Does the Farming Method Influence the Porcine Vomeronasal Organ Condition? A Histological Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
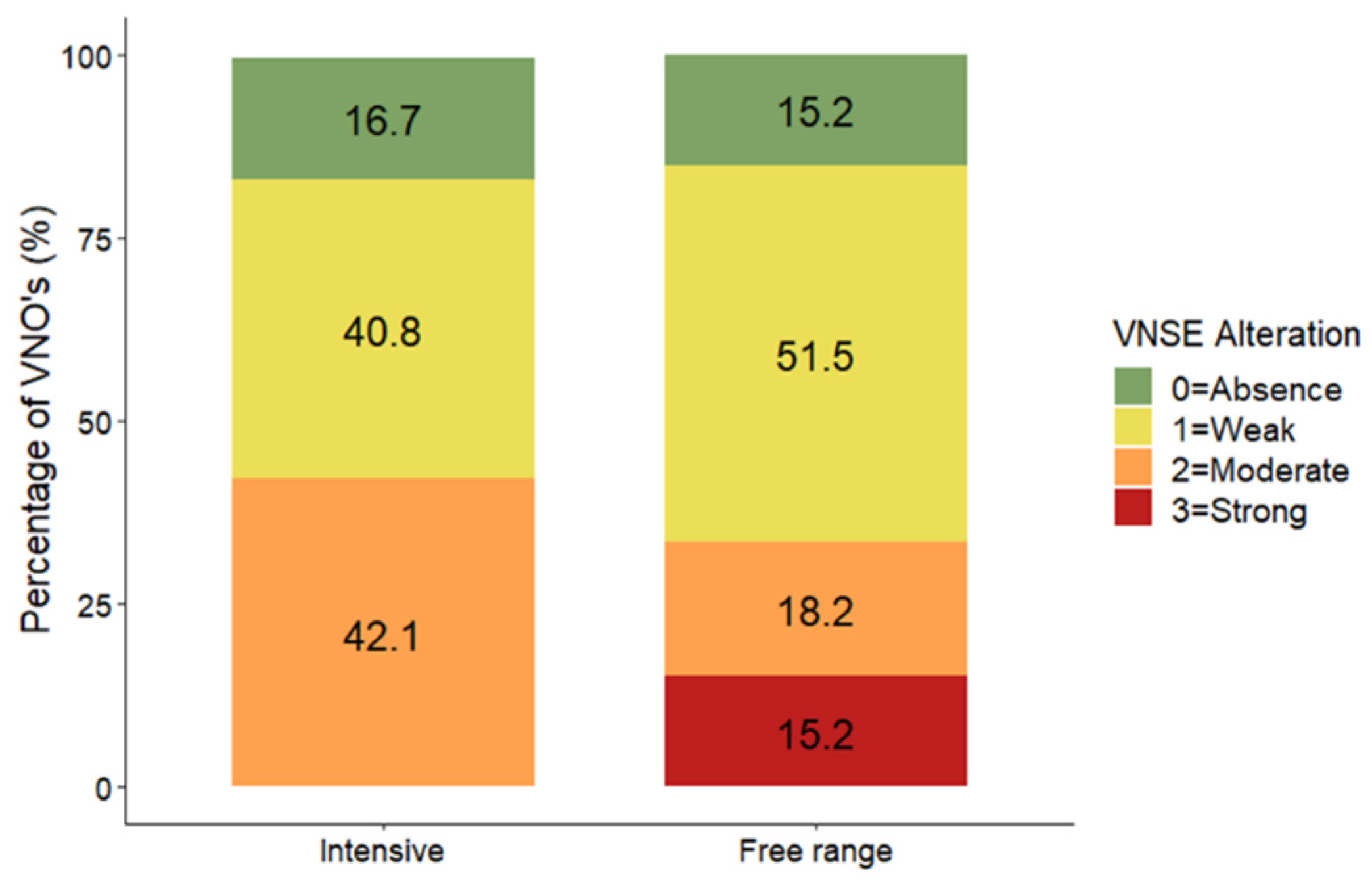
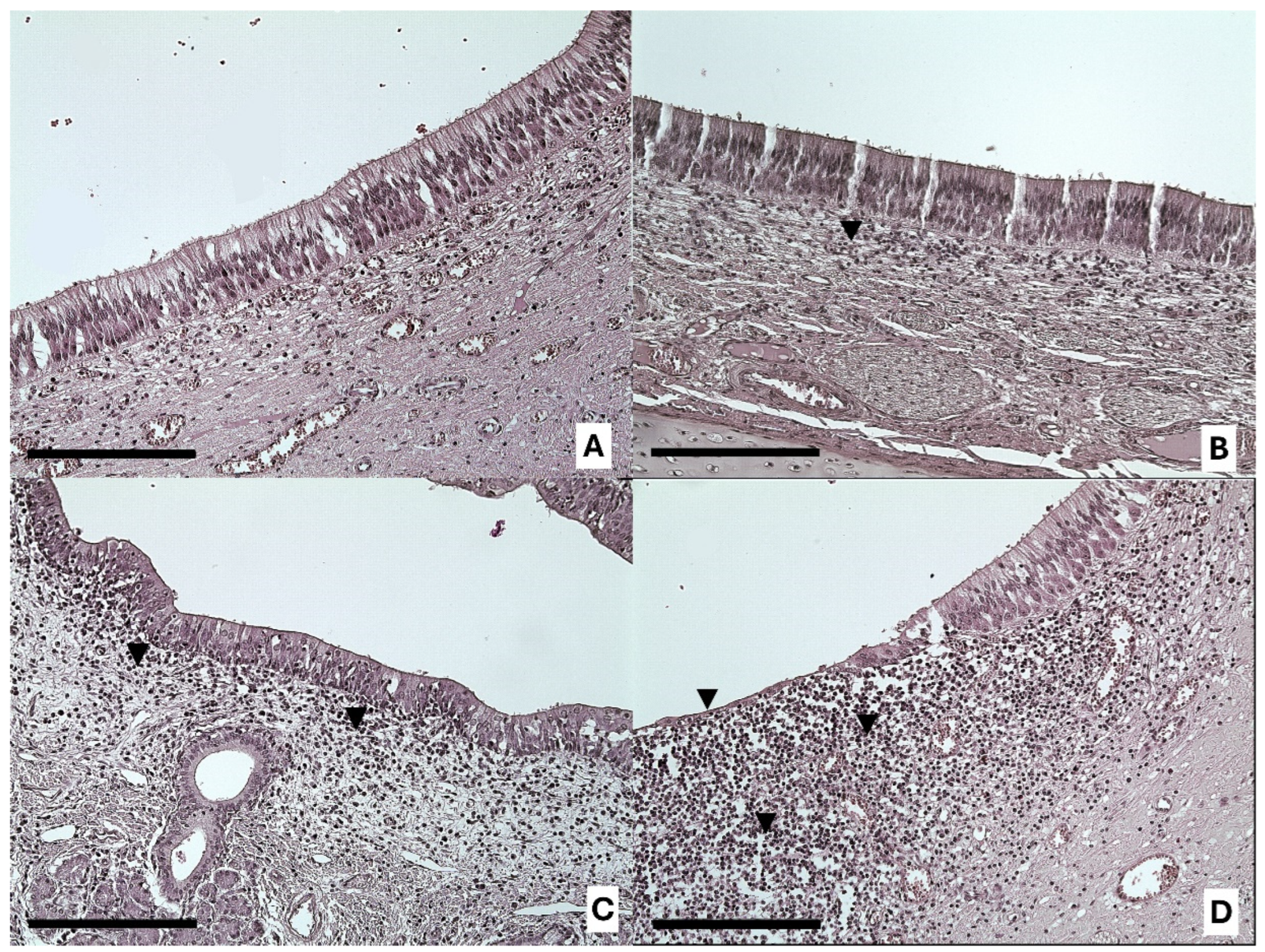
3.1. Vomeronasal Sensory Epithelium (VNSE) Alterations
3.2. Vomeronasal Nonsensory Epithelium (NSE) Alterations
3.3. Collagenolysis
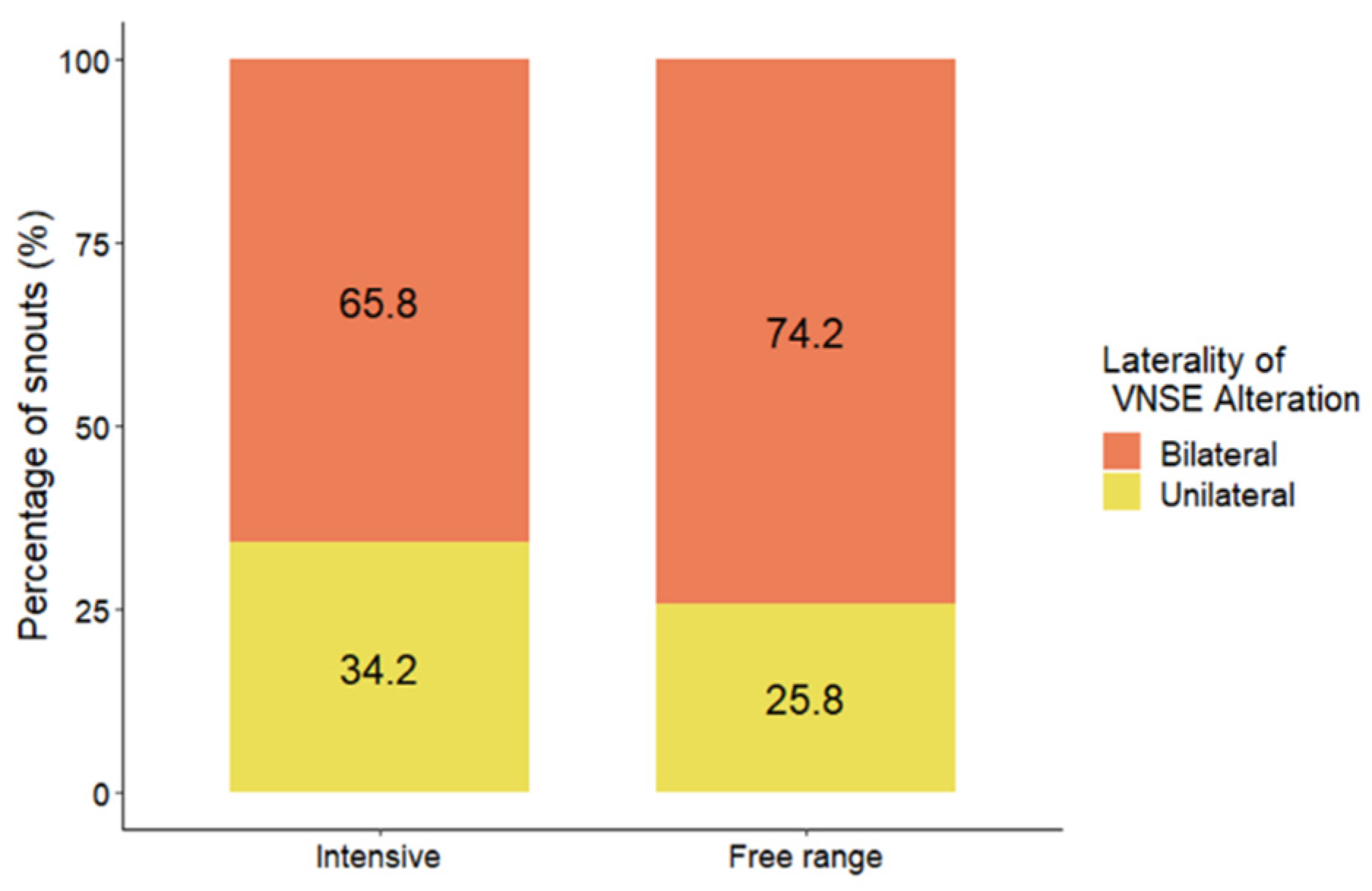
3.4. Laterality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zufall, F.; Kelliher, K.R.; Leinders-Zufall, T. Pheromone detection by mammalian vomeronasal neurons. Microsc. Res. Tech. 2002, 58, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Martínez-Marcos, A. Structure and function of the vomeronasal system: An update. Prog. Neurobiol. 2003, 70, 245–318. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Pheromones and Animal Behavior; Cambridge University Press: Cambridge, UK, 2014; pp. 685–700. [Google Scholar] [CrossRef]
- Wysocki, C.J.; Lepri, J.J. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 1991, 39 Pt 2, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Booth, K.; Katz, L.S. Role of the vomeronasal Organ in neonatal offspring recognition in sheep. Biol. Reprod. 2000, 63, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Booth, K.K.; Webb, E.C. Effect of blockage of the ducts of the vomeronasal Organ on LH plasma levels during the whitten effect in does. Vet. Med. Int. 2011, 2011, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, Y.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Removal of the vomeronasal organ blocks the stress-induced hyperthermia response to alarm pheromone in male rats. Chem. Sens. 2007, 32, 57–64. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Cherry, J.A.; Baum, M.J. Effect of Vomeronasal Organ Removal From Male Mice on Their Preference for and Neural Fos Responses to Female Urinary Odors. Behav. Neurosci. 