Detection and Phenotypic Antimicrobial Susceptibility of Salmonella enterica Serotypes in Dairy Cattle Farms in the Po Valley, Northern Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Cattle Population
2.2. Sample Collection
2.3. Pathological Examination
2.4. Microbiological Analyses
2.5. Serogroup and Serotype Identification
2.6. Antimicrobial Susceptibility Testing
2.7. Autogenous Vaccine Development
2.8. Data Analysis
3. Results
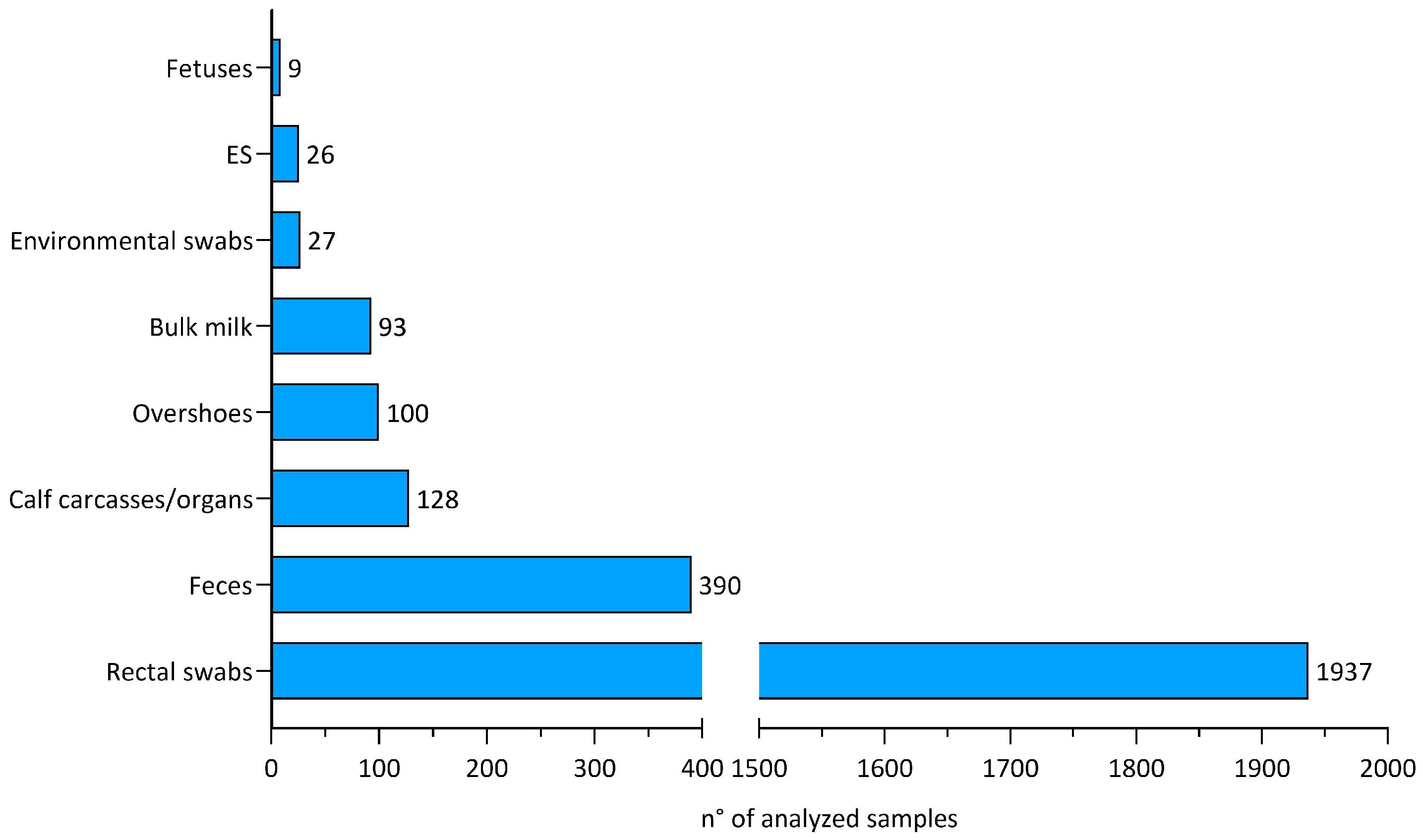
3.1. Overall Samples
3.2. Isolated Salmonella Serotypes
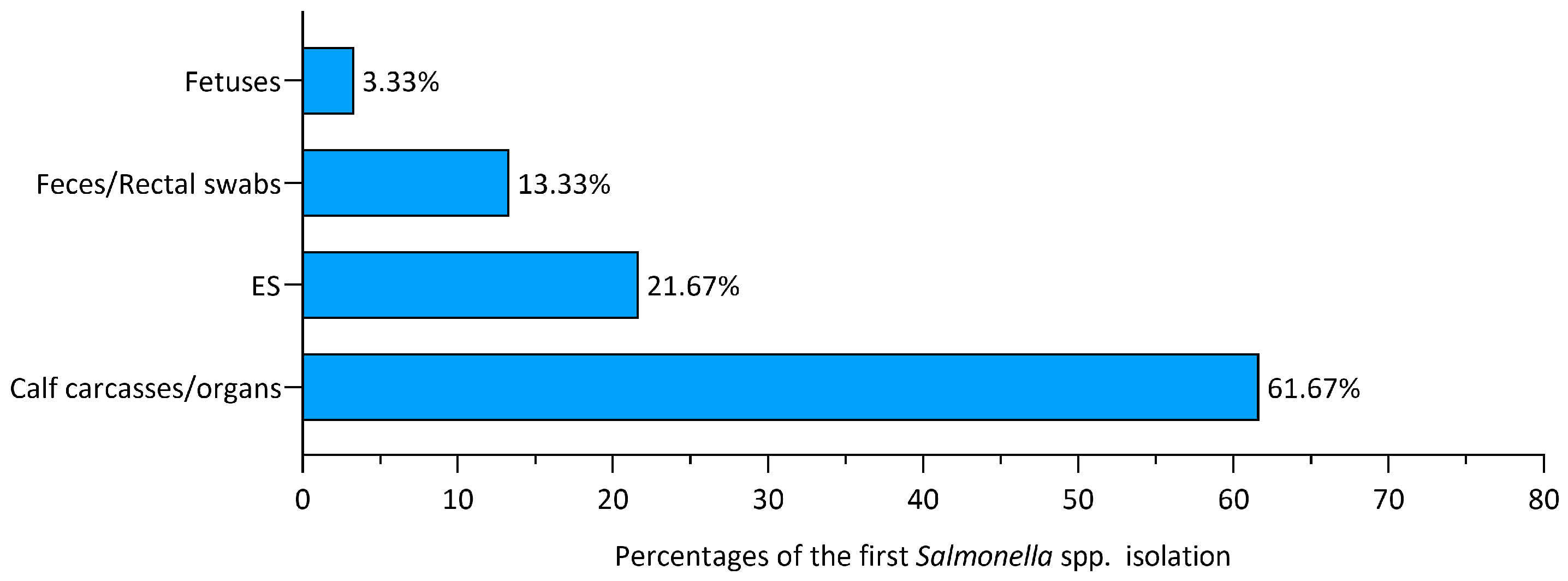
3.3. First Isolation Sources
3.4. Anatomopathological Examination
3.5. Follow-Up Analyses
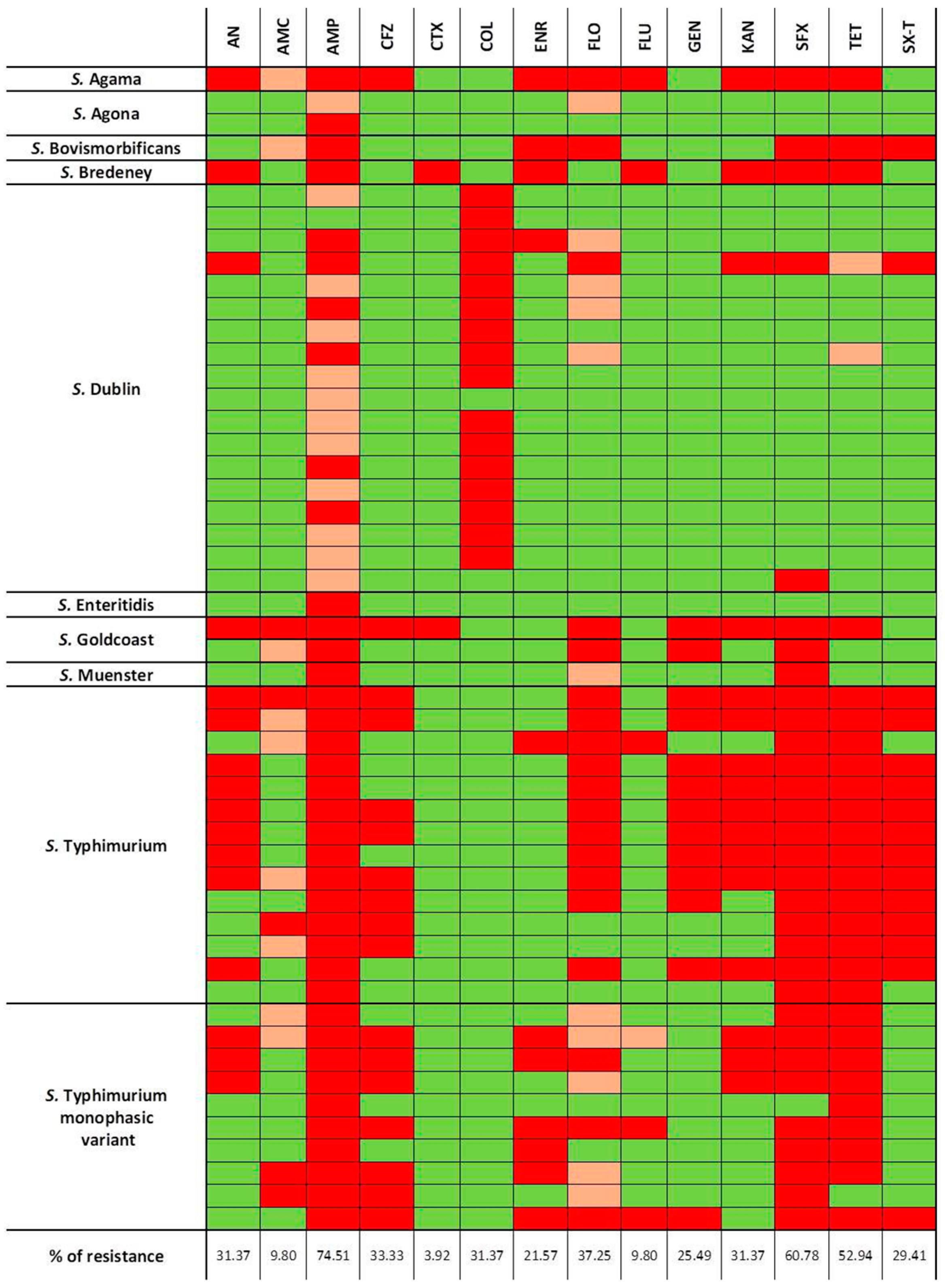
3.6. Antimicrobial Susceptibility Testing
3.7. Autogenous Vaccine Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Old, D.C. Nomenclature of Salmonella. J. Med. Microbiol. 1992, 37, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.E.; Langridge, G.C.; Lauer, A.C.; Grant, K.; Maiden, M.C.J.; Chattaway, M.A. An evaluation of the species and subspecies of the genus Salmonella with whole