Differences in Milk Fatty Acids Profile of Two Breeds of Water Buffaloes Explained by Their Gastrointestinal Microbiota
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet and Animal Management
2.2. Measurement of Dry Matter Consumption, Milk Production, Milk Constituents, and Milk Fatty Acids Profile
2.3. Assessment of Rumen Fermentation Parameters, along with Fatty Acids Profiling
2.4. Feces Collection
2.5. 16S rDNA Gene Sequencing and Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
3.1. Milk Yield, Milk Composition, and Milk Fatty Acids
3.2. Rumen Fermentation Characteristics, and Ruminal Fatty Acids
3.3. Ruminal Bacterial Communities
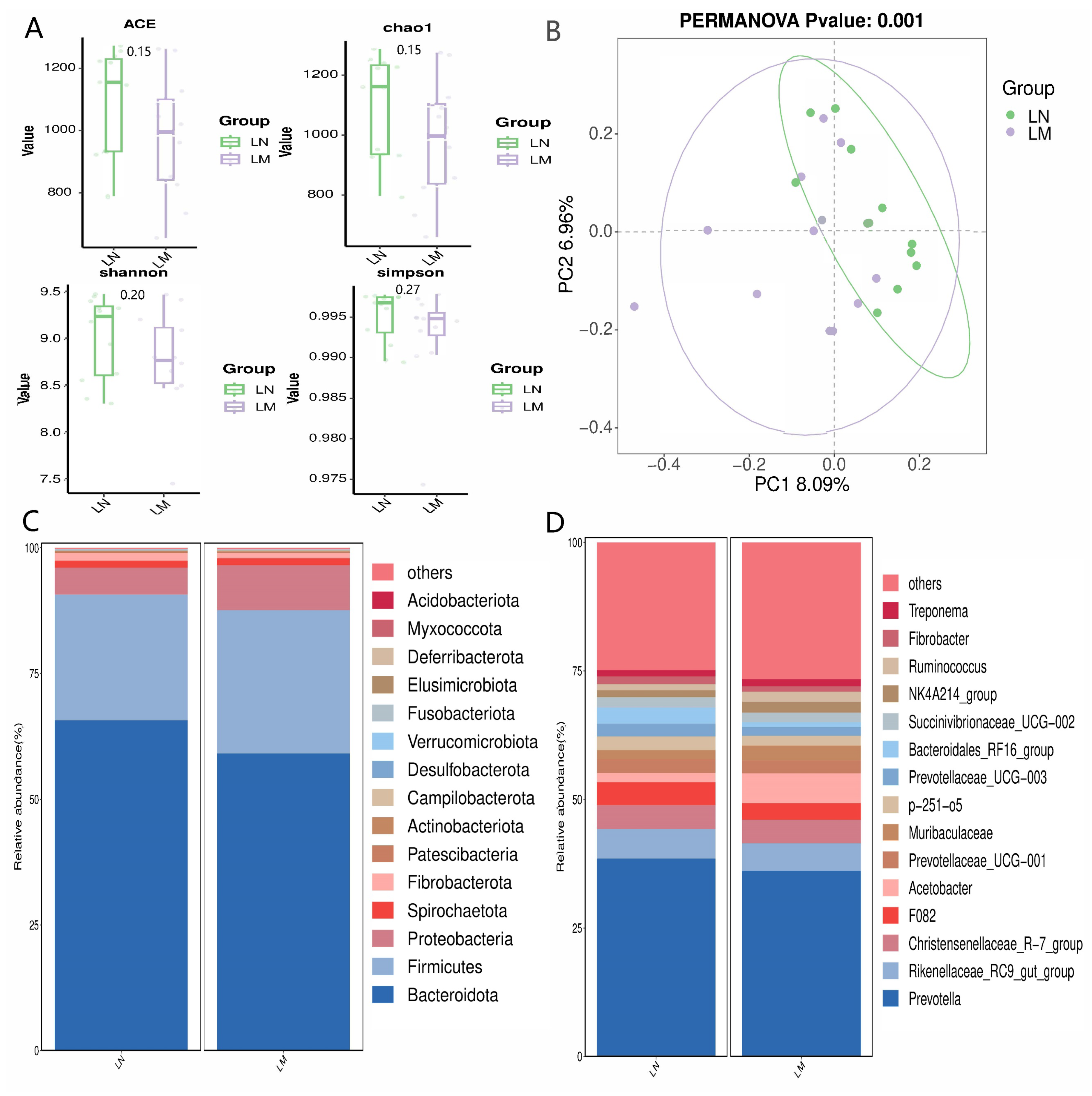
3.3.1. Alpha and Beta Diversity Assessments, along with the Proportional Abundance of Ruminal Bacterial Communities
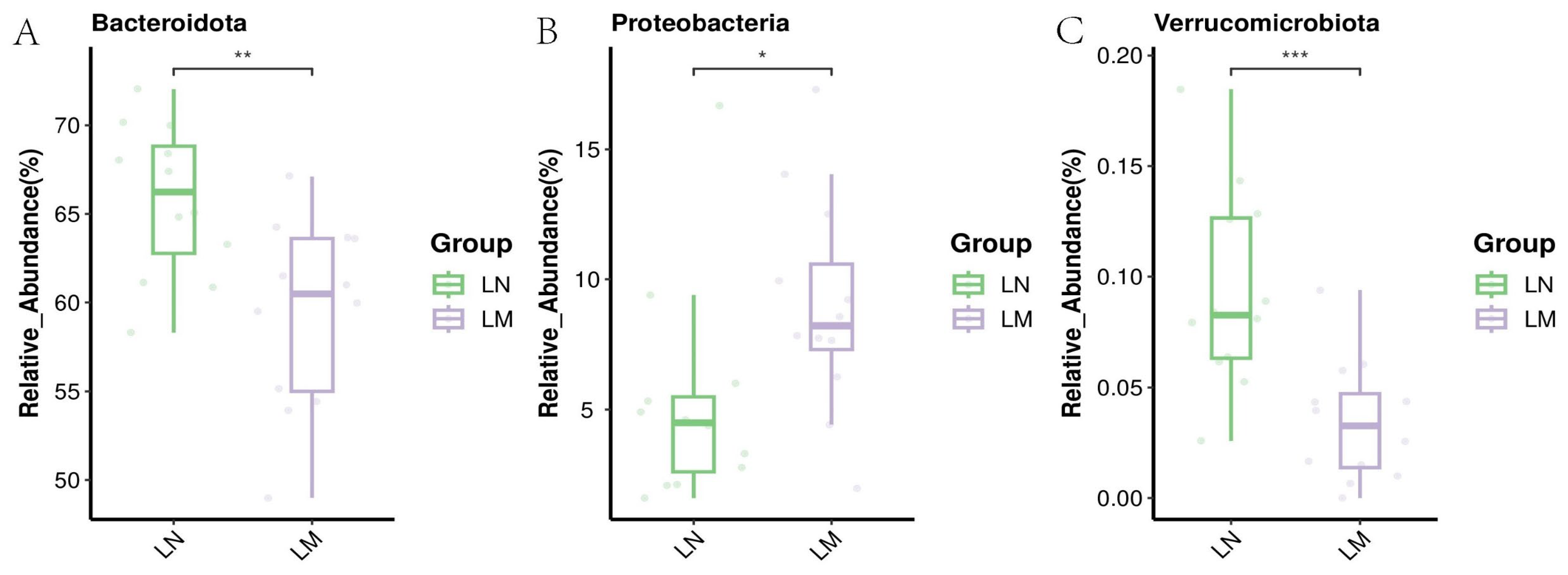