2006, 120, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Asproni, P.; Cozzi, A.; Verin, R.; Lafont-Lecuelle, C.; Bienboire-Frosini, C.; Poli, A.; Pageat, P. Pathology and behaviour in feline medicine: Investigating the link between vomeronasalitis and aggression. J. Feline Med. Surg. 2016, 18, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Asproni, P.; Mainau, E.; Cozzi, A.; Carreras, R.; Bienboire-Frosini, C.; Teruel, E.; Pageat, P. Is There a Link between Vomeronasalitis and Aggression in Stable Social Groups of Female Pigs? Animals 2022, 12, 303. [Google Scholar] [CrossRef]
- Martin, S.W.; Willoughby, R.A. Organic dusts, sulfur dioxide, and the respiratory tract of swine. Arch. Environ. Health 1972, 25, 158–165. [Google Scholar] [CrossRef]
- Curtis, S.E.; Anderson, C.R.; Simon, J.; Jensen, A.H.; Day, D.L.; Kelley, K.W. Effects of aerial ammonia, hydrogen sulfide and swine-house dust on rate of gain and respiratory-tract structure in swine. J. Anim. Sci. 1975, 41, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. The Effect of Stress on Livestock and Meat Quality Prior to and during Slaughter. Int. J. Study Anim. Probl. 1980, 1, 313–337. [Google Scholar]
- Grosso, L.; Battini, M.; Wemelsfelder, F.; Barbieri, S.; Minero, M.; Dalla Costa, E.; Mattiello, S. On-farm Qualitative Behaviour Assessment of dairy goats in different housing conditions. Appl. Anim. Behav. Sci. 2016, 180, 51–57. [Google Scholar] [CrossRef]
- Russell, A.L.; Randall, L.V.; Kaler, J.; Eyre, N.; Green, M.J. Use of qualitative behavioural assessment to investigate affective states of housed dairy cows under different environmental conditions. Front. Vet. Sci. 2023, 10, 1099170. [Google Scholar] [CrossRef] [PubMed]
- Mechin, V.; Asproni, P.; Bienboire-Frosini, C.; Cozzi, A.; Chabaud, C.; Arroub, S.; Mainau, E.; Meillour, P.N.-L.; Pageat, P. Inflammation interferes with chemoreception in pigs by altering the neuronal layout of the vomeronasal sensory epithelium. Front. Vet. Sci. 2022, 9, 936838. [Google Scholar] [CrossRef] [PubMed]
