Arginine Promoted Ovarian Development in Pacific White Shrimp Litopenaeus vannamei via the NO-sGC-cGMP and TORC1 Signaling Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: 12-Week Arginine Feeding Experiment
2.1.1. Farming Experiment
2.1.2. Shrimp Culture and Condition Monitoring
2.1.3. Sample Collection
2.2. Experiment 2: RNA Interference Experiment for Silencing TOR and NOS Genes
2.2.1. Double-Stranded RNA (dsRNA) Preparation
2.2.2. RNA Interference Treatment
2.3. Experimental Parameters
2.3.1. Hormone Concentrations and Enzyme Activity
2.3.2. Ovarian Histological Examination
2.3.3. Crude Protein Content and Amino Acid Composition Analysis in Hepatopancreas and Ovary
2.3.4. Gene Expression Analysis by Real-Time Quantitative PCR (qPCR)
2.4. Statistical Analysis
3. Results
3.1. Experiment 1: 12-Week Arginine Feeding Experiment
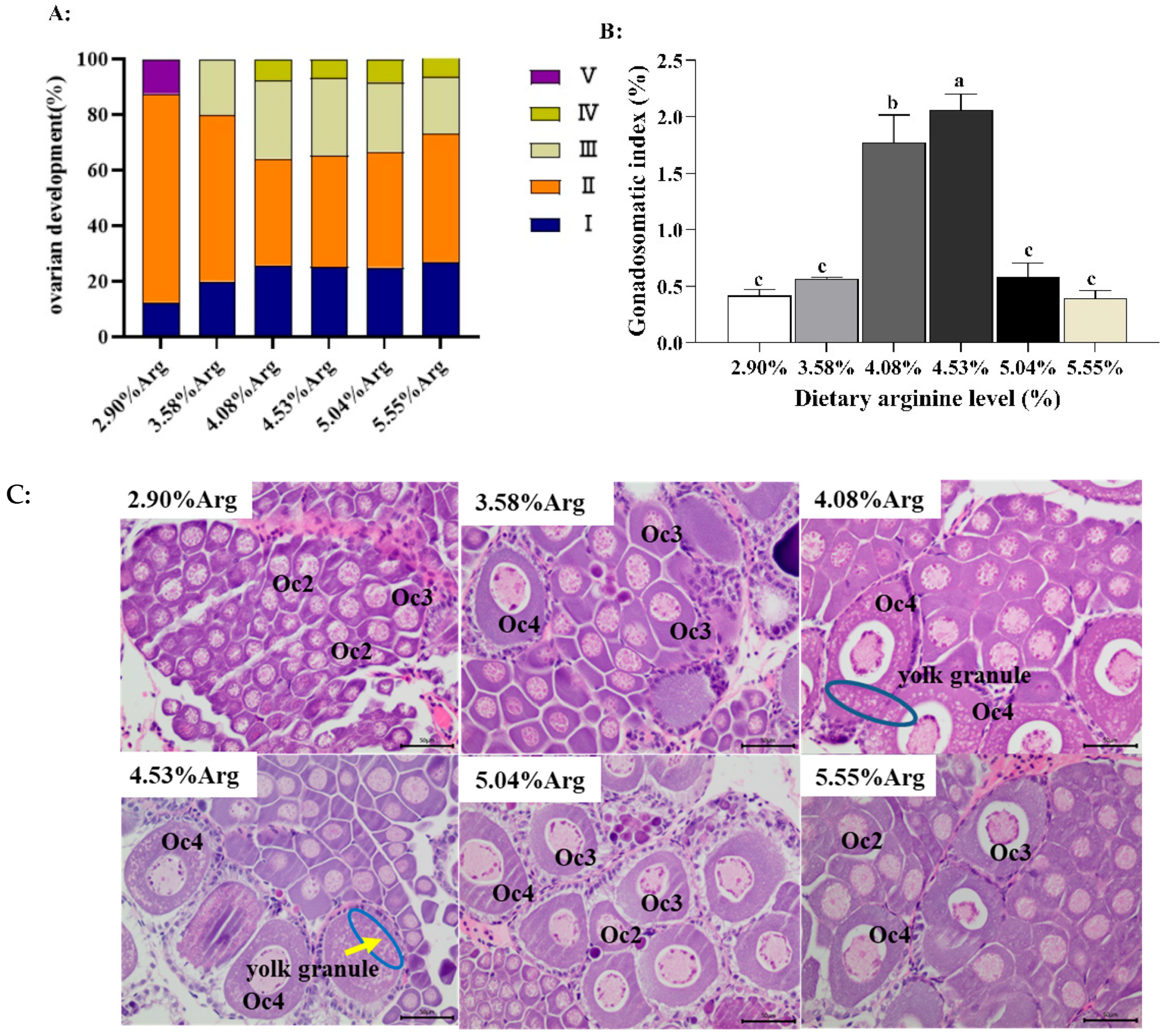
3.1.1. Statistics of Ovarian Development Stages and Ovary Histological Evaluation
3.1.2. Serum Hormone Concentrations Associated with Ovarian Development
3.1.3. Expression of Ovarian Development and Arg-Transported Genes in the Ovarian Tissue
3.1.4. The Crude Protein Content and Amino Acid Composition of the Hepatopancreas and Ovary
3.1.5. Gene Expression Related to mTORC1 Signaling Pathway in the Ovary and Hepatopancreas
3.1.6. Expression of Vitellogenin and Vgr Genes in the Ovary and Hepatopancreas
3.1.7. Vitellogenin Concentration in Serum and Hepatopancreas
3.1.8. Arg Metabolism Associated Enzyme Activity and Gene Expression
3.2. Experiment 2 RNA Interference Experiment for Silencing TOR and NOS Genes
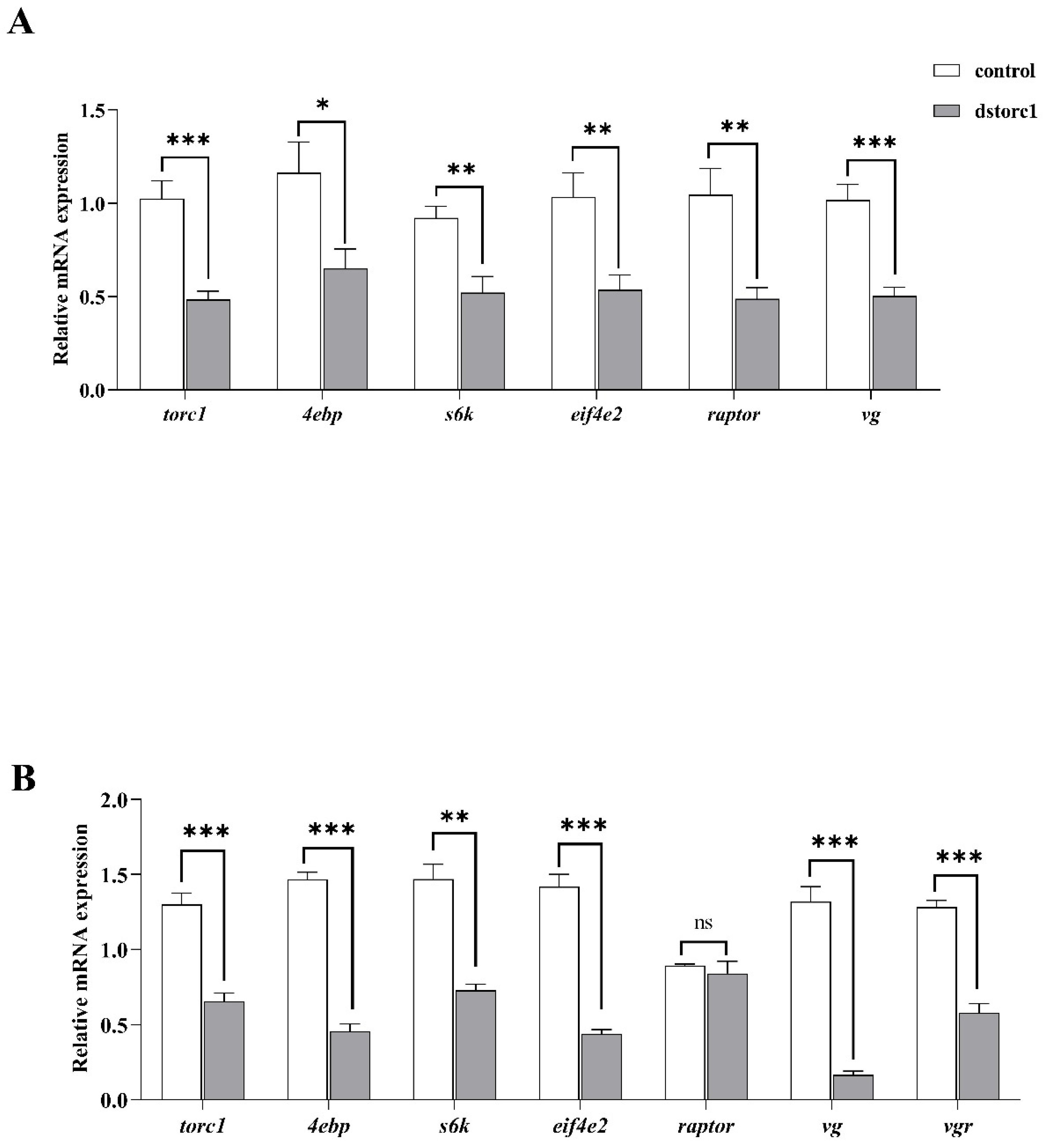
3.2.1. TOR Knockdown Experiment
3.2.2. NOS Knockdown Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- FAO. World Food Situation. 2020. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 9 October 2023).
- Yang, D.Z.; Wang, C.J.; Kou, N.; Xing, J.B.; Li, X.; Zhao, H.; Luo, M. Gonadal maturation in Litopenaeus vannamei fed on four different polychaetes. Aquacult. Rep. 2022, 22, 100920. [Google Scholar] [CrossRef]
- Wouters, R.; Lavens, P.; Nieto, J.; Sorgeloos, P. Penaeid shrimp broodstock nutrition: An updated review on research and development. Aquaculture 2001, 202, 1–21. [Google Scholar] [CrossRef]
