Biological Significance of the Komodo Dragon’s Tail (Varanus komodoensis, Varanidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Methods and Procedures
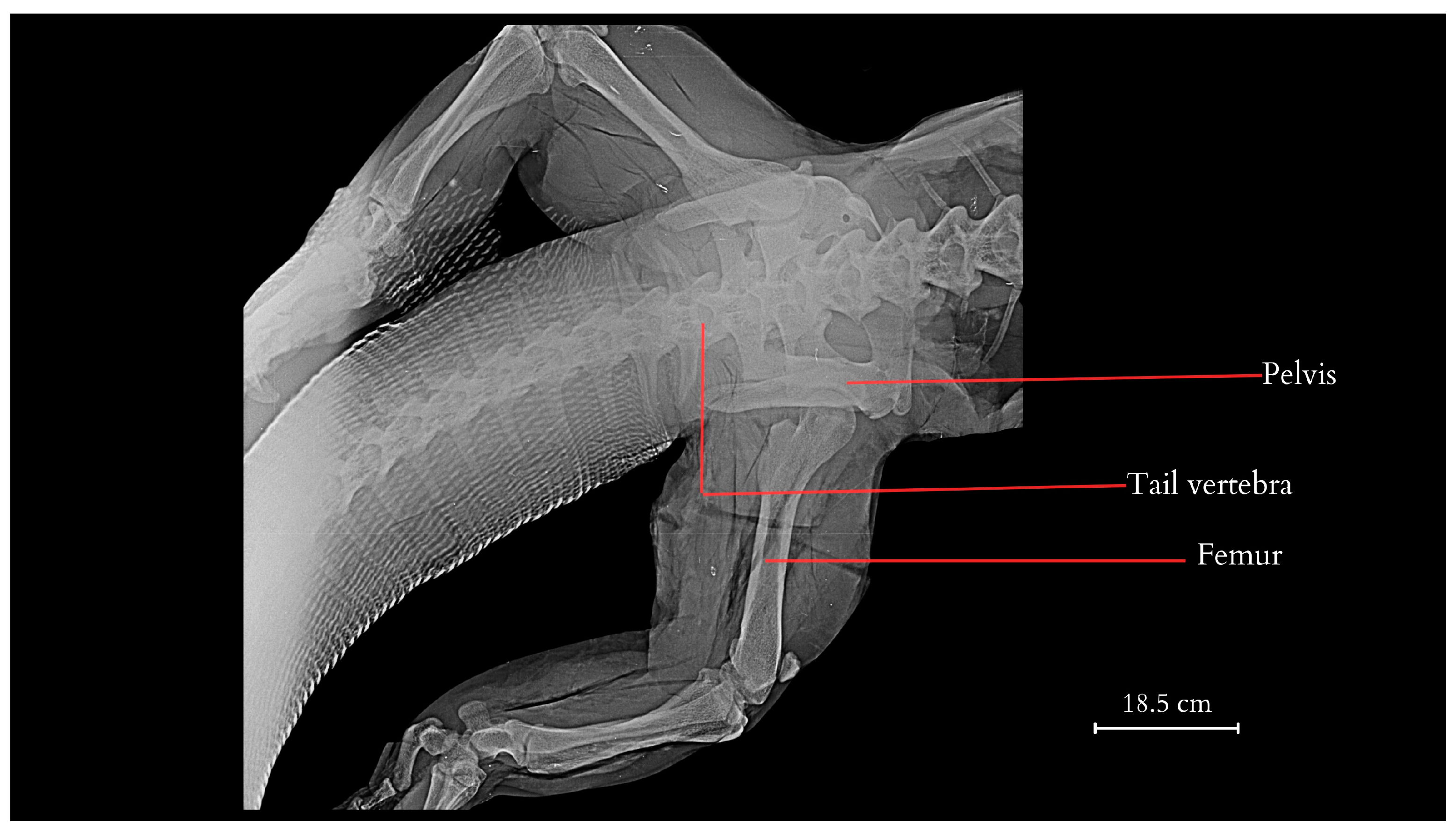
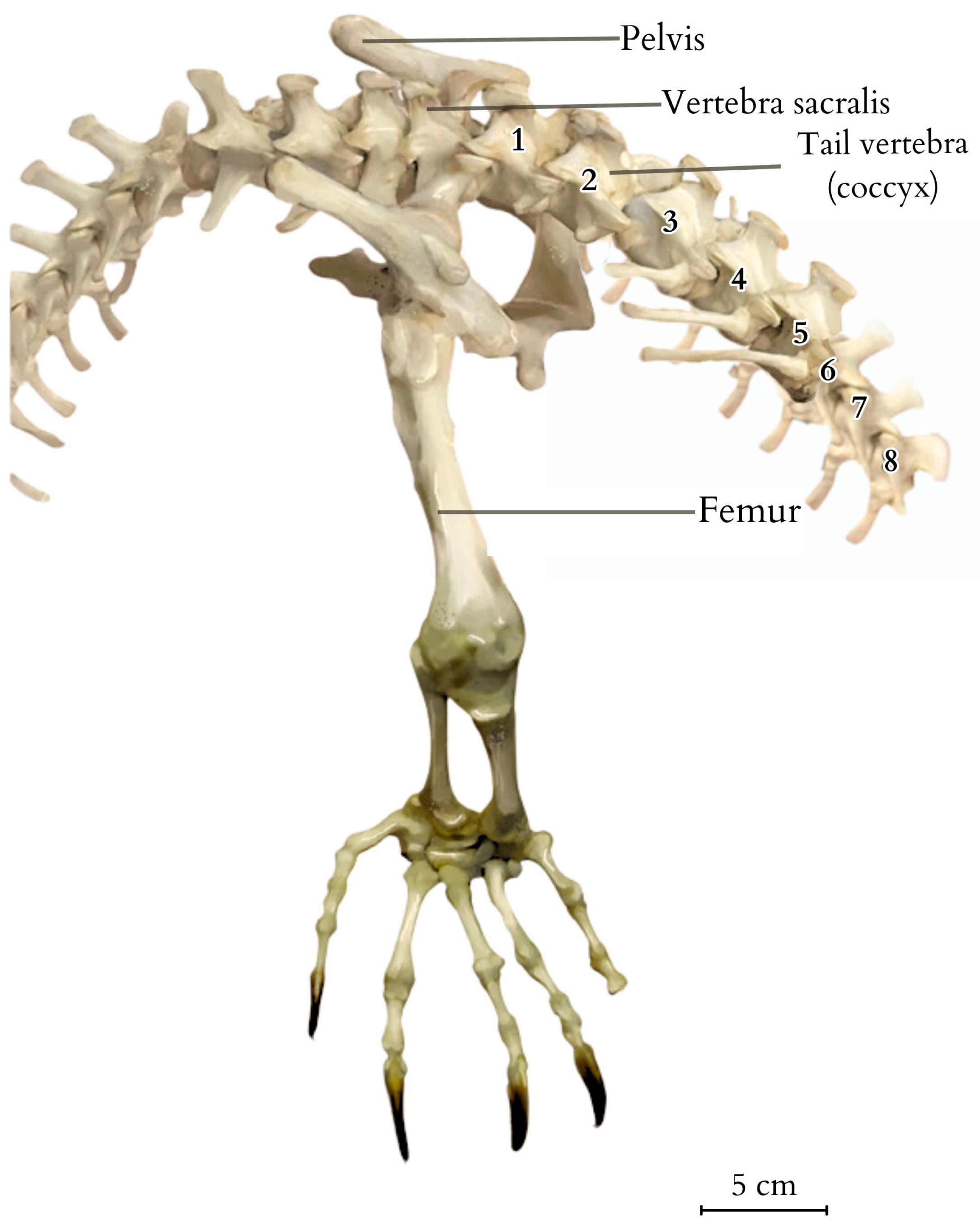
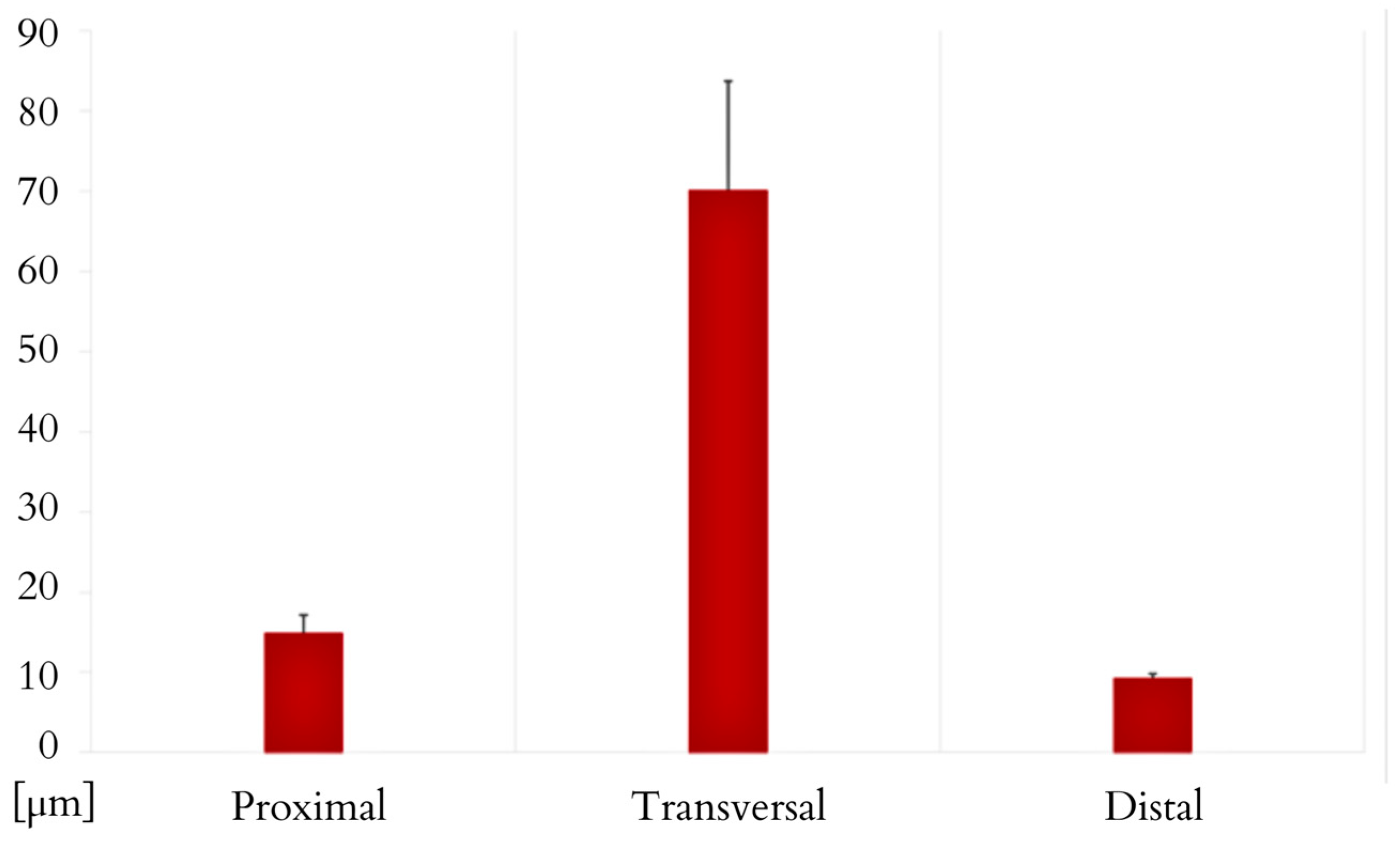
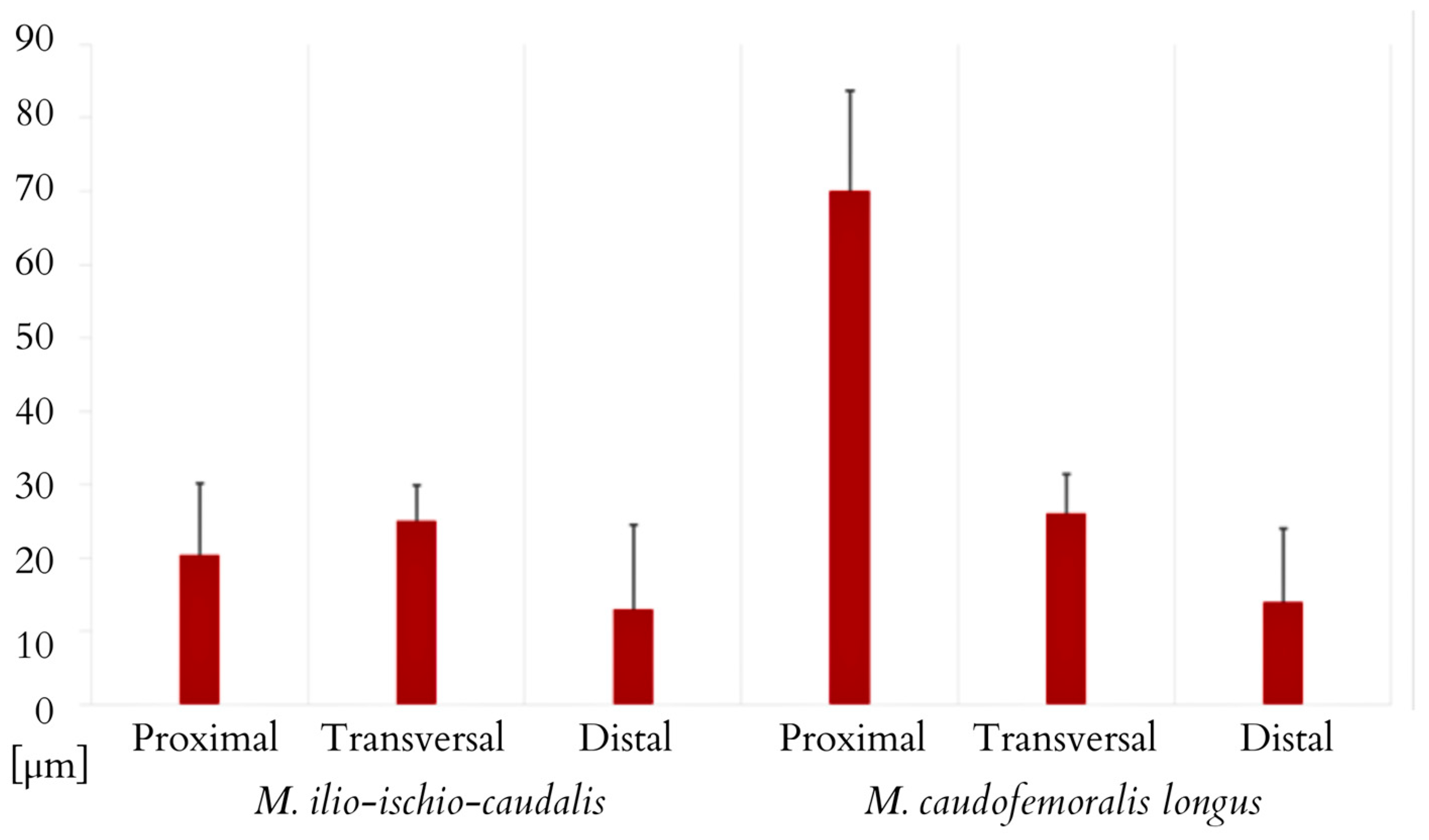
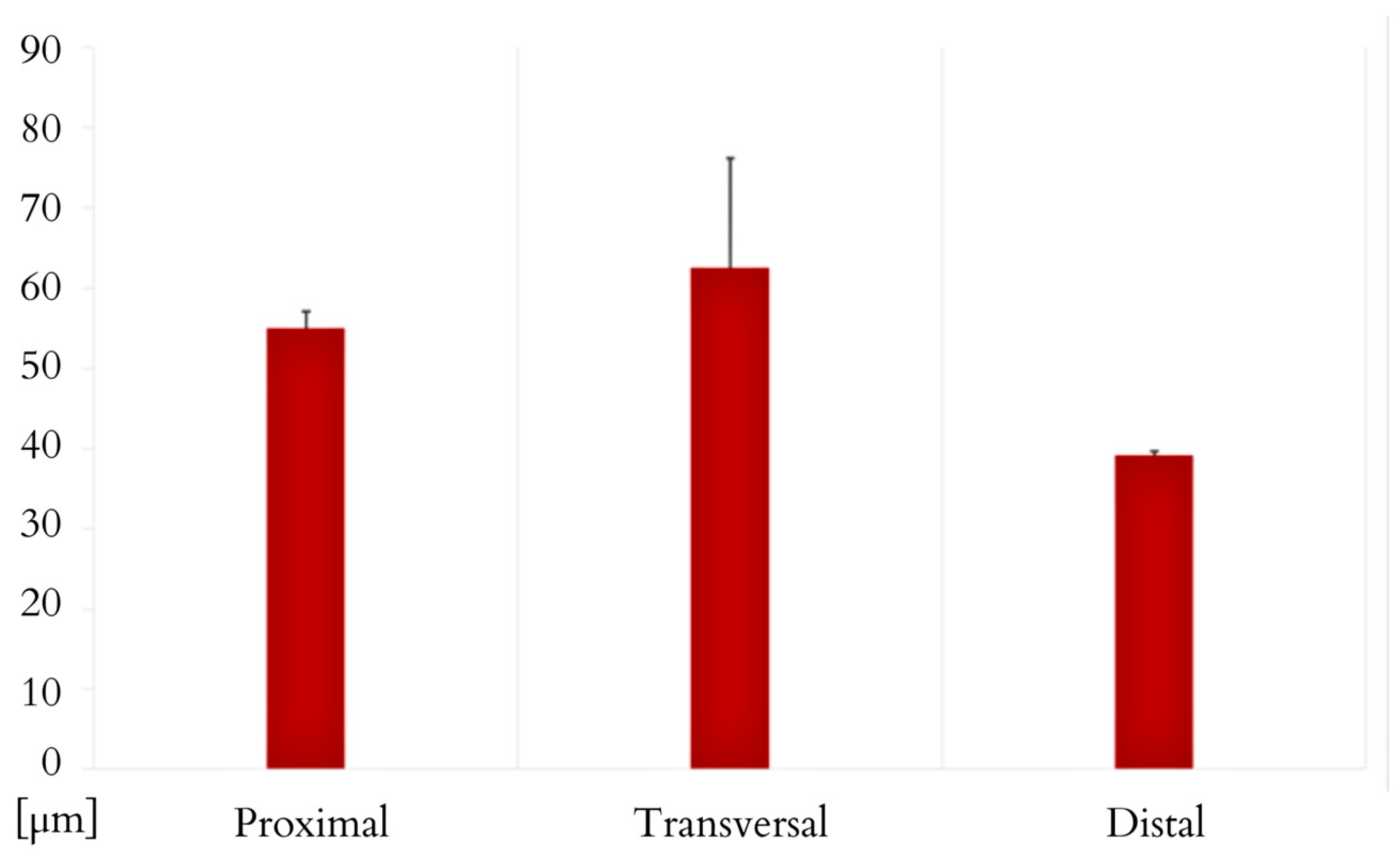
3. Results
4. Discussion
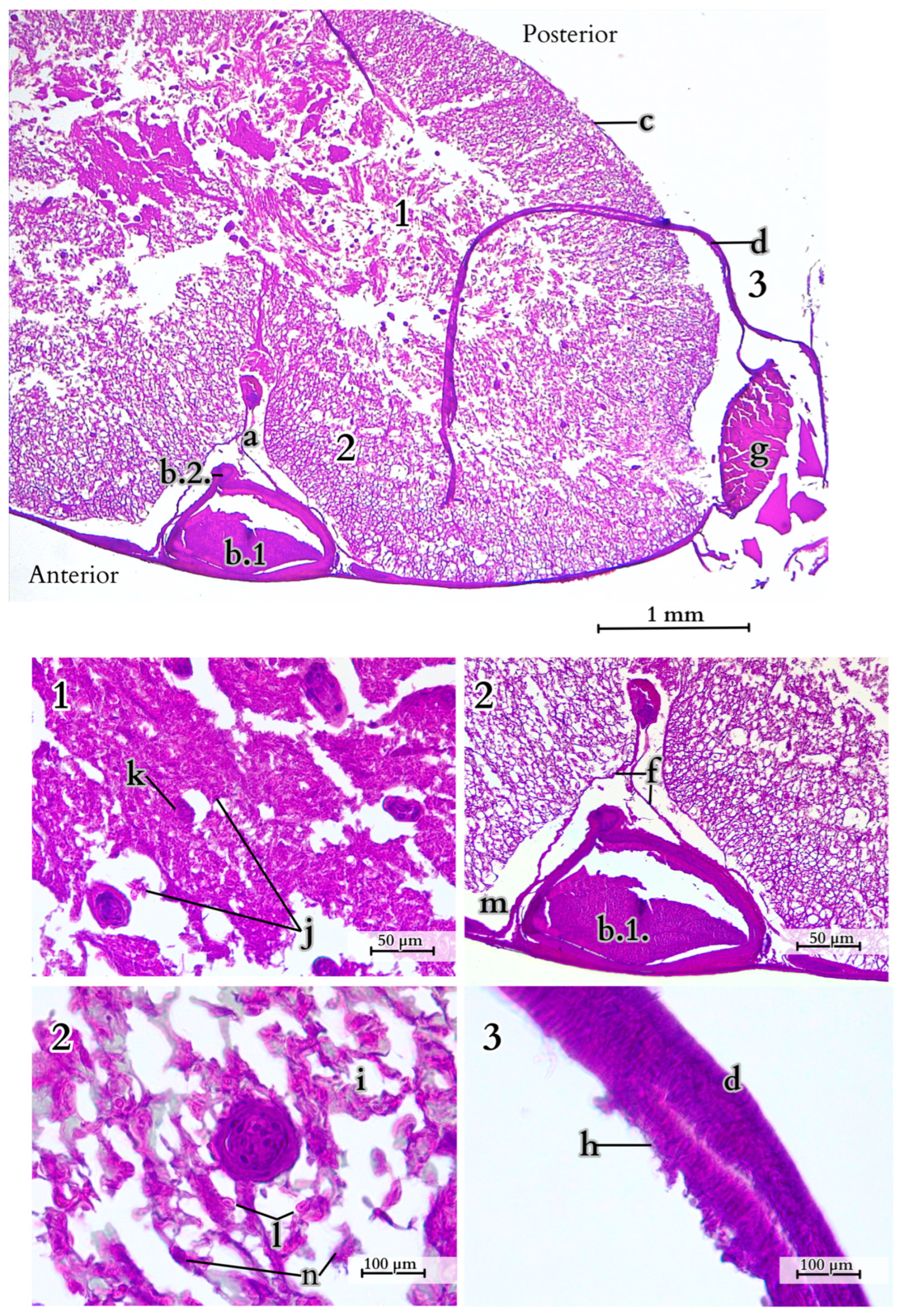
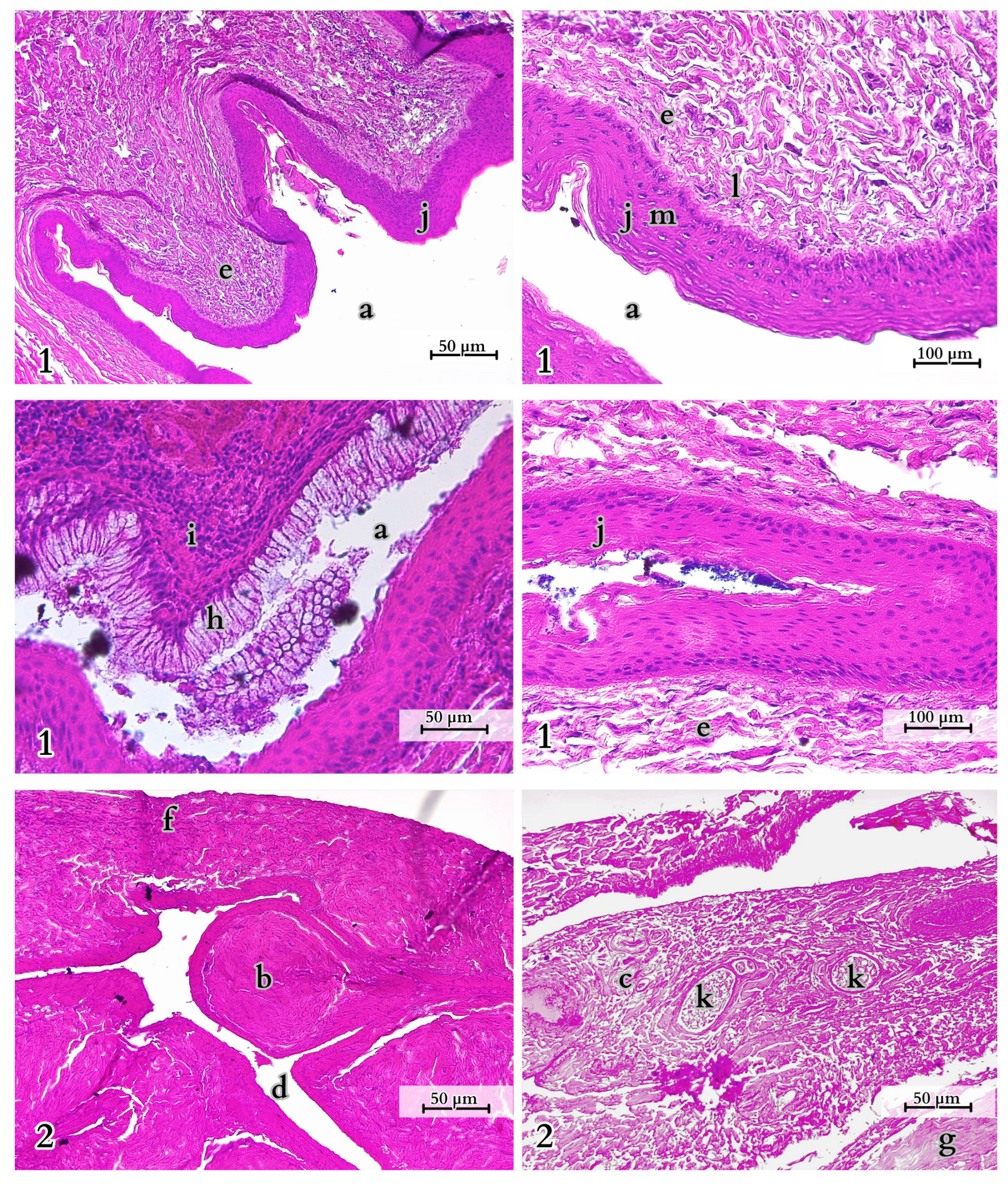
4.1. Overview of the Morphology and Features of the Tail
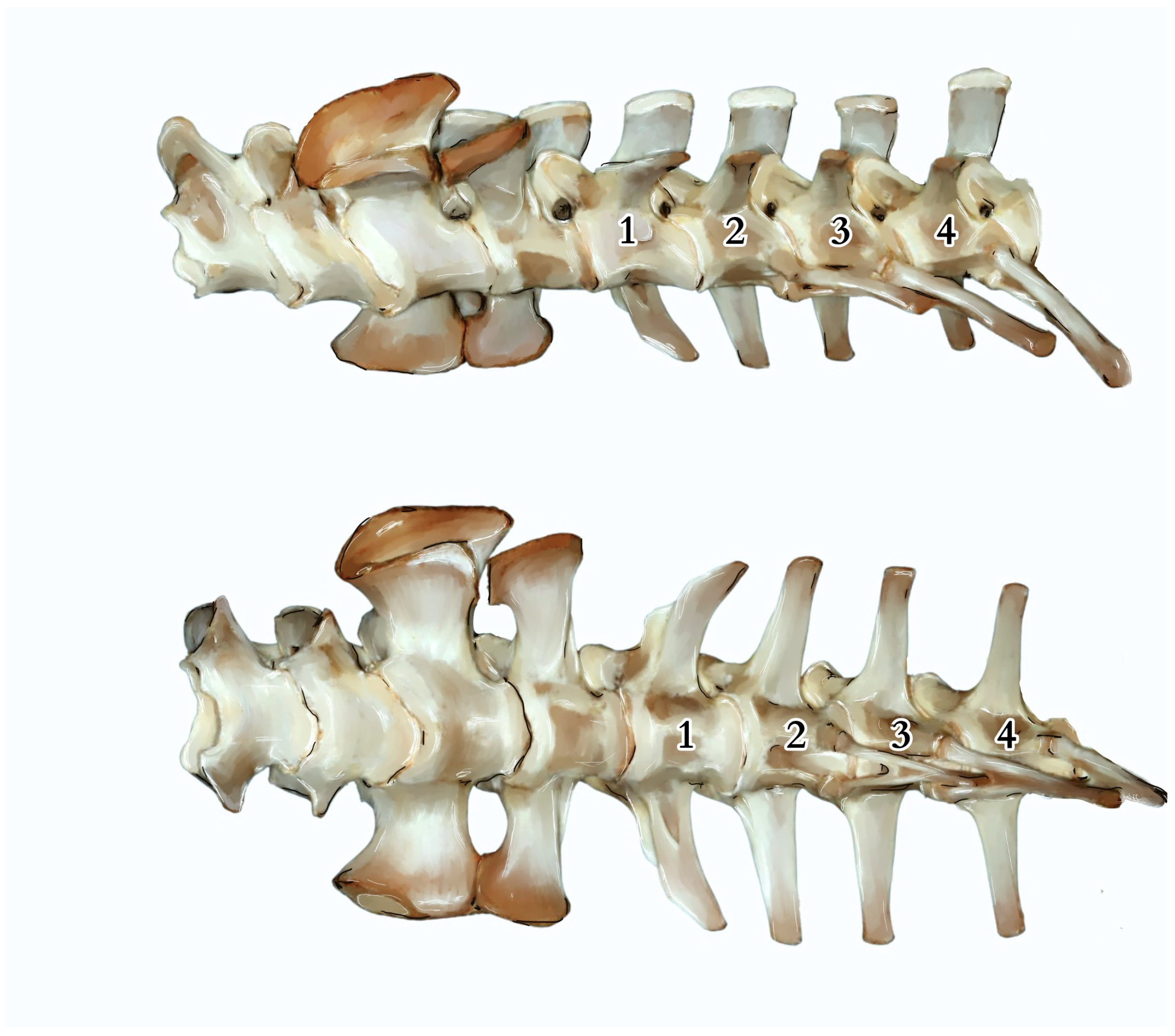
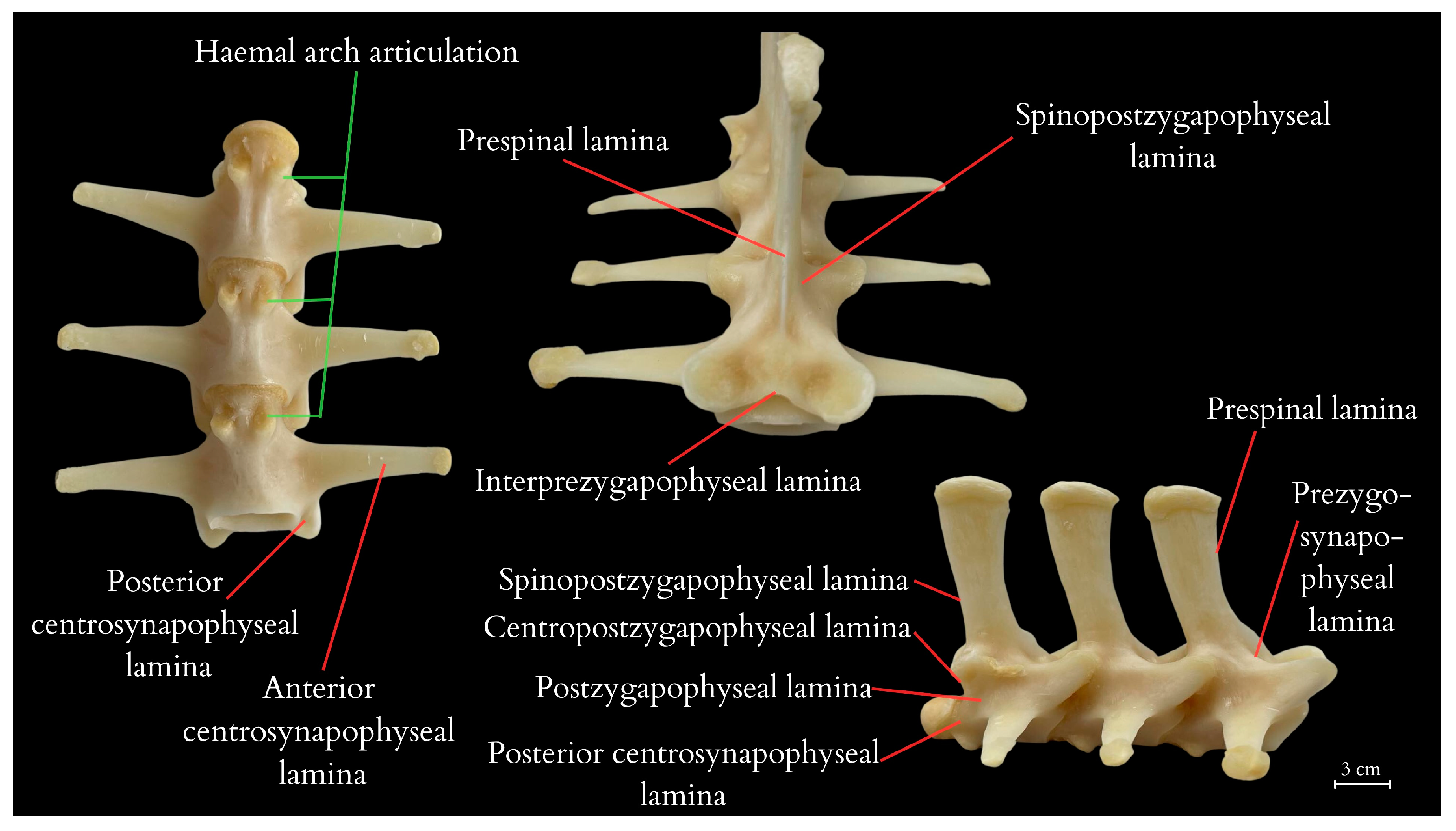
4.2. Anatomical Analysis of the Caudal Vertebrae, Species-Specific Characteristics
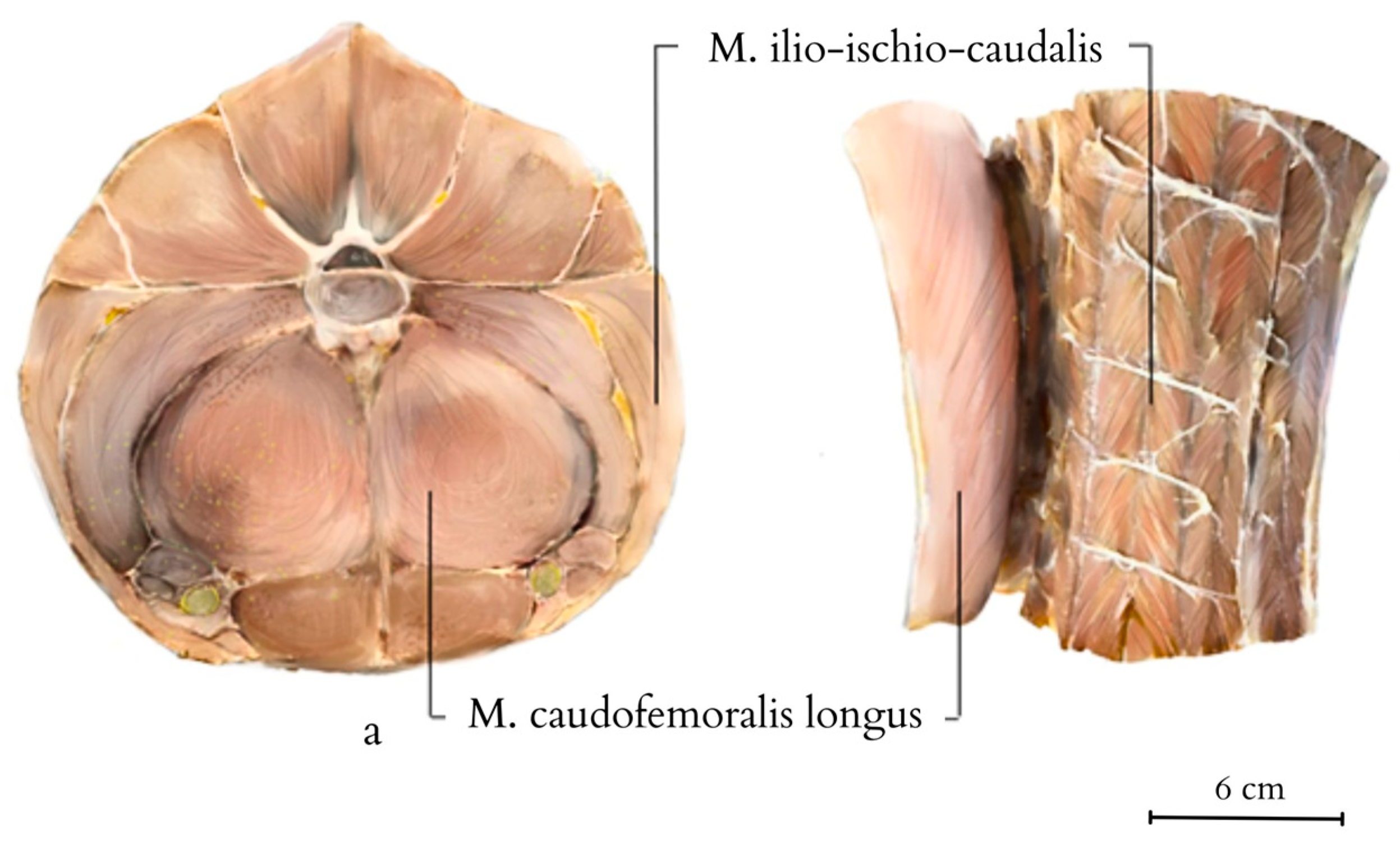