- Mechin, V.; Pageat, P.; Boutry, M.; Teruel, E.; Portalier, C.; Asproni, P. Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming. Animals 2023, 13, 1902. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. The olfactory system and Its disorders. Semin. Neurol. 2009, 29, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Eduard, W.; Pearce, N.; Douwes, J. Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest 2009, 136, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Skogstad, A.; Madsø, L.; Eduard, W. Classification of particles from the farm environment by automated sizing, counting and chemical characterisation with scanning electron microscopy-energy dispersive spectroscopy. J. Environ. Monit. 1999, 1, 379–382. [Google Scholar] [CrossRef]
- Riskowski, G.L.; Harrison, P.C.; Memarzadeh, F. Mass generation rates of ammonia, moisture, and heat production in mouse cages with two bedding types, two mouse strains, and two room relative humidities. ASHRAE Trans. 2006, 112 Pt 1, 134–144. [Google Scholar]
- Ferrecchia, C.E.; Jensen, K.; Van Andel, R. Intracage ammonia levels in static and individually ventilated cages housing C57BL/6 mice on 4 bedding substrates. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 146–151. [Google Scholar]
- Shi, Z.; Sun, X.; Lu, Y.; Xi, L.; Zhao, X. Emissions of ammonia and hydrogen sulfide from typical dairy barns in central China and major factors influencing the emissions. Sci. Rep. 2019, 9, 13821. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.M. Ventilation and temperature criteria for pigs. In Environmental Aspects of Housing for Animal Production; Clarck, J.A., Ed.; Butterworth: Boston, MA, USA, 1981; pp. 197–216. [Google Scholar]
- Buckley, L.A.; Jiang, X.Z.; James, R.A.; Morgan, K.T.; Barrow, C.S. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol. Appl. Pharmacol. 1984, 74, 417–429. [Google Scholar] [CrossRef]
- Vogelweid, C.M.; Zapien, K.A.; Honigford, M.J.; Li, L.; Li, H.; Marshall, H. Effects of a 28-day cage-change interval on intracage ammonia levels, nasal histology, and perceived welfare of CD1 mice. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 868–878. [Google Scholar] [PubMed Central]