- Gao, H. Amino acids in reproductive nutrition and health. Adv. Exp. Med. Biol. 2020, 1265, 111–131. [Google Scholar] [PubMed]
- Zhang, G.M.; Guo, Y.X.; Cheng, C.Y.; El-Samahy, M.A.; Tong, R.; Gao, X.X.; Deng, K.P.; Wang, F.; Lei, Z.H. Arginine infusion rescues ovarian follicular development in feed-restricted Hu sheep during the luteal phase. Theriogenology 2020, 158, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, V.A.; Xin, Q.; Sun, M.F.; Zhao, J.P.; Wang, X.J.; Jiao, H.C.; Onagbesan, O.M.; Lin, H. Research Note: Effects of dietary L-arginine on the production performance and gene expression of reproductive hormones in laying hens fed low crude protein diets. Poult. Sci. 2022, 101, 101816. [Google Scholar] [CrossRef] [PubMed]
- Wassef, E.A.; El-Husseiny, O.M.; El-Kasheif, M.A.; Aboseif, A.M.; Suloma, A. Effect of different dietary lipid sources and arginine supplementation on body-composition and gonadal development of young Nile tilapia (Oreochromis niloticus). J. Egypt. Acad. Soc. Environ. Develop. 2017, 18, 59–69. [Google Scholar]
- Qi, C.; Wang, X.; Han, F.; Jia, Y.; Lin, Z.; Wang, C.; Lu, J.; Yang, L.; Wang, X.; Li, E.; et al. Arginine supplementation improves growth, antioxidant capacity, immunity and disease resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2019, 93, 463–473. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Zeng, W.P.; Wang, H.L.; Wang, T.; Wang, Y.L.; Xie, F.J. Dietary arginine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2012, 364, 252–258. [Google Scholar] [CrossRef]
- Palma, J.; Andrade, J.P.; Lemme, A.; Bureau, D.P. Quantitative dietary requirement of juvenile Atlantic ditch shrimp Palaemonetes varians for lysine, methionine and arginine. Aquac. Res. 2015, 46, 1822–1830. [Google Scholar] [CrossRef]
- Ngernsoungnern, A.; Ngernsoungnern, P.; Weerachatyanukul, W.; Chavadej, J.; Sobhon, P.; Sretarugsa, P.J.A. The existence of gonadotropin-releasing hormone (GnRH) immunoreactivity in the ovary and the effects of GnRHs on the ovarian maturation in the black tiger shrimp Penaeus monodon. Aquaculture 2008, 279, 197–203. [Google Scholar] [CrossRef]