3.3.2. Biomarker Bacteria Taxa
3.3.3. Association of Rumen Bacteria with Ruminal Fermentation Parameters and Milk Fatty Acid Contents
3.4. Fecal Bacterial Communities
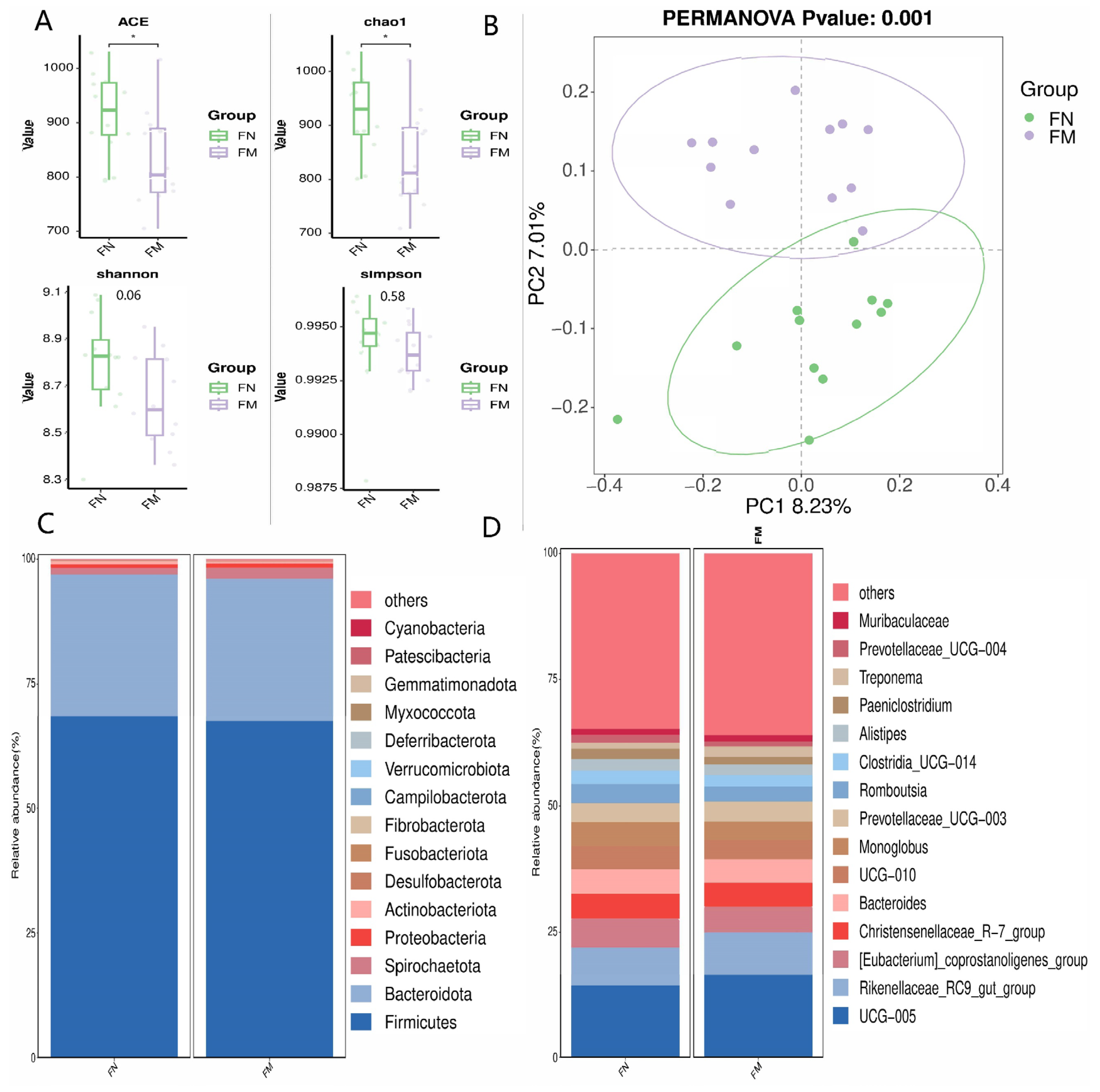
3.4.1. Alpha and Beta Diversity Analysis, and Relative Abundance of Fecal Bacterial Populations
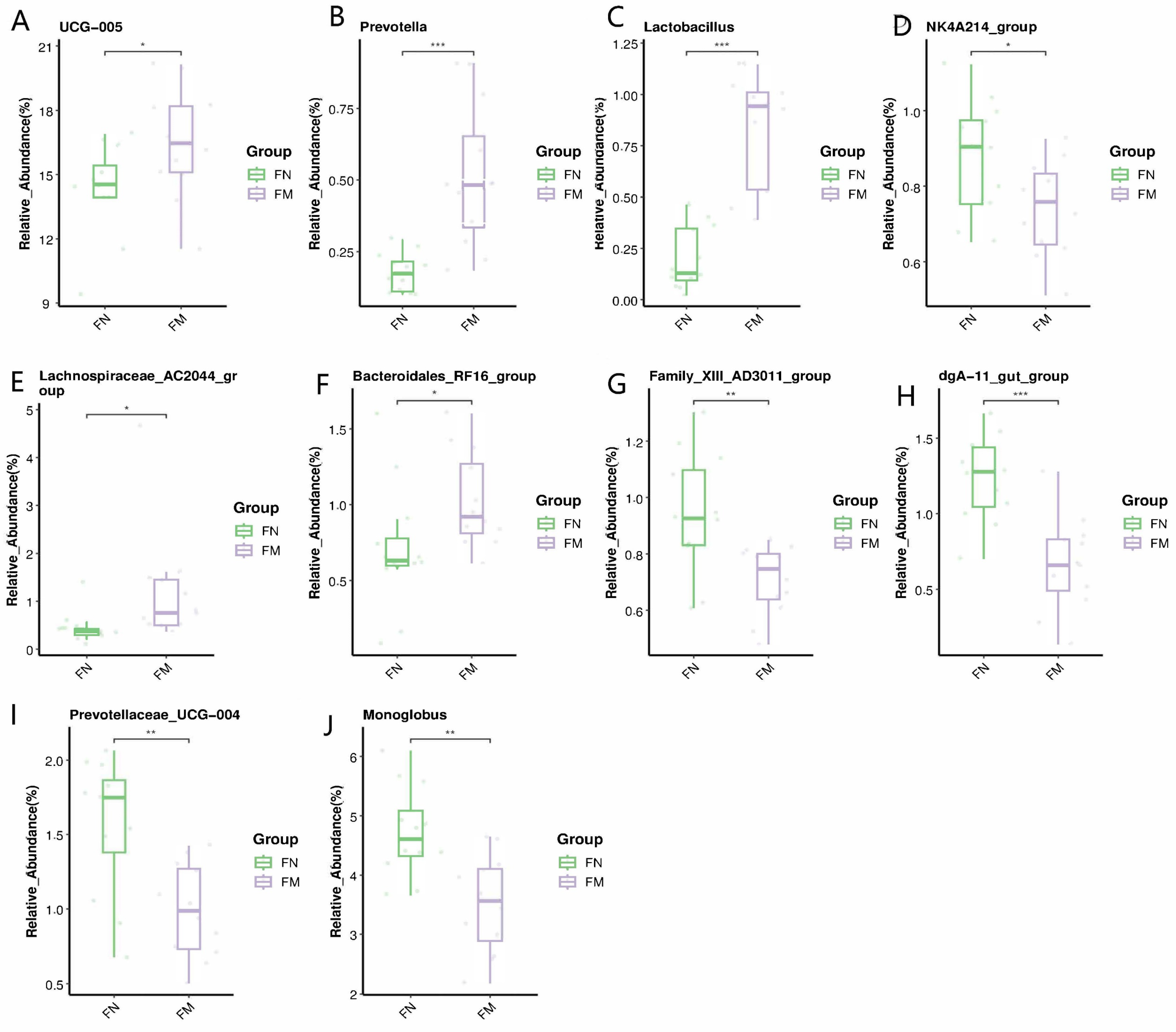
3.4.2. Biomarker Bacteria Taxa
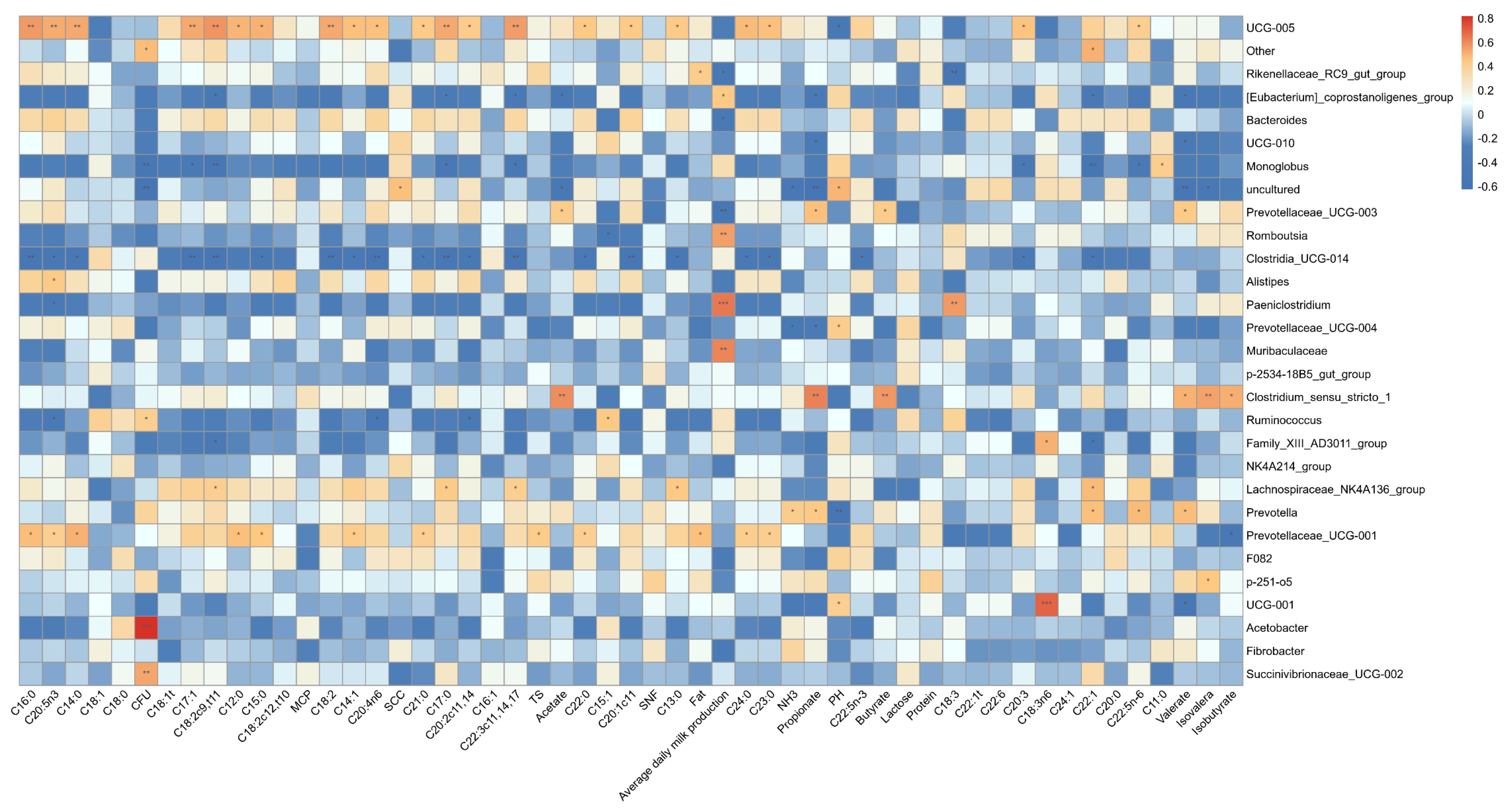
3.4.3. Correlation of Fecal Microbiota with Fermentation Indices and Milk Fatty Acids Composition
4. Discussion
4.1. Differences in the Milk Composition of Different Breeds
4.2. Differences in Gastrointestinal Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasr, M.; Awad, A.; El Araby, I.E. Associations of Leptin and Pituitary-Specific Transcription Factor Genes’ Polymorphisms with Reproduction and Production Traits in Dairy Buffalo. Reprod. Domest. Anim. Zuchthyg. 2016, 51, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Neglia, G.; Casale, R.; Cautela, D.; Marrelli, M.; Limone, A.; Campanile, G.; Balestrieri, M.L. Carnitine Precursors and Short-Chain Acylcarnitines in Water Buffalo Milk. J. Agric. Food Chem. 2018, 66, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- FAO. Available online: https://www.fao.org/faostat/en/#home (accessed on 1 July 2023).