- Mexas, A.M.; Brice, A.K.; Caro, A.C.; Hillanbrand, T.S.; Gaertner, D.J. Nasal histopathology and intracage ammonia levels in female groups and breeding mice housed in static isolation cages. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 478–486. [Google Scholar] [PubMed Central]
- Honda, A.; Matsuda, Y.; Murayama, R.; Tsuji, K.; Nishikawa, M.; Koike, E.; Yoshida, S.; Ichinose, T.; Takano, H. Effects of Asian sand dust particles on the respiratory and immune system. J. Appl. Toxicol. 2014, 34, 250–257. [Google Scholar] [CrossRef]
- Hernandez, M.; Harrington, A.; Ma, Y.; Galdanes, K.; Halzack, B.; Zhong, M.; Vaughan, J.; Sebasco, E.; Gordon, T.; Lippmann, M.; et al. World Trade Center Dust induces airway inflammation while promoting aortic endothelial dysfunction. Toxicol. Appl. Pharmacol. 2020, 400, 115041. [Google Scholar] [CrossRef]
- Lewkowich, I.; Ahlbrand, R.; Johnson, E.; McAlees, J.; Nawreen, N.; Raman, R.; Lingel, I.; Hargis, J.; Hoover, C.; Sah, R. Modulation of fear behavior and neuroimmune alterations in house dust mite exposed A/J mice, a model of severe asthma. Brain Behav. Immun. 2020, 88, 688–698. [Google Scholar] [CrossRef]
- Dibattista, M.; Reisert, J. The odorant receptor-dependent role of olfactory marker protein in olfactory receptor neurons. J. Neurosci. 2016, 36, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Molière, S.; Jaulin, A.; Tomasetto, C.-L.; Dali-Youcef, N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. Int. J. Mol. Sci. 2023, 24, 10649. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.J.; Farndale, R.W. Collagens and atherosclerosis. Exp. Gerontol. 1999, 34, 513–525. [Google Scholar] [CrossRef]
- Capo-Chichi, C.D.; Smith, E.R.; Yang, D.-H.; Roland, I.H.; Vanderveer, L.; Cohen, C.; Hamilton, T.C.; Godwin, A.K.; Xu, X. Dynamic alterations of the extracellular environment of ovarian surface epithelial cells in premalignant transformation, tumorigenicity, and metastasis. Cancer 2002, 95, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Stolba, A.; Wood-Gush, D.G.M. The behaviour of pigs in a semi-natural environment. Anim. Prod. 1989, 48, 419–425. [Google Scholar] [CrossRef]