4.3. Specific Muscle Anatomy and Function of the Tail
4.4. Multifunctionality of Fat Storage in the Komodo Dragon’s Tail
4.5. Observations on Cloacal Glands and Spinal Cord
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryder, O.; Feistner, A.T. Research in zoos: A growth area in conservation. Biodivers. Conserv. 1995, 4, 671–677. [Google Scholar] [CrossRef]
- Kleiman, D.G.; Thompson, K.V.; Baer, C.K. Wild Mammals in Captivity. Principles and Techniques for Zoo Management, 2nd ed.; The University of Chicago Press: Chicago, IL, USA, 2012; pp. 281–283. [Google Scholar]
- Cowie, R.H.; Bouchet, P.; Fontaine. The Sixth Mass Extinction: Fact, fiction or speculation? Biol. Rev. 2022, 97, 640–663. [Google Scholar] [CrossRef]
- Schwaner, M.J.; Hsieh, S.T.; Braasch, I.; Bradley, S.; Campos, C.B.; Collins, C.E.; Donatelli, C.M.; Fish, F.E.; Fitch, O.E.; Flammang, B.E.; et al. Future Tail Tales: A Forward-Looking, Integrative Perspective on Tail Research. Integr. Comp. Biol. 2021, 61, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Gallina, P.A.; Canale, J.I.; Haluza, A. Sauropod haemal arches: Morphotypes, new classification and phylogenic aspects. Historical Biology. Int. J. Paleobiol. 2012, 24, 243–256. [Google Scholar]
- Tschopp, E. Nomenclature of Vertebral Laminae in Lizards, with Comments on Ontogenetic and Serial Variation in Lacertini (Squamata, Lacertidae). PLoS ONE 2016, 11, e0149445. [Google Scholar] [CrossRef]