- Tinikul, Y.; Poljaroen, J.; Tinikul, R.; Anuracpreeda, P.; Chotwiwatthanakun, C.; Senin, N.; Poomtong, T.; Hanna, P.J.; Sobhon, P.J.A. Effects of gonadotropin-releasing hormones and dopamine on ovarian maturation in the Pacific white shrimp, Litopenaeus vannamei, and their presence in the ovary during ovarian development. Aquaculture 2014, 420–421, 79–88. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, T.; Wang, C.; Chan, S.; Wang, W. Deciphering the molecular regulatory mechanism orchestrating ovary development of the Pacific whiteleg shrimp Litopenaeus vannamei through integrated transcriptomic analysis of reproduction-related organs. Aquaculture 2021, 533, 736160. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Ruan, Y.; Zhou, M.; Liu, J.; Li, Z.; Wu, X.; Ren, C.; Zhang, X.; Zhang, J.; et al. Pacific white shrimp (Litopenaeus vannamei) vitelline membrane outer layer protein 1 (VMO1) is produced in the hepatopancreas and transported into ovarian oocytes during vitellogenesis. Gene 2023, 851, 147027. [Google Scholar] [CrossRef]
- Yao, C.L.; Ji, P.F.; Wang, Z.Y.; Li, F.H.; Xiang, J.H. Molecular cloning and expression of NOS in shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2010, 28, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Tinikul, Y.; Poljaroen, J.; Nuurai, P.; Anuracpreeda, P.; Chotwiwatthanakun, C.; Phoungpetchara, I.; Kornthong, N.; Poomtong, T.; Hanna, P.J.; Sobhon, P. Existence and distribution of gonadotropin-releasing hormone-like peptides in the central nervous system and ovary of the Pacific white shrimp, Litopenaeus vannamei. Cell Tissue Res. 2011, 343, 579–593. [Google Scholar] [CrossRef]
- Swetha, C.H.; Girish, B.P.; Reddy, P.S. Elucidation of the role of estradiol and progesterone in regulating reproduction in the edible crab, Oziothelphusa senex senex. RSC Adv. 2016, 6, 24959–24967. [Google Scholar] [CrossRef]
- Jayasankar, V.; Tomy, S.; Wilder, M.N. Insights on molecular mechanisms of ovarian development in decapod crustacea: Focus on vitellogenesis-stimulating factors and pathways. Front. Endocrinol. 2020, 11, 577925. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Yue, D.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S.; Wu, Y. Effects of dietary supplementation with 17 beta-estradiol and 17 alpha-methyltestosterone on growth performance and gonadal development of the juvenile oriental river prawn (Macrobrachium nipponense). Aquacult. Rep. 2022, 23, 101042. [Google Scholar]