genome sequence data: Proposal of type strains and epithets for novel S. enterica subspecies VII, VIII, IX, X and XI. Genomics 2021, 113, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Holschbach, C.L.; Peek, S.F. Salmonella in Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 133–154. [Google Scholar] [CrossRef]
- Velasquez-Munoz, A.; Castro-Vargas, R.; Cullens-Nobis, F.M.; Mani, R.; Abuelo, A. Review: Salmonella Dublin in dairy cattle. Front. Vet. Sci. 2024, 10, 1331767. [Google Scholar] [CrossRef] [PubMed]
- Mohler, V.L.; Izzo, M.M.; House, J.K. Salmonella in calves. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 37–54. [Google Scholar] [CrossRef]
- Uzzau, S.; Brown, D.J.; Wallis, T.; Rubino, S.; Leori, G.; Bernard, S.; Casadesús, J.; Platt, D.J.; Olsen, J.E. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 2000, 125, 229–255. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J 2023, 21, e8442. [Google Scholar] [CrossRef]
- Santos, R.L.; Zhang, S.; Tsolis, R.M.; Kingsley, R.A.; Garry Adams, L.; Bäumler, A.J. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microb. Infect. 2001, 3, 1335–1344. [Google Scholar] [CrossRef]
- Loeb, E.; Toussaint, M.J.M.; Rutten, V.P.M.G.; Koeman, J.P. Dry Gangrene of the Extremities in Calves Associated with Salmonella dublin Infection; a Possible Immune-mediated Reaction. J. Comp. Pathol. 2006, 134, 366–369. [Google Scholar] [CrossRef]
- 2003/99/CE of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 2023, 325, 31–40. Available online: https://eur-lex.europa.eu/eli/dir/2003/99/oj (accessed on 1 January 2021).
- Peters, A.R. An estimation of the economic impact of an outbreak of Salmonella dublin in a calf rearing unit. Vet. Rec. 1985, 117, 667–668. [Google Scholar] [CrossRef] [PubMed]
- House, J.K.; Ontiveros, M.M.; Blackmer, N.M.; Dueger, E.L.; Fitchhorn, J.B.; McArthur, G.R.; Smith, B.P. Evaluation of an autogenous Salmonella bacterin and a modified live Salmonella serotype Choleraesuis vaccine on a commercial dairy farm. Am. J. Vet. Res. 2001, 62, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Mulchandani, R.; Van Boeckel, T.P. Global surveillance of antimicrobial resistance in food animals using priority drugs maps. Nat. Commun. 2024, 15, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Zhu, B.; Lyu, N.; Liu, Y.; Ma, S.; Jia, S.; Wan, B.; Du, Y.; Zhang, G.; et al. Genomic analysis of almost 8,000 Salmonella genomes reveals drivers and landscape of antimicrobial resistance in China. Microbiol. Spectr. 2023, 11, e0208023-23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Lyu, N.; Li, Z.; Ma, S.; Cao, D.; Pan, Y.; Hu, Y.; Huang, H.; Gao, G.F.; et al. The temporal dynamics of antimicrobial-resistant Salmonella enterica and predominant serovars in China. Natl. Sci. Rev. 2022, 10, nwac269. [Google Scholar] [CrossRef] [PubMed]
- ISO 6579-1:2017/Amd 1:2020; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.—Amendment 1: Broader Range of Incubation Temperatures, Amendment to the Status of Annex D, and Correction of the Composition of MSRV and SC. Available online: https://www.iso.org/standard/76671.html (accessed on 1 January 2021).
- ISO/TR 6579-3:2014; Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Part 3: Guidelines of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2014.