- Jiang, M.; Meng, Z.; Cheng, Z.; Zhan, K.; Ma, X.; Yang, T.; Huang, Y.; Yan, Q.; Gong, X.; Zhao, G. Effects of Buffalo Milk and Cow Milk on Lipid Metabolism in Obese Mice Induced by High Fat. Front. Nutr. 2022, 9, 841800. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Milk exosome-derived miRNAs from water buffalo are implicated in immune response and metabolism process. BMC Vet. Res. 2020, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Deng, T.; Zhou, Y.; Ye, T.; Zhou, Z.; Zhang, S.; Shao, B.; Wei, P.; Sun, H.; Khan, F.A.; et al. Systematic analyses for candidate genes of milk production traits in water buffalo (Bubalus bubalis). Anim. Genet. 2019, 50, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Basilicata, M.G.; Pepe, G.; Sommella, E.; Ostacolo, C.; Manfra, M.; Sosto, G.; Pagano, G.; Novellino, E.; Campiglia, P. Peptidome profiles and bioactivity elucidation of buffalo-milk dairy products after gastrointestinal digestion. Food Res. Int. 2018, 105, 1003–1010. [Google Scholar] [CrossRef]
- Zicarelli, L. Buffalo milk: Its properties, dairy yield and mozzarella production. Vet. Res. Commun. 2004, 28 (Suppl. S1), 127–135. [Google Scholar] [CrossRef]
- Murtaza, M.A.; Pandya, A.J.; Khan, M.M.H. Buffalo Milk Utilization for Dairy Products: Handbook of Milk of Non–Bovine Mammals; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.L.; Grimm, N.K.; Pariza, M.W. Anticarcinogens from fried ground beef: Heat-altered derivatives of linoleic acid. Carcinogenesis 1987, 8, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Zhong, W.J.; Zheng, C.T.; Lin, Y.C.; Yang, L.; Jiang, S.Q. Conjugated linoleic acid differentially regulates fat deposition in backfat and longissimus muscle of finishing pigs. J. Anim. Sci. 2010, 88, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Pariza, M.W.; Hargraves, W.A. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis 1985, 6, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Tabashsum, Z.; Patel, P.; Bernhardt, C.; Biswas, D. Linoleic Acids Overproducing Lactobacillus casei Limits Growth, Survival, and Virulence of Salmonella Typhimurium and Enterohaemorrhagic Escherichia coli. Front. Microbiol. 2018, 9, 2663. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xu, H.; Xia, J.; Lu, Y.; Xu, D.; Sun, J.; Wang, Y.; Liao, W.; Sun, G. Effect of Alpha-Linolenic Acid Supplementation on Cardiovascular Disease Risk Profile in Individuals with Obesity or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Huang, W.; Guo, Y.; Ma, X.; Yang, J. α-Linolenic Acid Suppresses Proliferation and Invasion in Osteosarcoma Cells via Inhibiting Fatty Acid Synthase. Molecules 2022, 27, 2741. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020, 11, 110. [Google Scholar] [CrossRef]
- Stergiadis, S.; Cabeza-Luna, I.; Mora-Ortiz, M.; Stewart, R.D.; Dewhurst, R.J.; Humphries, D.J.; Watson, M.; Roehe, R.; Auffret, M.D. Unravelling the Role of Rumen Microbial Communities, Genes, and Activities on Milk Fatty Acid Profile Using a Combination of Omics Approaches. Front. Microbiol. 2020, 11, 590441. [Google Scholar] [CrossRef]
