Lupin Seed Supplementation as a Functional Feed Additive: In Vitro Ruminal Gas, Methane and Carbon Dioxide Production, Fermentation Kinetics, and Nutrient Degradability
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients and Treatments
2.2. In Vitro Fermentation and Biodegradation
2.3. Sampling and Analysis of Fermentation Variables
2.4. Chemical Analysis
2.5. Calculations and Statistical Analyses
3. Results
3.1. Lupin Seeds
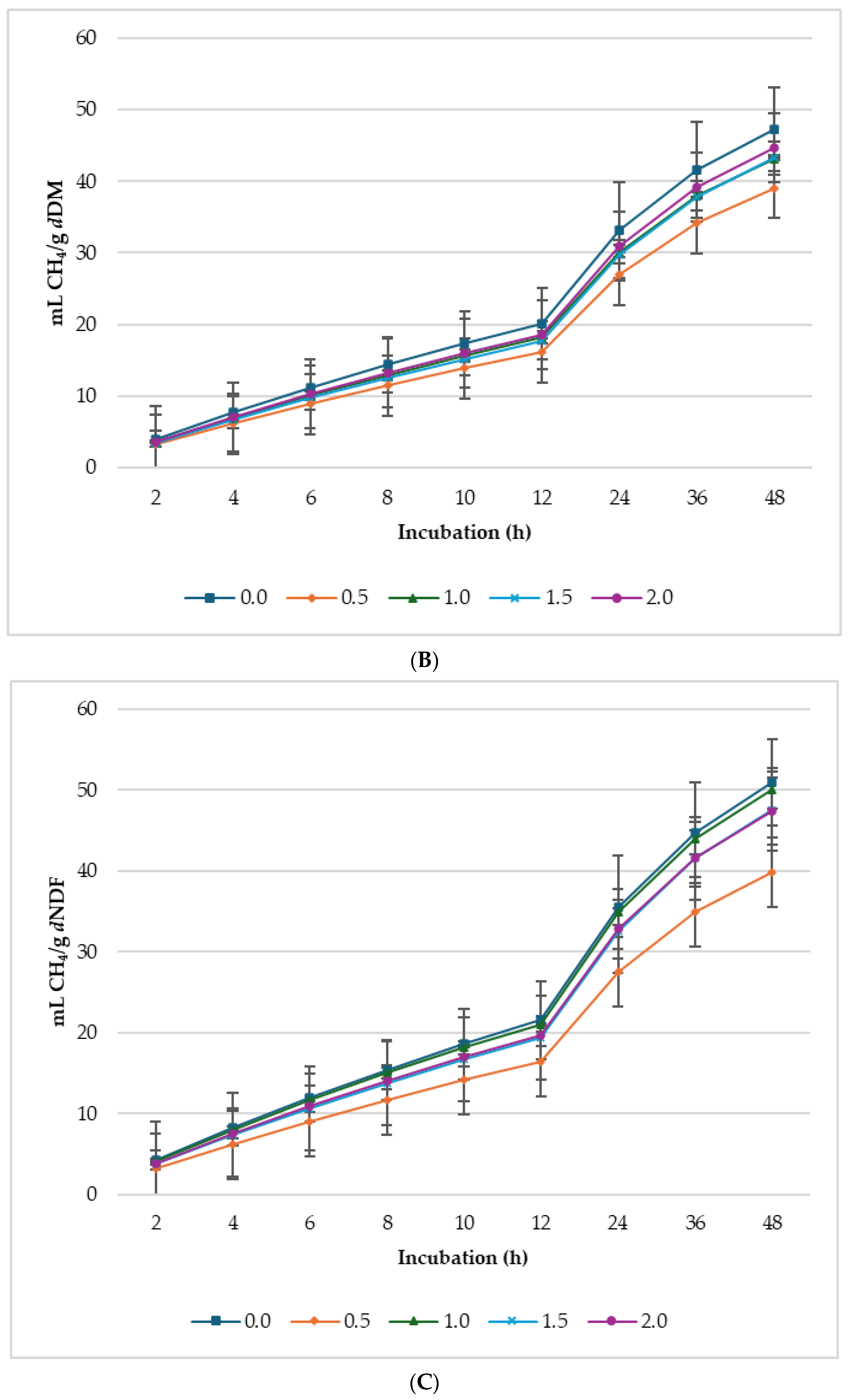
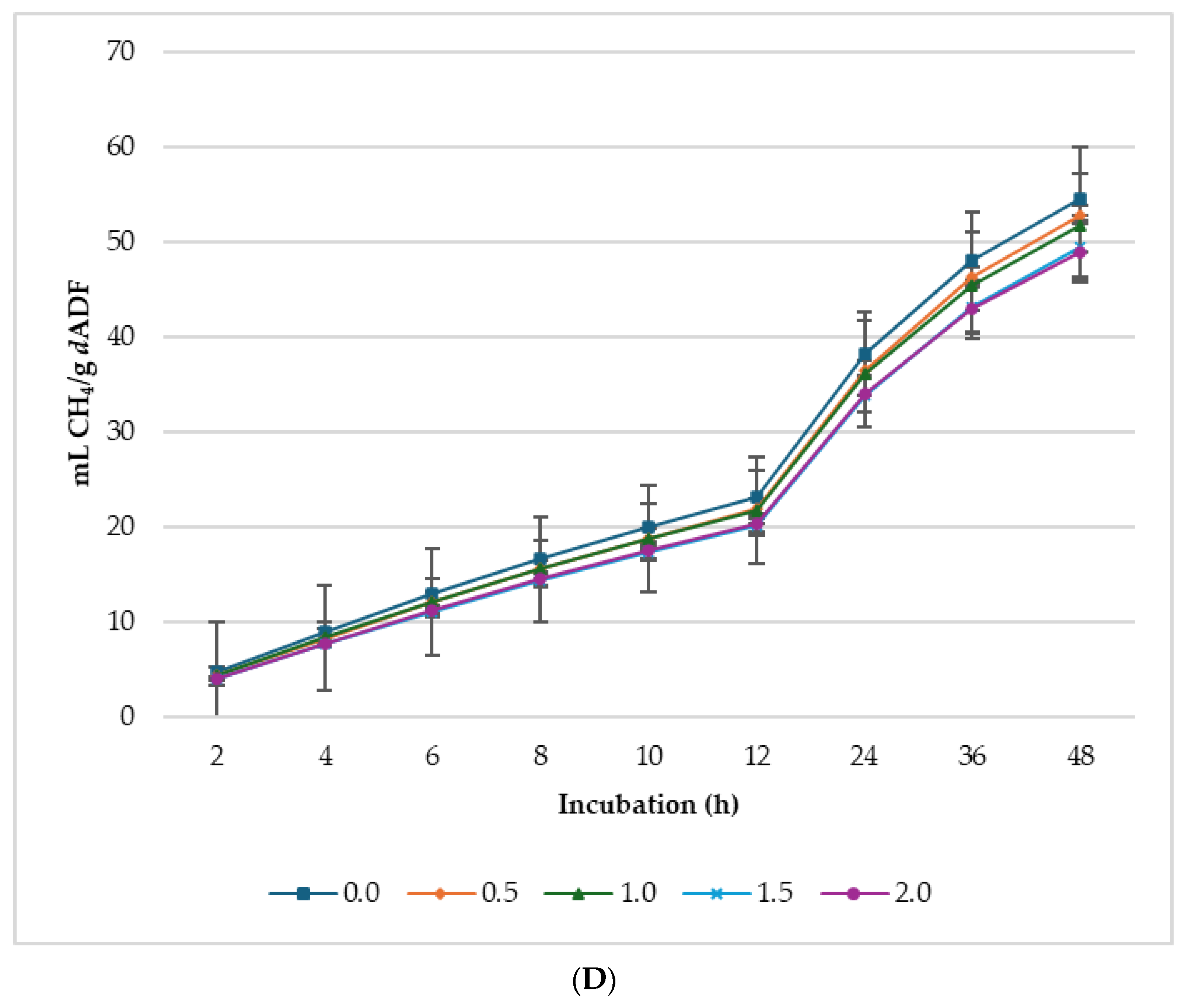
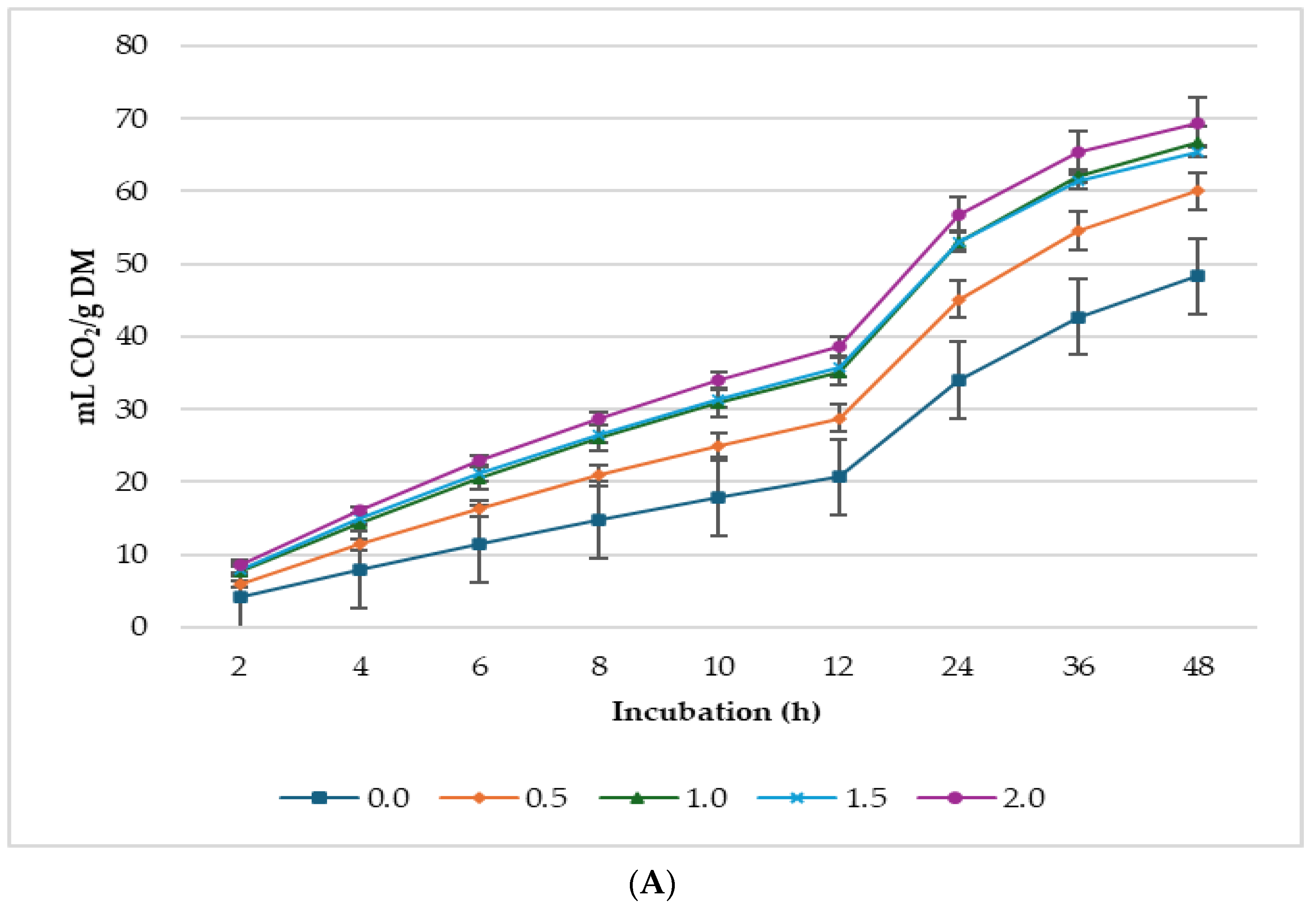
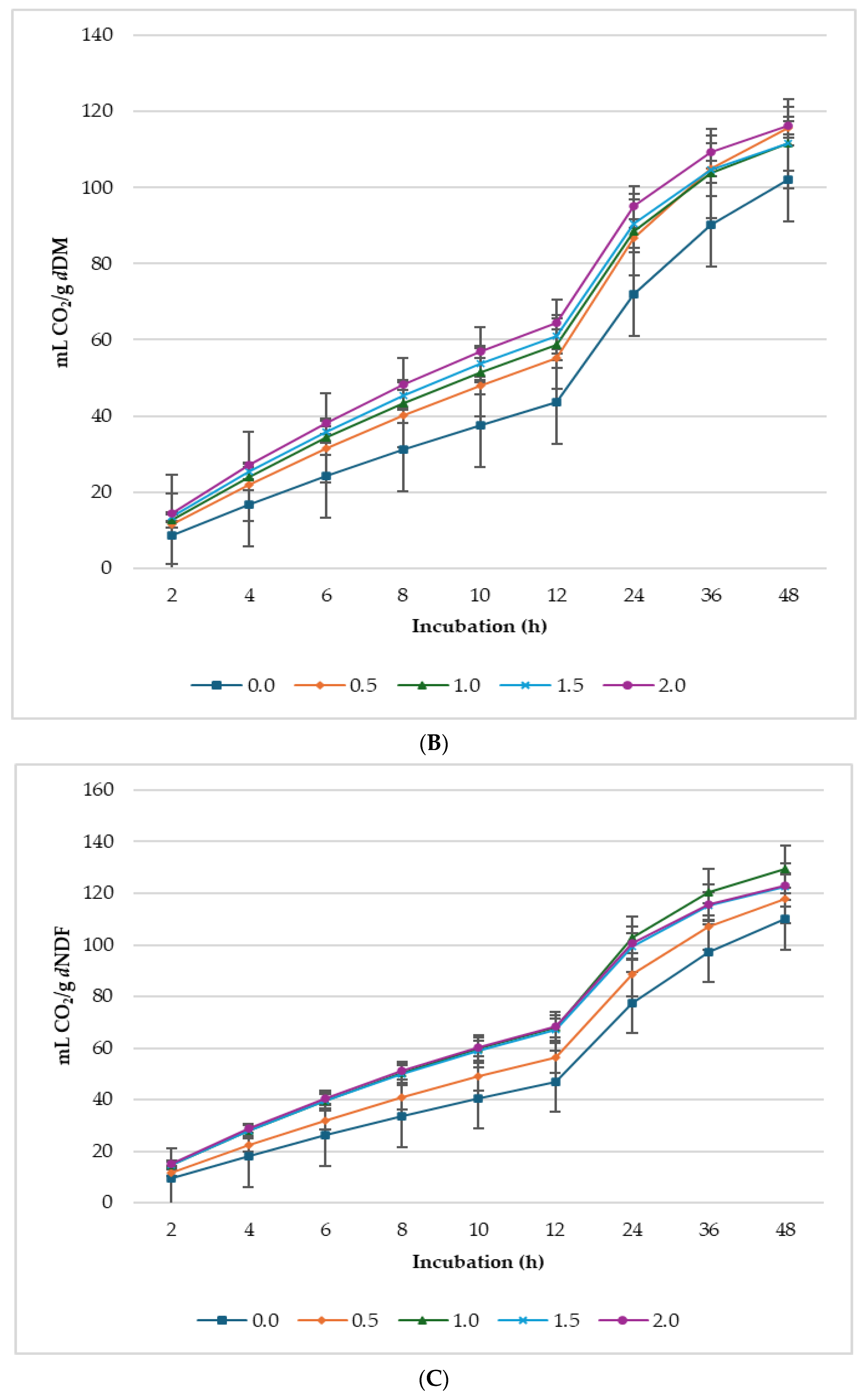
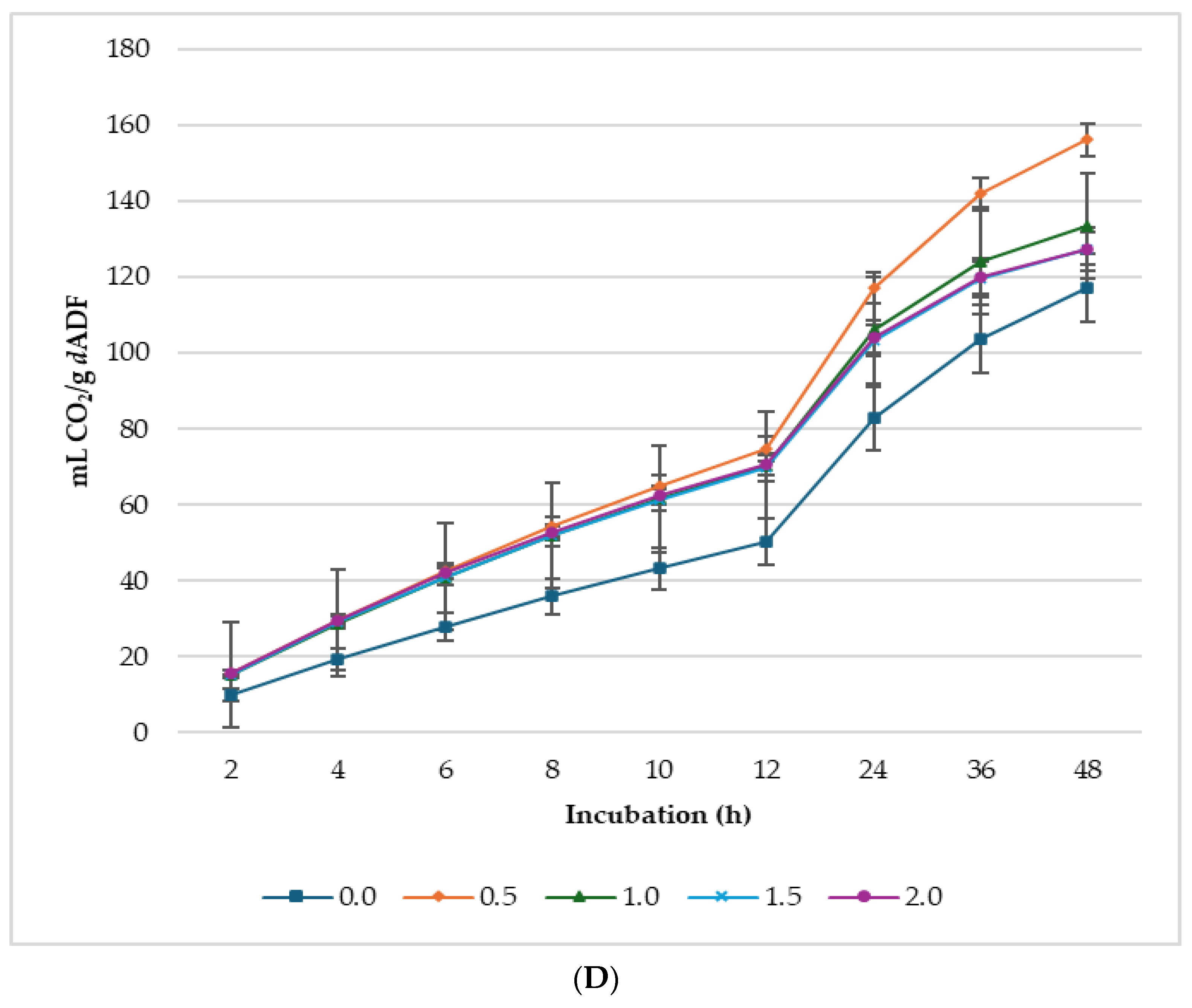
3.2. Biogas Production
3.3. Degradability and Fermentation
4. Discussion
4.1. Lupin Seeds
4.2. Gas Production
4.3. Degradability and Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited Review: Enteric Methane in Dairy Cattle Production: Quantifying the Opportunities and Impact of Reducing Emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, D.S.; Linnemann, A.R.; Van Boekel, T.A.J.S. Towards Sustainable Production of Protein-Rich Foods: Appraisal of Eight Crops for Western Europe. PART II: Analysis of the Technological Aspects of the Production Chain. Crit. Rev. Food Sci. Nutr. 2003, 43, 481–506. [Google Scholar] [CrossRef] [PubMed]
- van Barneveld, R.J. Understanding the Nutritional Chemistry of Lupin (Lupinus spp.) Seed to Improve Livestock Production Efficiency. Nutr. Res. Rev. 1999, 12, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.K.; Clements, J.; Villarino, C.B.J.; Coorey, R. Lupins: Their Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains; Taylor, J.R.N., Awika, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 179–221. [Google Scholar]
- Yanez, E.; Ivanovic, D.; Owen, D.F.; Ballester, D. Chemical and Nutritional Evaluation of Sweet Lupines. Ann. Nutr. Metab. 1983, 27, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Wroblewska, K.; Czyzniejewski, M.; Górnaś, P.; Kachlicki, P.; Siger, A. Bioaccessibility of Defatted Lupin Seed Phenolic Compounds in a Standardized Static In Vitro Digestion System. Food Res. Int. 2019, 116, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Olafadehan, O.A. Essential Oils and Phytogenic Feed Additives in Ruminant Diet: Chemistry, Ruminal Microbiota and Fermentation, Feed Utilization and Productive Performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Kholif, A.E. A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. Vet. Sci. 2023, 10, 450. [Google Scholar] [CrossRef]