- Zhang, Z.P.; Shen, B.L.; Wang, Y.L.; Chen, Y.; Wang, G.D.; Lin, P.; Zou, Z.H. Molecular cloning of proliferating cell nuclear antigen and its differential expression analysis in the developing ovary and testis of penaeid shrimp Marsupenaeus japonicus. DNA Cell Biol. 2010, 29, 163–170. [Google Scholar] [CrossRef]
- Okutsu, T.; Kang, B.J.; Miwa, M.; Yoshizaki, G.; Maeno, Y.; Wilder, M.N. Molecular cloning and characterization of Dmc1, a gene involved in gametogenesis, from the whiteleg shrimp Litopenaeus vannamei. Fish Sci. 2010, 76, 961–969. [Google Scholar] [CrossRef]
- Inada, M.; Mekata, T.; Sudhakaran, R.; Okugawa, S.; Kono, T.; Asely, A.M.E.; Linh, N.T.; Yoshida, T.; Sakai, M.; Yui, T.; et al. Molecular cloning and characterization of the nitric oxide synthase gene from kuruma shrimp, Marsupenaeus japonicus. Fish Shellfish Immunol. 2010, 28, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Girish, B.P.; Swetha, C.H.; Reddy, P.S. Expression of RXR, EcR, E75 and VtG mRNA levels in the hepatopancreas and ovary of the freshwater edible crab, Oziothelphusa senex senex (Fabricius, 1798) during different vitellogenic stages. Naturwissenschaften 2015, 102, 20. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Yu, K.; Shu, L.; Ye, H.H.; Li, S.J.; Zeng, C.S. Evaluating the effects of temperature, salinity, starvation and autotomy on molting success, molting interval and expression of ecdysone receptor in early juvenile mud crabs, Scylla paramamosain. J. Exp. Mar. Biol. Ecol. 2015, 464, 11–17. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.Y.; Ruan, Y.; Chen, X.L.; Ren, C.H.; Yang, H.; Zhang, X.; Liu, J.S.; Li, H.; Zhang, L.; et al. Transcriptomic analysis reveals yolk accumulation mechanism from the hepatopancreas to ovary in the Pacific white shrimp Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 948105. [Google Scholar] [CrossRef]
- Tiu, S.; Benzie, H.J.; Chan, S.M. From hepatopancreas to ovary: Molecular characterization of a shrimp vitellogenin receptor involved in the processing of vitellogenin. Biol. Reprod. 2008, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.Q.; Wang, C.; Yang, L.; Wang, W.; Deng, W.; Wu, G. Structural mechanism for the arginine sensing and regulation of CASTOR1 in the mTORC1 signaling pathway. Cell Discov. 2016, 2, 16051. [Google Scholar] [CrossRef] [PubMed]