- Spicer, C.C. A quick method of identifying Salmonella H antigens. J. Clin. Pathol. 1956, 9, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.K.; Steele, C.D.; Wells, J.G. Evaluation of plastic multi-well plates for serological screening of Salmonella cultures with Spicer-Edwards pooled antisera. Appl. Microbiol. 1972, 24, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Grimont, P.A.D.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; WHO Collaborating Center for Reference and Research on Salmonella, Institut Pasteur: Paris, France, 2007; Available online: https://www.pasteur.fr/sites/www.pasteur.fr/files/wklm_en.pdf (accessed on 1 January 2024).
- VET08: CLSI; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 4th ed. CLSI Supplement VET08. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2018.
- M100: CLSI; Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI Supplement M100. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2019.
- SFM: Societé Francaise de Microbiologie. Comité de l’antibiogramme de la societé Francaise de Microbiologie Comité de L’antibiogramme de la Societé Francaise de Microbiologie. Recommendations 2014. Available online: https://www.departement-information-medicale.com/wp-content/uploads/2015/04/CASFM_EUCAST_V1_0_2014.pdf (accessed on 1 January 2024).
- SFM-VET: Societé Francaise de Microbiologie Comité de L’antibiogramme de la Societé Francaise de Microbiologie. Recomm. Vet. 2020. Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2020/09/CASFM_VET2020.pdf (accessed on 1 January 2024).
- EUCAST Breakpoint Tables for Interpretation of MICs and Xone Diameters Version 11.0. Eur. Comm. Antimicrob. Susceptibility Test. 2021. Available online: http://www.eucast.org (accessed on 1 January 2024).
- El-Gazzar, F.; Marth, E.H. Salmonellae, Salmonellosis, and Dairy Foods: A Review. J. Dairy. Sci. 1992, 75, 2327–2343. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar]
- McInerney, J.P.; Howe, K.S.; Schepers, J.A. A framework for the economic analysis of disease in farm livestock. Prev. Vet. Med. 1992, 13, 137–154. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Kudahl, A.B.; Østergaard, S.; Nielsen, L.R. Gross margin losses due to Salmonella Dublin infection in Danish dairy cattle herds estimated by simulation modelling. Prev. Vet. Med. 2013, 111, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tamba, M.; Pallante, I.; Petrini, S.; Feliziani, F.; Iscaro, C.; Arrigoni, N.; Di Sabatino, D.; Barberio, A.; Cibin, V.; Santi, A.; et al. Overview of Control Programs for Twenty-Four Infectious Cattle Diseases in Italy. Front. Vet. Sci. 2021, 8, 665607. [Google Scholar]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Guizelini, C.C.; Tutija, J.F.; Morais, D.R.; Bacha, F.B.; Ramos, C.A.N.; Leal, C.R.B.; Zaquetti, M.E.; Lemos, R.A.A. Outbreak investigation of septicemic salmonellosis in calves. J. Infect. Dev. Ctries. 2020, 14, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Casaux, M.L.; Neto, W.S.; Schild, C.O.; Costa, R.A.; Macías-Rioseco, M.; Caffarena, R.D.; Silveira, C.S.; Aráoz, V.; Díaz, B.D.; Giannitti, F.; et al. Epidemiological and clinicopathological findings in 15 fatal outbreaks of salmonellosis in dairy calves and virulence genes in the causative Salmonella enterica Typhimurium and Dublin strains. Braz. J. Microbiol. 2023, 54, 475–490. [Google Scholar] [CrossRef]