- Um, K.H.; Shin, J.S.; Park, B.K. Effect of Lupin Flake Supplementation on Rumen Fermentation and Meat Composition of Hanwoo Steers. S. Afr. J. Anim. Sci. 2023, 52, 563–576. [Google Scholar] [CrossRef]
- Chaves, A.V.; He, M.L.; Yang, W.Z.; Hristov, A.N.; McAllister, T.A.; Benchaar, C. Effects of Essential Oils on Proteolytic, Deaminative and Methanogenic Activities of Mixed Ruminal Bacteria. Can. J. Anim. Sci. 2008, 88, 117–122. [Google Scholar] [CrossRef]
- Sallam, S.M.A.; Bueno, I.C.S.; Brigide, P.; Godoy, P.B.; Vitti, D.M.S.; Abdalla, A.L. Production in Efficacy of Eucalyptus Oil on In Vitro Ruminal Fermentation and Methane Production. In Options Méditerranéennes: Série A. Séminaires Méditerranéens; Papachristou, T.G., Parissi, Z.M., Ben Salem, H., Morand-Fehr, P., Eds.; CIHEAM/FAO/NAGREF: Zaragoza, Spain, 2009; Volume 272, pp. 267–272. [Google Scholar]
- Kumar, R.; Kamra, D.N.; Agarwal, N.; Chaudhary, L.C. Effect of Eucalyptus (Eucalyptus globulus) Oil on In Vitro Methanogenesis and Fermentation of Feed with Buffalo Rumen Liquor. Anim. Nutr. Feed Technol. 2009, 9, 237–243. [Google Scholar]
- Hynd, P.I.; Valentine, S.C.; Bartsch, B.D. Rumen Protozoa Numbers in Dairy Cows Fed Barley or Lupins. Proc. Nutr. Soc. 1985, 10, 147–150. [Google Scholar]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen Methanogens and Mitigation of Methane Emission by Anti-Methanogenic Compounds and Substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Kridli, R.T.; Ata, M.; Mahmoud, K.Z.; Bartlewski, P.M. Nutrient Intake, in Vivo Digestibility, Growth Performance and Carcass Quality of Growing Lambs Fed Concentrate Diets Containing Sweet Lupin Grain (Lupinus angustifolius). Small Rumin. Res. 2021, 204, 106510. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A.; Gouda, G.A.; Fahmy, M.; Morsy, T.A.; Ammar, H.; Hamdon, H.A.; Chahine, M. Turmeric Rhizomes Reduced In Vitro Methane Production and Improved Gas Production and Nutrient Degradability. Anim. Biotechnol. 2024, 35, 2371519. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Fahmy, M.; Morsy, T.A.; Abdelsattar, M.M.; Vargas-Bello-Pérez, E. Fennel Seeds Dietary Inclusion as a Sustainable Approach to Reduce Methane Production and Improve Nutrient Utilization and Ruminal Fermentation. Anim. Sci. J. 2024, 95, e13910. [Google Scholar] [CrossRef]