- Villegas, S.N.; Gombos, R.; García-López, L.; Gutiérrez-Pérez, I.; García-Castillo, J.; Vallejo, D.M.; Da Ros, V.G.; Ballesta-Illán, E.; Mihály, J.; Dominguez, M. PI3K/Akt cooperates with oncogenic notch by inducing nitric oxide-dependent inflammation. Cell Rep. 2018, 22, 2541–2549. [Google Scholar] [CrossRef]
- Budani, M.C.; Tiboni, G.M. Novel insights on the role of nitric oxide in the ovary: A review of the literature. Int. J. Environ. Res. Public Health 2021, 18, 980. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Wang, Y.; Niu, C. The dual role of cGMP in oocyte maturation of zebrafish. Biochem. Biophys. Res. Commun. 2018, 499, 998–1003. [Google Scholar] [CrossRef]
- Liang, X.; Luo, X.; Lin, H.; Han, F.; Qin, J.G.; Chen, L.; Xu, C.; Li, E. Effects and mechanism of different phospholipid diets on ovary development in female broodstock Pacific white shrimp, Litopenaeus vannamei. Front. Nutr. 2022, 9, 830934. [Google Scholar] [CrossRef]
- Basini, G.; Grasselli, F. Nitric oxide in follicle development and oocyte competence. Reproduction 2015, 150, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, P.; Jafari Atrabi, M.; Akbarinejad, V.; Khanbabaei, R.; Fathi, R. Incorporation of arginine, glutamine or leucine in culture medium accelerates in vitro activation of primordial follicles in 1-day-old mouse ovary. Zygote 2020, 28, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Grazul-Bilska, A.T.; Bass, C.S.; Kaminski, S.L.; Ebel, K.K.; Leke, E.; Thammasiri, J.; Kraisoon, A.; Navanukraw, C.; Holst, M.; Shelton, M.; et al. Effects of plane of nutrition and arginine on ovarian follicles in non-pregnant sheep: Cell proliferation, and expression of endothelial nitric oxide and its receptor. Acta. Histochem. 2019, 121, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Dubeibe, D.F.; Caldas-Bussiere, M.C.; Maciel, V.L., Jr.; Sampaio, W.V.; Quirino, C.R.; Gonçalves, P.B.; De Cesaro, M.P.; Faes, M.R.; Paes de Carvalho, C.S. L-arginine affects the IVM of cattle cumulus-oocyte complexes. Theriogenology 2017, 88, 134–144. [Google Scholar] [CrossRef] [PubMed]





| Ingredients (g/100 g) | 2.90 %Arg | 3.58 %Arg | 4.08 %Arg | 4.53 %Arg | 5.04 %Arg | 5.55 %Arg |
|---|---|---|---|---|---|---|
| Fish meal (Peru) | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 |
| Soy protein concentrate | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Krill meal | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 |