- McClendon, C.J.; Gerald, C.L.; Waterman, J.T. Farm animal models of organic dust exposure and toxicity: Insights and implications for respiratory health. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.D.; Shin, T.H.; Bae, C.S.; Ahn, T.; Shin, S.S.; Kim, H.J.; Lee, C.M.; Suh, G.H. Respiratory and systemic toxicity of inhaled artificial asian sand dust in pigs. Life 2021, 11, 25. [Google Scholar] [CrossRef]
- Takami, S. Recent progress in the neurobiology of the vomeronasal organ. Microsc. Res. Tech. 2002, 58, 228–250. [Google Scholar] [CrossRef]
- Martínez-Macipe, M.; Mainau, E.; Manteca, X.; Dalmau, A. Environmental and management factors affecting the time budgets of free-ranging iberian pigs reared in Spain. Animals 2020, 10, 798. [Google Scholar] [CrossRef]
- Arey, D.S.; Edwards, S.A. Factors influencing aggression between sows after mixing and the consequences for welfare and production. Livest. Prod. Sci. 1998, 56, 61–70. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechin, V.; Asproni, P.; Teruel, E.; Boutry, M.; Cozzi, A.; Pageat, P. Does the Farming Method Influence the Porcine Vomeronasal Organ Condition? A Histological Study. Animals 2024, 14, 2105. https://doi.org/10.3390/ani14142105
Mechin V, Asproni P, Teruel E, Boutry M, Cozzi A, Pageat P. Does the Farming Method Influence the Porcine Vomeronasal Organ Condition? A Histological Study. Animals. 2024; 14(14):2105. https://doi.org/10.3390/ani14142105
Chicago/Turabian StyleMechin, Violaine, Pietro Asproni, Eva Teruel, Marion Boutry, Alessandro Cozzi, and Patrick Pageat. 2024. "Does the Farming Method Influence the Porcine Vomeronasal Organ Condition? A Histological Study" Animals 14, no. 14: 2105. https://doi.org/10.3390/ani14142105
APA StyleMechin, V., Asproni, P., Teruel, E., Boutry, M., Cozzi, A., & Pageat, P. (2024). Does the Farming Method Influence the Porcine Vomeronasal Organ Condition? A Histological Study. Animals, 14(14), 2105. https://doi.org/10.3390/ani14142105