- Kholif, A.E.; Rahman, M.A.; Abo El-Nor, S.A.H.; Morsy, T.A.; Gouda, G.A.; Fahmy, M.; Chahine, M. Efficacy of Salvia officinalis Shrub as a Sustainable Feed Additive for Reducing Ruminal Methane Production and Enhancing Fermentation in Ruminants. Animals 2024, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.-M.; Wang, X.-B.; Zou, N.; Han, C.; Xu, J. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Volatile Oil of Cichorium Glandulosum Boiss et Huet and Its Effects on Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Med. Sci. Monit. 2019, 25, 3591–3604. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses; ARS-USDA: Washington, USA, USA, 1975. [Google Scholar]
- Fortina, R.; Glorio Patrucco, S.; Barbera, S.; Tassone, S. Rumen Fluid from Slaughtered Animals: A Standardized Procedure for Sampling, Storage and Use in Digestibility Trials. Methods Protoc. 2022, 5, 59. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Washington, DC, USA, 1997; ISBN 9780935584547. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the Extent of Degradation of Ruminant Feeds from a Description of Their Gas Production Profiles Observed In Vitro: Derivation of Models and Other Mathematical Considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Blümmel, M.; Steingaβ, H.; Becker, K. The Relationship between In Vitro Gas Production, In Vitro Microbial Biomass Yield and 15 N Incorporation and Its Implications for the Prediction of Voluntary Feed Intake of Roughages. Br. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor In Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Tadele, Y. White Lupin (Lupinus albus) Grain, a Potential Source of Protein for Ruminants: A Review. Res. J. Agric. Environ. Manag. 2015, 4, 180–188. [Google Scholar]
- Maia, M.R.G.; Monteiro, A.; Valente, I.M.; Sousa, C.; Miranda, C.; Castro, C.; Cortez, P.P.; Cabrita, A.R.J.; Trindade, H.; Fonseca, A.J.M. Upcycling Post-Harvest Biomass Residues from Native European Lupinus Species: From Straws and Pod Shells Production to Nutritive Value and Alkaloids Content for Ruminant Animals. Front. Nutr. 2023, 10, 1195015. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Nishida, T.; Ishida, M.; Hosoda, K.; Bayaru, E. Effect of Peppermint Feeding on the Digestibility, Ruminal Fermentation and Protozoa. Livest. Prod. Sci. 2003, 82, 245–248. [Google Scholar] [CrossRef]