| Baker’s yeast hydrolysate | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Domestic fish meal | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Squid offal powder | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Squid powder | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Sandworms | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Wheat flour | 22.19 | 22.19 | 22.19 | 22.19 | 22.19 | 22.19 |
| Fish oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soy lecithin | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Cholesterol | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Mineral premix 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix 2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Ca (H2PO4)2 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Choline chloride | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Non-essential amino acid premix 3 | 5.51 | 4.41 | 3.31 | 2.20 | 1.10 | 0.00 |
| Arginine | 0.00 | 0.50 | 1.00 | 1.50 | 2.00 | 2.50 |
| Cellulose | 0.00 | 0.60 | 1.20 | 1.81 | 2.41 | 3.01 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Crude nutrient levels 4 | ||||||
| Protein (%) | 54.00 | 54.38 | 54.71 | 55.25 | 54.07 | 54.00 |
| Crude lipid (%) | 9.62 | 9.54 | 9.66 | 9.56 | 9.53 | 9.60 |
| Moisture (%) | 8.48 | 9.00 | 8.76 | 8.32 | 8.88 | 8.76 |
| Ash (%) | 10.95 | 10.91 | 11.27 | 10.75 | 11.48 | 10.96 |
| Arginine (%) | 2.90 | 3.58 | 4.08 | 4.53 | 5.04 | 5.55 |
| Primers | Sequence (5′-3′) | TM |
|---|---|---|
| T7-dsGFP-F | TAATACGACTCACTATAGGGCGACGTAAACGGCCACAAGT | 70.31 |
| T7-dsGFP-R | TAATACGACTCACTATAGGGCTTGTACAGCTCGTCCATGC | 70.61 |
| T7-dsTOR-F | TAATACGACTCACTATAGGGGTTATGTCACCACGGAGTT | 67.43 |
| T7-dsTOR-R | TAATACGACTCACTATAGGGAGTGCCACCCGAAGA | 68.52 |
| T7-dsNOS-F | TAATACGACTCACTATAGGGTCACGCCCGTATTCCA | 67.76 |
| T7-dsNOS-R | TAATACGACTCACTATAGGGCGTTCCGCTAACTTTCAT | 68.32 |
| tor for qPCR | F: TTTGAAGTTGCCATGACCCG | 52.52 |
| tor for qPCR | R: GAGACGCCAATTCAGCAGAG | 54.62 |
| nos for qPCR | F: ATGAGGACGGGACCATCATC | 53.12 |
| nos for qPCR | R: TTGTATTCGGGGTGGCTGAT | 54.91 |
| Gene | Accession No. | Primers (5′-3′) |
|---|---|---|
| dmc1 | HQ116385.1 | F: CGAGGAATACAACGTGTCTGTC R: ATTCGGGGCTGTCGTAGAT |
| pcna | JN034913.1 | F: ATTGCCTTCTGGGGAGTTC R: CAAGCAAAGGTGAGCGTGA |