- Maxie, G. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier: St. Louis, MO, USA, 2015; pp. 1–654. [Google Scholar]
- Fossler, C.P.; Wells, S.J.; Kaneene, J.B.; Ruegg, P.L.; Warnick, L.D.; Eberly, L.E.; Godden, S.M.; Halbert, L.W.; Campbell, A.M.; Bolin, C.A.; et al. Cattle and environmental sample-level factors associated with the presence of Salmonella in a multi-state study of conventional and organic dairy farms. Prev. Vet. Med. 2005, 67, 39–53. [Google Scholar] [CrossRef]
- Creutzinger, K.C.; Proudfoot, K.L. Invited Review: Design and management of group maternity areas for dairy cows. Appl. Anim. Sci. 2020, 36, 124–132. [Google Scholar] [CrossRef]
- Horton, B.C.; Gehring, K.B.; Sawyer, J.E.; Arnold, A.N. Evaluation of Autogenous Vaccine Use in Mitigating Salmonella in Lymph Nodes from Feedlot Cattle in Texas. J. Food Prot. 2021, 84, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.W.; Alley, M.L.; Foster, D.M.; Smith, F.; Wileman, B.W. Passive immunity stimulated by vaccination of dry cows with a Salmonella bacterial extract. J. Vet. Intern. Med. 2014, 28, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- Otto, S.J.G.; Ponich, K.L.; Cassis, R.; Goertz, C.; Peters, D.; Checkley, S.L. Antimicrobial resistance of bovine Salmonella enterica ssp. enterica isolates from the Alberta Agriculture and Forestry Disease Investigation Program (2006–2014). Can. Vet. J. 2018, 59, 1195–1201. [Google Scholar] [PubMed]
- Mourão, J.; Machado, J.; Novais, C.; Antunes, P.; Peixe, L. Characterization of the emerging clinically-relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- (monophasic variant of S. Typhimurium) clones. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Agersø, Y.; Torpdahl, M.; Zachariasen, C.; Seyfarth, A.; Hammerum, A.M.; Nielsen, E.M. Tentative colistin epidemiological cut-off value for Salmonella spp. Foodborne Pathog. Dis. 2012, 9, 367–369. [Google Scholar] [CrossRef]
- Ricci, V.; Zhang, D.; Teale, C.; Piddock, L.J.V. The O-Antigen Epitope Governs Susceptibility to Colistin in Salmonella enterica. mBio 2020, 11, e02831-19. [Google Scholar] [CrossRef]

| Salmonella enterica susp. enterica Serovars | N° of Cases | Prevalence (%) |
|---|---|---|
| S. Dublin | 46 | 38.33 |
| S. Typhimurium | 28 | 23.33 |
| S. Typhimurium monophasic variant | 17 | 14.17 |
| Salmonella spp. | 2 | 1.67 |
| S. London | 2 | 1.67 |
| S. Agona | 2 | 1.67 |
| S. group O:9 (D1) | 2 | 1.67 |
| S. Muenster | 2 | 1.67 |
| S. Bredeney | 1 | 0.83 |
| S. Chester | 1 | 0.83 |
| S. Infantis | 1 | 0.83 |
| S. Goldcoast | 1 | 0.83 |
| S. Bovismorbificans | 1 | 0.83 |
| S. Enteritidis | 1 | 0.83 |
| S. Dublin and S. Typhimurium | 3 | 2.50 |
| S. Dublin and S. Goldcoast | 2 | 1.67 |
| S. Typhimurium and S. Bredeney | 1 | 0.83 |
| S. Newport and S. Kentucky | 1 | 0.83 |
| S. Bovismorbificans and S. Infantis | 1 | 0.83 |
| S. Dublin and S. Bredeney | 1 | 0.83 |
| S. Typhimurium and S. Senftenberg | 1 | 0.83 |
| S. Typhimurium and S. Typhimurium monophasic variant | 1 | 0.83 |