- Dixon, R.M.; Hosking, B.J. Nutritional Value of Grain Legumes for Ruminants. Nutr. Res. Rev. 1992, 5, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, Z.; Mikulski, D.; Jankowski, J.; Przybylska-Gornowicz, B.; Juskiewicz, J. Gastrointestinal Response of Laying Hens to Graded Dietary Inclusion Levels of Yellow Lupine Seeds. Anim. Feed. Sci. Technol. 2019, 255, 114214. [Google Scholar] [CrossRef]
- Gresta, F.; Oteri, M.; Scordia, D.; Costale, A.; Armone, R.; Meineri, G.; Chiofalo, B. White Lupin (Lupinus albus L.), an Alternative Legume for Animal Feeding in the Mediterranean Area. Agriculture 2023, 13, 434. [Google Scholar] [CrossRef]
- Bryszak, M.; Szumacher-Strabel, M.; Huang, H.; Pawlak, P.; Lechniak, D.; Kołodziejski, P.; Yanza, Y.R.; Patra, A.K.; Váradyová, Z.; Cieslak, A. Lupinus angustifolius Seed Meal Supplemented to Dairy Cow Diet Improves Fatty Acid Composition in Milk and Mitigates Methane Production. Anim. Feed. Sci. Technol. 2020, 267, 114590. [Google Scholar] [CrossRef]
- Patra, A.K. Recent Advances in Measurement and Dietary Mitigation of Enteric Methane Emissions in Ruminants. Front. Vet. Sci. 2016, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Callaway, T.R.; Lee, C.; Dowd, S.E. Rumen Bacterial, Archaeal, and Fungal Diversity of Dairy Cows in Response to Ingestion of Lauric or Myristic Acid. J. Anim. Sci. 2012, 90, 4449–4457. [Google Scholar] [CrossRef]
- Abubakr, A.R.; Alimon, A.R.; Yaakub, H.; Abdullah, N.; Ivan, M. Digestibility, Rumen Protozoa, and Ruminal Fermentation in Goats Receiving Dietary Palm Oil By-Products. J. Saudi Soc. Agric. Sci. 2013, 12, 147–154. [Google Scholar] [CrossRef]
- Galagan, J.E.; Nusbaum, C.; Roy, A.; Endrizzi, M.G.; Macdonald, P.; FitzHugh, W.; Calvo, S.; Engels, R.; Smirnov, S.; Atnoor, D.; et al. The Genome of M. Acetivorans Reveals Extensive Metabolic and Physiological Diversity. Genome Res. 2002, 12, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Clarke, W.P.; Blackall, L.L. Concurrent Microscopic Observations and Activity Measurements of Cellulose Hydrolyzing and Methanogenic Populations during the Batch Anaerobic Digestion of Crystalline Cellulose. Biotechnol. Bioeng. 2005, 91, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Hassan, A.A.; El Ashry, G.M.; Bakr, M.H.; El-Zaiat, H.M.; Olafadehan, O.A.; Matloup, O.H.; Sallam, S.M.A. Phytogenic Feed Additives Mixture Enhances the Lactational Performance, Feed Utilization and Ruminal Fermentation of Friesian Cows. Anim. Biotechnol. 2021, 32, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.M.Y.; Kholif, A.E.; Salem, A.Z.M.; Montes de Oca, R.; Barbabosa, A.; Mariezcurrena, M.; Olafadehan, O.A. Addressing Sustainable Ruminal Methane and Carbon Dioxide Emissions of Soybean Hulls by Organic Acid Salts. J. Clean. Prod. 2016, 135, 194–200. [Google Scholar] [CrossRef]