| torc1 | XM_027372359.1 | F: TGCCAACGGGTGGTAGA R: GGGTGTTTGTGGACGGA |
| vitellogenin | KM077131.1 | F: GAACCCTAAGGCTATCATCACTG R: AGGTCGCTCTTCCATCTTTACT |
| cyp18a1 | XM_027360159.1 | F: CTCCTGAGGTGCCTGTCG R: GGGATGTAGTTGGCGATG |
| ecr | XM_027356283.1 | F: GTTATGACGCAAAGACCAAT R: TTACGACAGAAGCGAAAGAG |
| rxra | Reference [13] | F: CCCTCTCCTCTCTCCCTC R: GGTTGCTGCTCCCATTAC |
| 4ebp | XM_027367939.1 | F: ATGTCTGCTTCGCCCGTCGCTCGCC R: GGTTCTTGGGTGGGCTCTT |
| vgr | MN807241.1 | F: TCTCCTCGTCTTGGCTCT R: GCAAACTGCGGCGGCTGG |
| eif4e | XM_027354395.1 | F: TGGAATCAAACCTATGTGGG R: GTCCTCCTGGAAGCGTA |
| cat-1 | F: CCTGTCTCCAGCCTGTTTGAAGTTG R: CCAGTCAATGCTCCCAAATGTGCTC | |
| vg1 | MN105878.1 | F: GCCGTAGAAGCCAAGGTA R: AAGGCAGTGAAAGGAGCA |
| β-actin akt rheb s6k raptor nos cgmp | Reference [14] XM_027364781.1 MG696863.1 XM_027368997.1 XM_027360909.1 Reference [15] XM_027376715.1 | F: CCTCGGTTCTATTTTGTCGGTTT R: GCAGATGCTTTCGCAGTAGGT F: AAATGACTATGGACGAGGTGTT R: GTTGATGGTGATGTAGAAGGGG F: AGGAAAGTGGCCGTTATGGG R: TACCAGCTCCAGGCCATACT F: GCAAGAGGAAGACGCCATA R: CCGCCCTTGCCCAAAACCT F: CTGCTTTCCAGGCTACTC R: TCACAATCCAAGGTCCAG F: GAGCAAGTTATTCGGCAAGGC R: TCTCTCCCAGTTTCTTGGCGT F: TTTTACAACCCCCACCCCAC R: AGAGAGAGAGGAGGGGCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yin, Y.; Fan, H.; Zhou, Q.; Jiao, L. Arginine Promoted Ovarian Development in Pacific White Shrimp Litopenaeus vannamei via the NO-sGC-cGMP and TORC1 Signaling Pathways. Animals 2024, 14, 1986. https://doi.org/10.3390/ani14131986
Zhang X, Yin Y, Fan H, Zhou Q, Jiao L. Arginine Promoted Ovarian Development in Pacific White Shrimp Litopenaeus vannamei via the NO-sGC-cGMP and TORC1 Signaling Pathways. Animals. 2024; 14(13):1986. https://doi.org/10.3390/ani14131986
Chicago/Turabian StyleZhang, Xin, Yanan Yin, Haitao Fan, Qicun Zhou, and Lefei Jiao. 2024. "Arginine Promoted Ovarian Development in Pacific White Shrimp Litopenaeus vannamei via the NO-sGC-cGMP and TORC1 Signaling Pathways" Animals 14, no. 13: 1986. https://doi.org/10.3390/ani14131986
APA StyleZhang, X., Yin, Y., Fan, H., Zhou, Q., & Jiao, L. (2024). Arginine Promoted Ovarian Development in Pacific White Shrimp Litopenaeus vannamei via the NO-sGC-cGMP and TORC1 Signaling Pathways. Animals, 14(13), 1986. https://doi.org/10.3390/ani14131986