- Bainbridge, M.L.; Saldinger, L.K.; Barlow, J.W.; Alvez, J.P.; Roman, J.; Kraft, J. Alteration of Rumen Bacteria and Protozoa Through Grazing Regime as a Tool to Enhance the Bioactive Fatty Acid Content of Bovine Milk. Front. Microbiol. 2018, 9, 904. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Alimon, A.R.; Yaakub, H.; Samsudin, A.A.; Candyrine, S.C.L.; Wan Mohamed, W.N.; Noh, A.M.; Fuat, M.A.; Mookiah, S. Effects of vegetable oil supplementation on rumen fermentation and microbial population in ruminant: A review. Trop. Anim. Health Prod. 2021, 53, 422. [Google Scholar] [CrossRef]
- Kupczyński, R.; Pacyga, K.; Lewandowska, K.; Bednarski, M.; Szumny, A. Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Fermentation. Animals 2024, 14, 1706. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, B.; Fievez, V.; Tamminga, S.; Dewhurst, R.J.; van Vuuren, A.; De Brabander, D.; Demeyer, D. Milk odd- and branched-chain fatty acids in relation to the rumen fermentation pattern. J. Dairy Sci. 2006, 89, 3954–3964. [Google Scholar] [CrossRef]
- Salzano, A.; Neglia, G.; D’Onofrio, N.; Balestrieri, M.L.; Limone, A.; Cotticelli, A.; Marrone, R.; Anastasio, A.; D’Occhio, M.J.; Campanile, G. Green feed increases antioxidant and antineoplastic activity of buffalo milk: A globally significant livestock. Food Chem. 2021, 344, 128669. [Google Scholar] [CrossRef]
- Serrapica, F.; Masucci, F.; Romano, R.; Santini, A.; Manzo, N.; Seidavi, A.; Omri, B.; Salem, A.Z.M.; Di Francia, A. Peas may be a candidate crop for integrating silvoarable systems and dairy buffalo farming in southern Italy. Agrofor. Syst. 2020, 94, 1345–1352. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Pang, K.; Zeng, Q.; Zhang, X.; Ren, F.; Guo, H. Changes in milk yield and composition of colostrum and regular milk from four buffalo breeds in China during lactation. J. Sci. Food Agric. 2019, 99, 5799–5807. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975. [Google Scholar]
- Van Soest, P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Zhang, Y.; Wang, S.; Shao, Y.; Xiong, X.; Hu, X.; Yu, R.; Lan, W.; Cui, Y.; et al. Peppermint extract improves egg production and quality, increases antioxidant capacity, and alters cecal microbiota in late-phase laying hens. Front. Microbiol. 2023, 14, 1252785. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Callaway, T.R.; Lourenco, J.M.; Ryman, V.E. Characterization of rumen, fecal, and milk microbiota in lactating dairy cows. Front. Microbiol. 2022, 13, 984119. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hvelplund, T.; Weisbjerg, M.R. The use of tannins as silage additives: Effects on silage composition and mobile bag disappearance of dry matter and protein. Anim. Feed Sci. Technol. 1999, 82, 243–259. [Google Scholar] [CrossRef]
- Makkar, H.P.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fan, Z.; Li, M.; Xie, H.; Peng, L.; Yang, C. Effects of Sodium Nitrate and Coated Methionine on Lactation Performance, Rumen Fermentation Characteristics, Amino Acid Metabolism, and Microbial Communities in Lactating Buffaloes. Microorganisms 2023, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, J.M.; Kieran, T.J.; Seidel, D.S.; Glenn, T.C.; Silveira, M.F.D.; Callaway, T.R.; Stewart, R.L. Comparison of the ruminal and fecal microbiotas in beef calves supplemented or not with concentrate. PLoS ONE 2020, 15, e0231533. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yuan, Y.; Ma, Y.; Luo, Y.; Zhou, W.; Deng, X.; Pu, J.; Hu, B.; Liu, S. Combined Intestinal Metabolomics and Microbiota Analysis for Acute Endometritis Induced by Lipopolysaccharide in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 791373. [Google Scholar] [CrossRef] [PubMed]