- Kalman, B. Endangered Komodo Dragons; Crabtree Publishing Company: New York, NY, USA, 2005; p. 11. [Google Scholar]
- Rookmaaker, L.C. The History of Some Komodo Dragons (Varanus Komodoensis) Captured on Rintja in 1927. Zool. Meded. 1975, 49, 65–71. [Google Scholar]
- Available online: https://komododragon.org/morphology/ (accessed on 19 September 2023).
- Rovatsos, M.; Johnson Pokorná, M.; Altmanová, M.; Kratchvíl, L.; Velenský, P.; Vodička, R.; Rehák, I. Sexing of Komodo Dragons, Varanus komodoensis. Gazella 2015, 42, 93–107. [Google Scholar]
- Sulandari, S.; Arifin Zein, M.S.; Ayu Arida, E.; Hamidy, A. Molecular Sex Determination of Captive Komodo Dragons (Varanus komodoensis) at Gembira Loka Zoo, Surabaya Zoo, and Rangunan Zoo, Indonesia. HAYATI J. Biosci. 2014, 21, 65–75. [Google Scholar] [CrossRef]
- Kirby, A.; Vickaryous, M.; Boyde, A.; Olivo, A.; Moazen, M.; Bertazzo, S.; Evans, S. A comparative histological study of the osteoderms in the lizards Heloderma suspectum (Squamata: Helodermatidae) and Varanus komodoensis (Squamata: Varanidae). J. Anat. 2020, 236, 1035–1043. [Google Scholar] [CrossRef]
- Etherdige, R. Lizard Caudal Vertebrae. Copeia 1967, 4, 699–721. [Google Scholar] [CrossRef]
- Arifin-Akbari, R. Studi Karakteristik Anatomi Skelet Aksial Biawak Air (Varanus salvator); Scientific Repository Collects; IPB University: Bogor, Indonesia, 2014; pp. 1–15. [Google Scholar]
- Cieri, R.L. The axial anatomy of monitor lizards (Varanidae). J. Anat. 2018, 233, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Collar, D.C.; Schulte, J.A.; Losos, J.B. Evolution of extreme body size disparity in monitor lizards (Varanus). Evolution 2011, 65, 2664–2680. [Google Scholar] [CrossRef] [PubMed]
- Rieppel, O. Studies on skeleton formation in reptiles. V. Patterns of ossification in the skeleton of Alligator mississippiensis DAUDIN (Reptilia, Crocodylia). Zool. J. Linn. Soc. 1993, 109, 301–325. [Google Scholar] [CrossRef]
- Cope, E.D. The Crocodilians, Lizards, and Snakes of North America; U.S. Government Printing Office: Washington, DC, USA, 1900; p. 1294.
