10.6 |
| F20 | 14.0 ± 0.47 e | 154 ± 15.8 e | 1.66 ± 0.11 e | 19.0 ± 1.43 | 38.6 ± 2.02 c | 0.84 ± 0.04 c | 82.7 ± 4.62 |
| F20G | 15.3 ± 0.34 d | 176 ± 7.28 e | 1.81 ± 0.05 e | 21.4 ± 1.19 | 39.7 ± 4.08 c | 0.86 ± 0.09 c | 81.3 ± 2.31 |
| F20S | 17.4 ± 1.30 c | 216 ± 21.7 d | 2.05 ± 0.12 d | 19.9 ± 1.48 | 56.4 ± 3.93 b | 1.23 ± 0.09 b | 89.3 ± 10.1 |
| Two-way ANOVA (p value) | |||||||
| FM level | 0.000 | 0.000 | 0.000 | 0.022 | 0.000 | 0.000 | 0.169 |
| GABA | 0.001 | 0.004 | 0.004 | 0.013 | 0.616 | 0.746 | 0.615 |
| SB | 0.000 | 0.000 | 0.000 | 0.025 | 0.000 | 0.000 | 0.346 |
| FM × GABA | 0.778 | 0.852 | 0.787 | 0.748 | 0.877 | 0.926 | 0.317 |
| FM × SB | 0.235 | 0.312 | 0.092 | 0.769 | 0.003 | 0.002 | 0.320 |
| Lysozyme 1 | SOD 2 | |
|---|---|---|
| F60 | 29.6 ± 0.81 ab | 76.7 ± 5.38 |
| F60G | 30.0 ± 0.85 ab | 75.2 ± 1.62 |
| F60S | 30.7 ± 0.49 a | 79.2 ± 4.64 |
| F40 | 27.8 ± 0.96 bc | 72.6 ± 2.87 |
| F40G | 29.1 ± 1.03 ab | 75.3 ± 1.50 |
| F40S | 29.3 ± 0.49 ab | 76.3 ± 1.11 |
| F20 | 26.3 ± 0.37 c | 72.0 ± 1.42 |
| F20G | 28.5 ± 0.67 ab | 75.3 ± 0.56 |
| F20S | 29.1 ± 0.37 ab | 76.6 ± 0.75 |
| Two-way ANOVA (p value) | ||
| FM level | 0.036 | 0.668 |
| GABA | 0.055 | 0.519 |
| SB | 0.004 | 0.203 |
| FM × GABA | 0.538 | 0.630 |
| FM × SB | 0.402 | 0.945 |
| GOT (U/L) 1 | ALP (U/L) 2 | T-CHO (mg/dL) 3 | TG (ng/dL) 4 | TP (g/dL) 5 | GLU (mg/dL) 6 | |
|---|---|---|---|---|---|---|
| F60 | 33.0 ± 0.82 | 141 ± 73.5 | 241 ± 6.53 ab | 344 ± 128 | 3.15 ± 0.29 ab | 110 ± 25.0 |
| F60G | 39.0 ± 1.63 | 231 ± 55.2 | 263 ± 6.74 ab | 165 ± 12.7 | 3.47 ± 0.09 a | 134 ± 28.8 |
| F60S | 30.0 ± 0.82 | 222 ± 108 | 296 ± 15.9 a | 273 ± 52.3 | 3.37 ± 0.07 a | 113 ± 8.37 |
| F40 | 42.0 ± 3.61 | 186 ± 24.5 | 216 ± 15.6 bc | 326 ± 90.7 | 3.27 ± 0.07 ab | 117 ± 19.4 |
| F40G | 29.0 ± 6.51 | 197 ± 7.00 | 206 ± 11.6 bcd | 221 ± 48.3 | 3.23 ± 0.12 ab | 99.7 ± 24.7 |
| F40S | 33.3 ± 6.96 | 170 ± 7.69 | 220 ± 27.6 bc | 183 ± 34.7 | 3.20 ± 0.10 ab | 97.0 ± 11.0 |
| F20 | 34.3 ± 1.45 | 220 ± 39.1 | 149 ± 16.4 de | 178 ± 12.9 | 2.87 ± 0.09 b | 97.3 ± 9.53 |
| F20G | 42.0 ± 6.35 | 184 ± 23.6 | 161 ± 27.5 cde | 186 ± 37.9 | 2.83 ± 0.20 b | 103 ± 9.84 |
| F20S | 32.7 ± 8.57 | 187 ± 55.9 | 138 ± 32.4 e | 246 ± 97.7 | 2.90 ± 0.15 b | 104 ± 25.6 |
| Two-way ANOVA (p value) | ||||||
| FM level | 0.087 | 0.427 | 0.000 | 0.297 | 0.001 | 0.692 |
| GABA | 0.306 | 0.487 | 0.532 | 0.077 | 0.422 | 0.792 |
| SB | 0.781 | 0.362 | 0.358 | 0.408 | 0.486 | 0.808 |
| FM × GABA | 0.089 | 0.273 | 0.602 | 0.309 | 0.410 | 0.573 |
| FM × SB | 0.236 | 0.159 | 0.286 | 0.332 | 0.589 | 0.677 |
| Condition Factor 1 | Hepatosomatic Index 2 | Viscerosomatic Index 3 | |
|---|---|---|---|
| F60 | 1.87 ± 0.02 | 1.20 ± 0.21 | 6.59 ± 0.69 |
| F60G | 1.77 ± 0.11 | 1.23 ± 0.27 | 6.73 ± 0.53 |
| F60S | 1.82 ± 0.09 | 1.22 ± 0.09 | 6.86 ± 0.10 |