- Garcia-Santos, S.; Almeida, M.; Closson, M.; Guedes, C.M.; Barros, A.; Ferreira, L.M.; Trindade, H.; Pinheiro, V. Effect of Total Replacement of the Soya Bean Meal by Lupine Seeds (L. Albus and L. Luteus) on Performance and Digestion Characteristics of Growing Rabbits. Anim. Feed. Sci. Technol. 2021, 278, 114996. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, B.; Zhao, X. Could Propionate Formation Be Used to Reduce Enteric Methane Emission in Ruminants? Sci. Total Environ. 2023, 855, 158867. [Google Scholar] [CrossRef]
- Park, S.J.; Beak, S.H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, Management, and Nutritional Factors Affecting Intramuscular Fat Deposition in Beef Cattle—A Review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Maheri-Sis, N.; Chamani, M.; Sadeghi, A.-A.; Mirza-Aghazadeh, A.; Aghajanzadeh-Golshani, A. Nutritional Evaluation of Kabuli and Desi Type Chickpeas (Cicer arietinum L.) for Ruminants Using In Vitro Gas Production Technique. Afr. J. Biotechnol. 2008, 7, 2946–2951. [Google Scholar]
- Azar, M.S.; Doust-Nobar, R.S.; Sis, N.M.; Shahryar, H.A.; Asadi, Y. Effects of Zataria multiflora Extract as Rumen Modifiers Using In Vitro Gas Production Technique. Curr. Res. J. Biol. Sci. 2012, 4, 350–354. [Google Scholar]
- Fellner, V. Rumen Microbes and Nutrient Management; Animal Science Department Report; North Carolina State University: Raleigh, NC, USA, 2004. [Google Scholar]
- Almeida, M.; Garcia-Santos, S.; Nunes, A.; Rito, S.; Azevedo, J.; Guedes, C.; Silva, S.; Ferreira, L. Introducing Mediterranean Lupins in Lambs’ Diets: Effects on Growth and Digestibility. Animals 2021, 11, 942. [Google Scholar] [CrossRef]
- Gomaa, A.S.; Kholif, A.E.; Kholif, A.M.; Salama, R.; El-Alamy, H.A.; Olafadehan, O.A. Sunflower Oil and Nannochloropsis oculata Microalgae as Sources of Unsaturated Fatty Acids for Mitigation of Methane Production and Enhancing Diets’ Nutritive Value. J. Agric. Food Chem. 2018, 66, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Guo, T.; Cao, Y.; Guo, L.; Li, F.; Li, F.; Yang, G. Changes in the Fermentation and Bacterial Community by Artificial Saliva pH in RUSITEC System. Front. Nutr. 2021, 8, 760316. [Google Scholar] [CrossRef]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH Regulation and Nutritional Consequences of Low pH. Anim. Feed. Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Laporte-Uribe, J.A. The Role of Dissolved Carbon Dioxide in Both the Decline in Rumen pH and Nutritional Diseases in Ruminants. Anim. Feed. Sci. Technol. 2016, 219, 268–279. [Google Scholar] [CrossRef]
- Kamra, D.N. Rumen Microbial Ecosystem. Curr. Sci. 2005, 89, 124–135. [Google Scholar]
- Ososanya, T.O.; Odubola, O.T.; Shuaib-Rahim, A. Intake, Nutrient Digestibility and Rumen Ecology of West African Dwarf Sheep Fed Palm Kernel Oil and Wheat Offal Supplemented Diets. Int. J. Agrisci. 2013, 3, 380–386. [Google Scholar]
- Salem, A.Z.; Kholif, A.E.; Elghandour, M.M.Y.; Hernandez, S.R.; Domínguez-Vara, I.A.; Mellado, M. Effect of Increasing Levels of Seven Tree Species Extracts Added to a High Concentrate Diet on In Vitro Rumen Gas Output. Anim. Sci. J. 2014, 85, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.M.Y.; Kholif, A.E.; Salem, A.Z.M.; Olafadehan, O.A.; Kholif, A.M. Sustainable Anaerobic Rumen Methane and Carbon Dioxide Productions from Prickly Pear Cactus Flour by Organic Acid Salts Addition. J. Clean. Prod. 2016, 139, 1362–1369. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Adebayo, O.F. Nutritional Evaluation of Ammoniated Ensiled Threshed Sorghum Top as a Feed for Goats. Trop. Anim. Health Prod. 2016, 48, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Um, K.H.; Shin, J.S.; Son, G.H.; Park, B.K. Effect of Lupin Supplementation on the Growth, Carcass, and Meat Characteristics of Late-Fattening Hanwoo Steers. Animals 2024, 14, 324. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, J.A.; Urías-Estrada, J.D.; López-Soto, M.A.; Barreras, A.; Plascencia, A.; Montaño, M.; González-Vizcarra, V.M.; Estrada-Angulo, A.; Castro-Pérez, B.I.; Barajas, R.; et al. Evaluation of Isoquinoline Alkaloid Supplementation Levels on Ruminal Fermentation, Characteristics of Digestion, and Microbial Protein Synthesis in Steers Fed a High-Energy Diet. J. Anim. Sci. 2016, 94, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Thirumalesh, T.; Krishnamoorthy, U. Rumen Microbial Biomass Synthesis and Its Importance in Ruminant Production. Int. J. Livest. Res. 2013, 3, 5. [Google Scholar] [CrossRef]
- Rodríguez, R.; Sosa, A.; Rodríguez, Y. Microbial Protein Synthesis in Rumen and Its Importance to Ruminants. Cuba. J. Agric. Sci. 2007, 41, 287–294. [Google Scholar]