- Cope, E.D. On Fossil Remains of Reptilia and Fishes from Illinois. Proc. Acad. Nat. Sci. Phila. 1875, 27, 404–408. [Google Scholar] [CrossRef]
- Akita, K. An anatomical investigation of the muscles of the pelvic outlet in Iguanas (Iguanidae Iguana iguana) and varanus (Varanidae Varanus (dumerillii)) with special reference to their nerve supply. Ann. Anat.–Anat. Anz. 1992, 174, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Rakhmiyati; Jafar Luthfi, M. Study Anatomy of Vertebrae Caudalis Asiatic Water Monitor (Varanus salvator). Proc. Int. Conf. Sci. Eng. 2020, 3, 109–112. [Google Scholar] [CrossRef]
- Ritzman, T.B.; Stroik, L.K.; Julik, E.; Hutchins, E.D.; Lasku, E.; Denardo, D.F.; Wilson-Rawls, J.; Rawls, A.; Kusumi, K.; Fisher, R.E. The Gross Anatomy of the Original and Regenerated Tail in the Green Anole (Anolis carolinensis). Anat. Rec. 2012, 295, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Woodward, H.N.; Farlow, J.O. Ruling Reptiles: Crocodylian Biology and Paleobiology; Indiana University Press: Bloomington, IN, USA, 2023; pp. 1–424. [Google Scholar]
- Villa, A.; Abella, J.; Alba, D.M.; Almécija, S.; Bolet, A.; Koufos, G.D.; Knoli, F.; Luján, A.H.; Morales, J.; Robles, J.M.; et al. Revision of Varanus marathonensis (Squamata, Varanidae) based on historical and new material: Morphology, systematics, and paleobiogeography of the European monitor lizards. PLoS ONE 2018, 13, e0207719. [Google Scholar] [CrossRef]
- Romer, A.S. The Osteology of the Reptiles; University of Chicago Press: Chicago, IL, USA, 1956. [Google Scholar]
- Erickson, G.M.; Lappin, A.K.; Larson, P. Androgynous rex—The utility of chevrons for determining the sex of crocodilians and non-avian dinosaurs. Zoology 2005, 108, 277–286. [Google Scholar] [CrossRef]
- Mallison, H.; Pittman, M.; Schwarz, D. Using crocodilian tails as models for dinosaur tails. PeerJ. PrePrints 2015, 3, e1339v1. [Google Scholar]
- Auliya, M.; Koch, A. Visual Identification Guide to the Monitor Lizard Species of the World (Genus Varanus); Federal Agency Nature Conservation, Bundesamt für Naturschutz: Bonn, Germany, 2020; pp. 178–180. [Google Scholar]
- Hoff, K.S.; Wassersburg, R.J. Tadpole Locomotion: Axial Movement and Tail Functions in a Largely Vertebraeless Vertebrate. Integr. Comp. Biol. 2000, 40, 62–76. [Google Scholar] [CrossRef]
- Gilmore, C.W. On a newly mounted skeleton of Diplodocus in the United States National Museum. Proc. U. S. Natl. Mus. 1932, 81, 1–21. [Google Scholar] [CrossRef]
- Pfeffer, P. Observations sur le Varan de Komodo. Varanus komodoensis. Ouwens 1912. 1912; pp. 195–243. Available online: https://www.usda.gov/ (accessed on 16 September 2023).
- Murphy, J.B. Portrait of a Herpetologist as an Older Man. Chapter 5. Monitor Lizards and the Leader of Them All, the Komodo Dragon. Bull. Chic. Herpetol. Soc. 2022, 57, 71–79. [Google Scholar]
- Wines, M. Komodo Dragon Trappers. 2017. Available online: https://reptilesmagazine.com/komodo-dragon-trappers/ (accessed on 19 September 2023).
- Xu, C.; Palade, J.; Fisher, R.E.; Smith, C.I.; Clark, A.R.; Sampson, S.; Bourgeois, R.; Rawls, A.; Elsey, R.M.; Wilson-Rawls, J.; et al. Anatomical and histological analyses reveal that tail repair is coupled with regrowth in wild-caught, juvenile Americal Alligators (Alligatos mississippiensis). Sci. Rep. 2020, 10, 20122. [Google Scholar] [CrossRef] [PubMed]
- Muzammil Ali, S. Studies on the anatomy of the tail in Sauria and Rhynchocephalia. Proc. Indian Acad. Sci. 1949, 30, 155–167. [Google Scholar]
- Hoser, R.T. Monitor Lizard reclassified with some common sense (Squamata: Sauria Varanidae). Aust. J. Herpetol. 2013, 21, 41–58. [Google Scholar]
- Hocknull, S.A.; Piper, P.J.; van den Bergh, G.D.; Awe Due, R.; Morwood, M.J.; Kurniawan, I. Dragon’s Paradise Lost: Palaeobiogeography, Evolution and Extinction of the Largest-Ever Terrestrial Lizards (Varanidae). PLoS ONE 2009, 4, e7241. [Google Scholar] [CrossRef] [PubMed]
- Libby, T.; Moore, Y.T.; Siu, E.C.; Liu, D.; Cohen, D.; Jusufi, A.; Full, R.J. Tail-assisted pitch control in lizards, robots and dinosaurs. Nature 2012, 481, 181–184. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B. Clinical Anatomy and Physiology of Exotic Species. Structure and Function of Mammals, Birds, Reptiles, and Amphibians; Chapter 4—Lizards; Saunders, Ltd.: London, UK, 2005; pp. 57–75. [Google Scholar]
- Harlow, H.J. Size-Related Differences in the Thermoregulatory Habits of Free-Ranging Komodo Dragons. Int. J. Zool. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Harlow, H.J.; Purwandana, D.; Jessop, T.S.; Phillips, J.A. Body temperature and thermoregulation of Komodo dragons in the field. J. Therm. Biol. 2010, 35, 338–347. [Google Scholar] [CrossRef]
- McNab, B.K.; Auffenberg, W. The effect of large body size on the temperature regulation of the komodo dragon, Varanus komodoensis. Comp. Biochem. Physiol. Part A Physiol. 1976, 55, 345–350. [Google Scholar] [CrossRef]
- Ariefiandy, A.; Purduwana, D.; Seno, A.; Ciofi, C.; Jessop, T.S. Can Camera Traps Monitor Komodo Dragons a Large Ectothermic Predator? PLoS ONE 2013, 8, e58800. [Google Scholar] [CrossRef] [PubMed]
- Jessop, T.S.; Purduwana, D.; Imansyah, M.J.; Ciofi, C.; Benu, Y.J.; Ariefiandy, A. The influence of tropical seasonality on breeding phenology, growth, survival and movement of a large reptile (Varanus komodoensis). Biol. J. Linn. Soc. 2022, 136, 1–14. [Google Scholar] [CrossRef]
- Simandle, E.T.; Espinoza, R.E.; Nussear, K.E.; Tracy, R. Lizards, Lipids, and Dietary Links to Animal Function. Physiol. Biochem. Zool. 2001, 74, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Price, E.R. The physiology of lipid storage and use in reptiles. Biol. Rev. 2016, 92, 1406–1426. [Google Scholar] [CrossRef] [PubMed]
- Jessop, T.S.; Madsen, T.; Purduwana, D.; Imansyah, M.J.; Rudiharto, H.; Ciofi, C. Evidence for Energetic Constraints Affecting a Small Island Komodo Dragon Population. Report from the Zoological Society of San Diego, Komodo National Park, and The Nature Conservancy. Labuan Bajo, Flores. 2005, pp. 1–9. Available online: https://www.researchgate.net/publication/237720499_Evidence_for_energetic_constraints_affecting_a_small_island_Komodo_dragon_population (accessed on 10 September 2023).