| F40 | 1.87 ± 0.03 | 1.25 ± 0.41 | 6.99 ± 0.18 |
| F40G | 1.77 ± 0.06 | 1.21 ± 0.32 | 6.86 ± 0.77 |
| F40S | 1.75 ± 0.06 | 1.22 ± 0.13 | 6.41 ± 0.41 |
| F20 | 1.72 ± 0.05 | 0.95 ± 0.06 | 5.70 ± 0.36 |
| F20G | 1.78 ± 0.11 | 1.06 ± 0.14 | 6.30 ± 1.08 |
| F20S | 1.70 ± 0.06 | 1.22 ± 0.17 | 6.52 ± 0.50 |
| Two-way ANOVA (p value) | |||
| FM level | 0.268 | 0.087 | 0.064 |
| GABA | 0.802 | 0.529 | 0.199 |
| SB | 0.402 | 0.413 | 0.039 |
| FM × GABA | 0.897 | 0.642 | 0.118 |
| FM × SB | 0.464 | 0.044 | 0.328 |
| Dry Matter | Protein | Lipid | Ash | |
|---|---|---|---|---|
| F60 | 25.6 ± 0.68 | 20.0 ± 0.60 | 4.24 ± 0.36 | 1.29 ± 0.08 |
| F60G | 25.8 ± 0.44 | 19.9 ± 1.26 | 4.26 ± 0.22 | 1.29 ± 0.09 |
| F60S | 25.3 ± 0.34 | 19.4 ± 1.07 | 3.78 ± 0.90 | 1.40 ± 0.06 |
| F40 | 25.0 ± 0.82 | 19.5 ± 0.91 | 2.65 ± 0.84 | 1.30 ± 0.06 |
| F40G | 25.2 ± 0.15 | 19.5 ± 0.35 | 3.03 ± 0.73 | 1.39 ± 0.01 |
| F40S | 24.9 ± 0.24 | 19.7 ± 0.39 | 3.64 ± 1.12 | 1.35 ± 0.05 |
| F20 | 25.0 ± 0.61 | 20.1 ± 0.69 | 3.11 ± 0.67 | 1.42 ± 0.03 |
| F20G | 24.9 ± 0.27 | 20.4 ± 0.79 | 4.05 ± 0.67 | 1.35 ± 0.13 |
| F20S | 24.8 ± 0.32 | 19.6 ± 0.38 | 3.87 ± 0.46 | 1.34 ± 0.05 |
| Two-way ANOVA (p value) | ||||
| FM level | 0.095 | 0.706 | 0.213 | 0.708 |
| GABA | 0.630 | 0.879 | 0.389 | 0.865 |
| SB | 0.761 | 0.607 | 0.507 | 0.526 |
| FM × GABA | 0.644 | 0.971 | 0.754 | 0.563 |
| FM × SB | 0.960 | 0.835 | 0.610 | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilaka, B.E.; Jeong, S.-M.; Kim, K.-W.; Lee, S.; Hur, S.-W.; You, S.-G.; Lee, S.-M. Evaluation of Gamma Aminobutyric Acid and Sodium Butyrate in Juvenile Red Seabream (Pagrus major) Diets Containing Graded Levels of Fish Meal and Soy Protein Concentrate. Animals 2024, 14, 1973. https://doi.org/10.3390/ani14131973
Gunathilaka BE, Jeong S-M, Kim K-W, Lee S, Hur S-W, You S-G, Lee S-M. Evaluation of Gamma Aminobutyric Acid and Sodium Butyrate in Juvenile Red Seabream (Pagrus major) Diets Containing Graded Levels of Fish Meal and Soy Protein Concentrate. Animals. 2024; 14(13):1973. https://doi.org/10.3390/ani14131973
Chicago/Turabian StyleGunathilaka, Buddhi E., Seong-Mok Jeong, Kang-Woong Kim, Seunghan Lee, Sang-Woo Hur, Sang-Guan You, and Sang-Min Lee. 2024. "Evaluation of Gamma Aminobutyric Acid and Sodium Butyrate in Juvenile Red Seabream (Pagrus major) Diets Containing Graded Levels of Fish Meal and Soy Protein Concentrate" Animals 14, no. 13: 1973. https://doi.org/10.3390/ani14131973
APA StyleGunathilaka, B. E., Jeong, S.-M., Kim, K.-W., Lee, S., Hur, S.-W., You, S.-G., & Lee, S.-M. (2024). Evaluation of Gamma Aminobutyric Acid and Sodium Butyrate in Juvenile Red Seabream (Pagrus major) Diets Containing Graded Levels of Fish Meal and Soy Protein Concentrate. Animals, 14(13), 1973. https://doi.org/10.3390/ani14131973