| Lupin Seeds | CFM 1 | Berseem Hay | Rice Straw | Diet 2,3 | |
|---|---|---|---|---|---|
| Dry matter | 927 | 903 | 890 | 940 | 893 |
| Organic matter | 965 | 923 | 884 | 851 | 819 |
| Crude protein | 200 | 165 | 128 | 42 | 136 |
| Ether extract | 144 | 47 | 54 | 19 | 62 |
| Nonstructural carbohydrates | 429 | 414 | 224 | 166 | 359 |
| Neutral detergent fiber | 192 | 297 | 478 | 624 | 379 |
| Acid detergent fiber | 179 | 175 | 381 | 394 | 240 |
| Peak | Compound 1 | Formula | RT 2 | Concentration 3 (%) | Concentration (mg/100 g DM) |
|---|---|---|---|---|---|
| 1 | α-Pinene | C10H16 | 3.702 | 4.33 | 142 |
| 2 | β-Pinene | C10H16 | 4.771 | 2.81 | 92 |
| 3 | Eucalyptol | C10H18O | 6.738 | 86.7 | 2835 |
| 4 | Camphor | C10H16O | 9.942 | 3.71 | 121 |
| 5 | trans-Caryophyllene | C15H24 | 15.221 | 2.48 | 81 |
| GP Parameters 1 | CH4 Parameters 2 | CO2 Parameters 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level (%, DM) | A | c | Lag | A | c | Lag | % 4 | A | c | Lag | % 4 | ||
| 0 | 78.1 c | 0.076 bc | 1.55 ab | 28.6 ab | 0.035 | 1.42 b | 29.3 a | 58.8 b | 0.036 c | 2.47 | 63.5 b | ||
| 0.5 | 90.5 b | 0.070 c | 1.61 a | 25.5 b | 0.033 | 1.44 b | 23.3 b | 67.8 ab | 0.047 bc | 2.22 | 68.9 a | ||
| 1 | 102.0 a | 0.082 ab | 1.59 a | 31.8 a | 0.035 | 1.49 ab | 25.9 ab | 71.6 a | 0.056 ab | 2.25 | 66.7 ab | ||
| 1.5 | 101.2 a | 0.088 a | 1.47 b | 32.3 a | 0.032 | 1.57 ab | 25.5 ab | 69.3 a | 0.061 a | 2.14 | 65.6 ab | ||
| 2 | 105.5 a | 0.090 a | 1.34 c | 33.2 a | 0.034 | 1.68 a | 25.6 ab | 73.0 a | 0.063 a | 2.19 | 66.7 ab | ||
| SEM | 1.82 | 0.002 | 0.06 | 1.03 | 0.0032 | 0.05 | 0.84 | 2.11 | 0.0029 | 0.17 | 0.94 | ||
| p value | |||||||||||||
| Treatment | <0.001 | 0.006 | 0.033 | 0.002 | 0.976 | 0.014 | 0.008 | 0.006 | 0.004 | 0.716 | 0.027 | ||
| Linear | <0.001 | 0.001 | 0.021 | 0.006 | 0.823 | 0.001 | 0.080 | 0.001 | <0.001 | 0.277 | 0.326 | ||
| Quadratic | 0.002 | 0.328 | 0.082 | 0.564 | 0.893 | 0.280 | 0.015 | 0.061 | 0.076 | 0.485 | 0.053 | ||
| Degradability (g/kg DM) 1 | SCFA (mmol/g DM ) 2 | Fermentation 3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level (%, DM) | dDM | dNDF | dADF | Total | C2 | C3 | C2:C3 | C4 | pH | NH3-N | ME | PF24 | MCP | GY24 | ||
| 0 | 473 b | 439 b | 412 b | 23.4 b | 11.43 b | 7.90 b | 1.47 b | 4.08 | 6.27 a | 10.43 a | 4.68 c | 7.22 | 328 b | 139 b | ||