| S. Agama, Typhimurium, and S. Typhimurium monophasic variant | 1 | 0.83 |
| S. Dublin, S. Typhimurium, and S. Typhimurium monophasic variant | 1 | 0.83 |
| Total | 120 | 100 |
| Salmonella co-presence | 13 | 10.83 |
| Salmonella Serovars | N° Herds | N° Positive Calves | Enteritis | Pneumonia | Hepatosplenomegaly |
|---|---|---|---|---|---|
| S. Agama | 1 | 1 | 1 | 1 | 0 |
| S. Agona | 2 | 2 | 2 | 1 | 0 |
| S. Bovismorbificans | 1 | 2 | 2 | 0 | 0 |
| S. Bredeney | 2 | 3 | 3 | 0 | 0 |
| S. Dublin | 24 | 29 | 14 | 9 | 15 |
| S. group O:9 (D1) | 2 | 2 | 2 | 0 | 1 |
| S. Goldcoast | 1 | 1 | 1 | 0 | 0 |
| S. Muenster | 2 | 3 | 1 | 0 | 0 |
| S. Typhimurium | 18 | 27 | 22 | 7 | 12 |
| S. Typhimurium monophasic variant | 11 | 21 | 20 | 5 | 1 |
| Samples Analyzed | N° Farms with Positive Samples/N° Total Farms Tested | N° Positive Samples/N° Total Samples |
|---|---|---|
| Feces/Rectal swabs | 26/50 | 206/2145 |
| Bulk milk | 3/32 | 3/93 |
| Calf carcasses/organs | 15/21 | 21/36 |
| Environmental overshoes | 7/16 | 34/100 |
| Environmental swabs/Sponges | 4/7 | 9/27 |
| Fetuses | 2/3 | 3/4 |
| Salmonella enterica subsp. enterica Serovars | N° of Tested Isolates |
|---|---|
| Salmonella Dublin | 18 |
| Salmonella Typhimurium | 14 |
| Salmonella Typhimurium monophasic variant | 10 |
| Salmonella Agona | 2 |
| Salmonella Goldcoast | 2 |
| Salmonella Agama | 1 |
| Salmonella Bovismorbificans | 1 |
| Salmonella Bredeney | 1 |
| Salmonella Muenster | 1 |
| Salmonella Enteritidis | 1 |
| Total | 51 |
| N° of Farms | Salmonella Serovars Present in the Vaccine |
|---|---|
| 6 | S. Dublin |
| 3 | S. Typhimurium |
| 3 | S. Typhimurium monophasic variant |
| 1 | S. Muenster |
| 1 | S. Typhimurium monophasic variant, S. Agama, and S. Typhimurium |
| 1 | S. Dublin and S. Typhimurium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parolini, F.; Ventura, G.; Rosignoli, C.; Rota Nodari, S.; D’incau, M.; Marocchi, L.; Santucci, G.; Boldini, M.; Gradassi, M. Detection and Phenotypic Antimicrobial Susceptibility of Salmonella enterica Serotypes in Dairy Cattle Farms in the Po Valley, Northern Italy. Animals 2024, 14, 2043. https://doi.org/10.3390/ani14142043
Parolini F, Ventura G, Rosignoli C, Rota Nodari S, D’incau M, Marocchi L, Santucci G, Boldini M, Gradassi M. Detection and Phenotypic Antimicrobial Susceptibility of Salmonella enterica Serotypes in Dairy Cattle Farms in the Po Valley, Northern Italy. Animals. 2024; 14(14):2043. https://doi.org/10.3390/ani14142043
Chicago/Turabian StyleParolini, Francesca, Giordano Ventura, Carlo Rosignoli, Sara Rota Nodari, Mario D’incau, Leonardo Marocchi, Giovanni Santucci, Massimo Boldini, and Matteo Gradassi. 2024. "Detection and Phenotypic Antimicrobial Susceptibility of Salmonella enterica Serotypes in Dairy Cattle Farms in the Po Valley, Northern Italy" Animals 14, no. 14: 2043. https://doi.org/10.3390/ani14142043
APA StyleParolini, F., Ventura, G., Rosignoli, C., Rota Nodari, S., D’incau, M., Marocchi, L., Santucci, G., Boldini, M., & Gradassi, M. (2024). Detection and Phenotypic Antimicrobial Susceptibility of Salmonella enterica Serotypes in Dairy Cattle Farms in the Po Valley, Northern Italy. Animals, 14(14), 2043. https://doi.org/10.3390/ani14142043