- Laver, R.J.; Purduwana, D.; Ariefiandy, A.; Imansyah, J.; Forsyth, D.; Ciofi, C.; Jessop, T.S. Life-History and Spatial Determinants of Somatic Growth Dynamics in Komodo Dragon Populations. PLoS ONE 2012, 7, e45398. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.C.; Jyrwa, J.D.; Jabin, G.; Sharma, L.K.; Thakur, M.; Chandra, K. Ethno-medicinal use of monitor lizard Varanus bengalensis (Daudin, 1802) by the ‘Adi’ tribe at East Siang, Arunachal Pradesh. Indian J. Tradit. Knowl. 2021, 20, 749–753. [Google Scholar]
- Huey, R.B.; Pianka, E.R.; Vitt, L.J. How Often Do Lizards “Run on Empty”? Ecology 2001, 82, 1–7. [Google Scholar]
- McCue, M.D. Fatty acid analyses may provide insight into the progression of starvation among squamate reptiles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 239–246. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; Govender, D.; Huchzermeyer, F.; Bouwman, H. Comparison of the Lipid Composition of Three Adipose Tissue Types of Male and Female Wild Nile Crocodiles (Crocodylus niloticus). J. Herpetol. 2014, 48, 525–531. [Google Scholar] [CrossRef]
- Li, O.; Yang, L.; Li, R.; Chen, G.; Dong, J.; Wu, L.; Fu, Y.; Yang, J. Lipid analysis of meat from Bactrian camel (Camelus bacterianus), beef, and tails of fat-tailed sheep using UPLC-Q-TOF/MS based lipidomics. Front. Nutr. 2023, 10, e1053116. [Google Scholar] [CrossRef]
- Kusuma, A. The organization of the spinal cord in reptiles with different locomotor patterns, Stichting Studentenpers Nijmegen. In Biology of the Reptilia (C. Gans, Red.), 10; Academic Press: London, UK, 1979; pp. 9–112. [Google Scholar]
- Yensen; Wulansari, R.; Handharyani, E. Gambaran Darah Komodo (Varanus komodoensis) Di Taman Margasatwa Ragunan. Bachelor’s Thesis, IPB University, Bogor, Indonesia, 2012; pp. 1–32.
- Divers, S.; Redmayne, G.; Aves, E. Haematological and biochemical values of 10 green iguanas (Iguana iguana). Vet. Rec. 1996, 138, 203–205. [Google Scholar] [CrossRef]
- Orakpoghenor, O.; Sambo, J.S.; Abdulsalam, H.; Markus, T.P. Haematological parameters of a monitor lizard. Comp. Clin. Pathol. 2019, 28, 513–516. [Google Scholar] [CrossRef]
- Barajas-Valero, S.; Rodríguez-Almonacid, C.; Rojas-Sereno, Z.; Moreno-Torres, C.; Matta, N.E. Hematology, Biochemistry Reference Intervals, and Morphological Description of Peripheral Blood Cells for a Captive Population of Crocodylus intermedius in Colombia. Front. Vet. Sci. 2021, 8, 694354. [Google Scholar] [CrossRef] [PubMed]
- Georoff, T.A.; Stacy, N.I.; Newton, A.N.; McAloose, D.; Post, G.S.; Raskin, R.E. Diagnosis and Treatment of Chronic T-Lymphocytic Leukemia in a Green Tree Monitor (Varanus prasinus). J. Herpetol. Med. Surg. 2009, 19, 106–114. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Nour-Eldin Ismail, M.; El Sayed, A.A.M.; Omran Ali, A.; Tsubota, T. Hematologic and Biochemical Parameters of Free-ranging Female Nile Monitors in Egypt. J. Wildl. Dis. 2013, 49, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.; Frye, F.L.; Stockham, L.; Fredeking, T. Blood Values in Wild and Captive Komodo Dragons (Varanus komodoensis). Zoo Biol. 2000, 19, 495–509. [Google Scholar] [CrossRef]
- Omonona, A.; Kareem, Y.O.; Odeniran, P.; Oluwafemi Ademola, I. Parasites and blood profile of Nile Monitor Lizards (Varanus niloticus) in Ibadan, Nigeria. Niger. J. Parasitol. 2019, 40, 303–308. [Google Scholar] [CrossRef]







| Minerals | Content [µg/g Fat] |
|---|---|
| Ca | 1732 ± 187 |
| P | 1359 ± 59 |
| K | 201.6 ± 12 |
| Na | 53.37 ± 2.13 |
| Mg | 27.08 ± 3.02 |
| Fe | 9.52 ± 0.42 |
| Zn | 3.74 ± 0.27 |
| Cu | 0.105 ± 0.438 |
| Cd | 0.034 ± 0.0067 |
| Hg | 0.0012 ± 0.00004 |
| Pb | <0.005 |
| Area [g/100 g] % | Cn:n | Fatty Acid |
|---|---|---|
| 0.05902 | C6:0 | Caproic acid |
| 0.09023 | C18:3 n6 | Gamma-linolenic acid (GLA) |
| 0.10943 | C8:0 | Caprylic acid |
| 0.14130 | C20:5 | Eicosapentaenoic acid (EPA) |
| 0.16512 | C17:1 | Heptadecenoic acid |
| 0.17593 | C18:3 n3 | Alpha-linolenic acid (ALA) |
| 0.19400 | C20:3 n3 | Eicosatrienoic acid (n-3) |
| 0.19803 | C20:2 | Eicosadienoic acid |
| 0.21951 | C20:0 | Arachidic acid |
| 0.26156 | C22:1 | Erucic acid |
| 0.29719 | C15:0 | Pentadecanoic acid |
| 0.46382 | C:17:0 | Margaric acid |
| 0.76226 | C20:1 | Eicosenoic acid |
| 1.40764 | C14:0 | Myristic acid |
| 4.42079 | C16:1 | Palmitoleic acid |
| 4.53312 | C18:1trans | Oleic acid (trans) |
| 6.40996 | C4:0 | Butyric acid |
| 8.76253 | C18:0 | Stearic acid |
| 9.77179 | C18:2 | Linoleic acid |
| 26.61068 | C16:0 | Palmitic acid |
| 34.94609 | C18:1cis | Oleic acid (cis) |
| Composition of Adipose Tissue in Animals | Komodo Dragon (V. komodoensis), Tail Fat | Nile Crocodile (Crocodylus niloticus), Tail Fat | Bactrian Camel (Camelus bacterianus), Camel Hump | Bactrian Camel (Camelus bacterianus), Tail Fat |
|---|---|---|---|---|
| Predominant fatty acids | C16:0 (26.61%) C18:1cis (34.94%) | C16:0 (28.7%) C18:1c9 (30.8%) | C16:0 (18.76%) C18:0 (23.23%) C18:1 n-9 cis (34.91%) | C16:0 (17.98%) C18:0 (17.18%) C18:1 n-9 cis (39.96%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomańska, A.; Stawinoga, M.; Szturo, K.; Styczyńska, M.; Klećkowska-Nawrot, J.; Janeczek, M.; Goździewska-Harłajczuk, K.; Melnyk, O.; Gębarowski, T. Biological Significance of the Komodo Dragon’s Tail (Varanus komodoensis, Varanidae). Animals 2024, 14, 2142. https://doi.org/10.3390/ani14152142
Tomańska A, Stawinoga M, Szturo K, Styczyńska M, Klećkowska-Nawrot J, Janeczek M, Goździewska-Harłajczuk K, Melnyk O, Gębarowski T. Biological Significance of the Komodo Dragon’s Tail (Varanus komodoensis, Varanidae). Animals. 2024; 14(15):2142. https://doi.org/10.3390/ani14152142
Chicago/Turabian StyleTomańska, Anna, Martyna Stawinoga, Kacper Szturo, Marzena Styczyńska, Joanna Klećkowska-Nawrot, Maciej Janeczek, Karolina Goździewska-Harłajczuk, Oleksii Melnyk, and Tomasz Gębarowski. 2024. "Biological Significance of the Komodo Dragon’s Tail (Varanus komodoensis, Varanidae)" Animals 14, no. 15: 2142. https://doi.org/10.3390/ani14152142