| 0.5 | 521 b | 512 ab | 386 b | 24.7 b | 12.83 ab | 8.11 b | 1.59 a | 3.81 | 6.30 a | 10.27 a | 5.33 b | 7.13 | 360 ab | 141 b | ||
| 1 | 599 a | 517 a | 501 a | 26.5 ab | 13.11 ab | 8.94 ab | 1.47 b | 4.45 | 6.13 b | 10.00 ab | 5.73 a | 6.82 | 406 a | 147 ab | ||
| 1.5 | 587 a | 534 a | 514 a | 28.8 a | 13.38 ab | 10.39 a | 1.29 c | 5.08 | 6.13 b | 9.63 b | 5.76 a | 6.60 | 391 a | 152 a | ||
| 2 | 598 a | 566 a | 546 a | 29.5 a | 13.94 a | 10.38 a | 1.34 c | 5.19 | 6.17 b | 9.43 b | 5.88 a | 6.41 | 393 a | 156 a | ||
| SEM | 11.7 | 16.2 | 13.0 | 0.84 | 0.467 | 0.406 | 0.100 | 0.418 | 0.039 | 0.201 | 0.051 | 0.208 | 11.5 | 3.8 | ||
| p value | ||||||||||||||||
| Treatment | <0.001 | 0.003 | <0.001 | 0.002 | 0.034 | 0.003 | 0.029 | 0.155 | 0.036 | 0.045 | <0.001 | 0.093 | 0.005 | 0.047 | ||
| Linear | <0.001 | 0.003 | <0.001 | 0.001 | 0.004 | 0.002 | 0.015 | 0.025 | 0.015 | 0.008 | <0.001 | 0.009 | 0.001 | 0.006 | ||
| Quadratic | 0.004 | 0.260 | 0.780 | 0.817 | 0.355 | 0.900 | 0.630 | 0.640 | 0.285 | 0.863 | <0.001 | 0.890 | 0.018 | 0.815 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsy, T.A.; Kholif, A.E.; Adegbeye, M.J.; Olafadehan, O.A.; Gouda, G.A.; Fahmy, M.; Chahine, M. Lupin Seed Supplementation as a Functional Feed Additive: In Vitro Ruminal Gas, Methane and Carbon Dioxide Production, Fermentation Kinetics, and Nutrient Degradability. Animals 2024, 14, 2119. https://doi.org/10.3390/ani14142119
Morsy TA, Kholif AE, Adegbeye MJ, Olafadehan OA, Gouda GA, Fahmy M, Chahine M. Lupin Seed Supplementation as a Functional Feed Additive: In Vitro Ruminal Gas, Methane and Carbon Dioxide Production, Fermentation Kinetics, and Nutrient Degradability. Animals. 2024; 14(14):2119. https://doi.org/10.3390/ani14142119
Chicago/Turabian StyleMorsy, Tarek A., Ahmed E. Kholif, Moyòsore J. Adegbeye, Olurotimi A. Olafadehan, Gouda A. Gouda, Mahmoud Fahmy, and Mireille Chahine. 2024. "Lupin Seed Supplementation as a Functional Feed Additive: In Vitro Ruminal Gas, Methane and Carbon Dioxide Production, Fermentation Kinetics, and Nutrient Degradability" Animals 14, no. 14: 2119. https://doi.org/10.3390/ani14142119
APA StyleMorsy, T. A., Kholif, A. E., Adegbeye, M. J., Olafadehan, O. A., Gouda, G. A., Fahmy, M., & Chahine, M. (2024). Lupin Seed Supplementation as a Functional Feed Additive: In Vitro Ruminal Gas, Methane and Carbon Dioxide Production, Fermentation Kinetics, and Nutrient Degradability. Animals, 14(14), 2119. https://doi.org/10.3390/ani14142119