- Gantner, V.; Mijic, P.; Baban, M.; Škrtić, Z.; Turalija, A. The overall and fat composition of milk of various species. Mljekarstvo/Dairy 2015, 65, 223–231. [Google Scholar] [CrossRef]
- Lopez, C.; Briard-Bion, V.; Ménard, O. Polar lipids, sphingomyelin and long-chain unsaturated fatty acids from the milk fat globule membrane are increased in milks produced by cows fed fresh pasture based diet during spring. Food Res. Int. 2014, 58, 59–68. [Google Scholar] [CrossRef]
- Agustinho, B.C.; Zeoula, L.M.; Santos, N.W.; Machado, E.; Yoshimura, E.H.; Ribas, J.C.R.; Bragatto, J.M.; Stemposki, M.R.; dos Santos, V.J.; Faciola, A.P. Effects of Flaxseed Oil and Vitamin E Supplementation on Digestibility and Milk Fatty Composition and Antioxidant Capacity in Water Buffaloes. Animals 2020, 10, 1294. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A. Dietary lipids and forages interactions on cow and goat milk fatty acid composition and sensory properties. Reprod. Nutr. Dev. 2004, 44, 467–492. [Google Scholar] [CrossRef]
- Madilindi, M.A.; Banga, C.B.; Zishiri, O.T. Prediction of dry matter intake and gross feed efficiency using milk production and live weight in first-parity Holstein cows. Trop. Anim. Health Prod. 2022, 54, 278. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Ma, X.; Li, S.; Wang, W. Producing natural functional and low-carbon milk by regulating the diet of the cattle-The fatty acid associated rumen fermentation, biohydrogenation, and microorganism response. Front. Nutr. 2022, 9, 955846. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Huang, L.; Huang, Z.; Romeih, E.; Yang, P.; Zeng, Q.; Li, L. Effect of Buffalo Breed on the Detailed Milk Composition in Guangxi, China. Foods 2023, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lv, J.-P.; Liu, L.; Zhang, S.-W.; Liang, X.; Lu, J. Comparison of milk samples collected from some buffalo breeds and crossbreeds in China. Dairy Sci. Technol. 2014, 94, 387–395. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Temme, E.H.; Hornstra, G. Dietary saturated and trans fatty acids and lipoprotein metabolism. Ann. Med. 1994, 26, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Palmonari, A.; Stevenson, D.M.; Mertens, D.R.; Cruywagen, C.W.; Weimer, P.J. pH dynamics and bacterial community composition in the rumen of lactating dairy cows. J. Dairy Sci. 2010, 93, 279–287. [Google Scholar] [CrossRef]
- Russell, J.B. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J. Dairy Sci. 1998, 81, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Abeni, F.; Calamari, L.; Stefanini, L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int. J. Biometeorol. 2007, 52, 87–96. [Google Scholar] [CrossRef]
- Lin, X.; Hu, Z.; Zhang, S.; Cheng, G.; Hou, Q.; Wang, Y.; Yan, Z.; Shi, K.; Wang, Z. A Study on the Mechanism Regulating Acetate to Propionate Ratio in Rumen Fermentation by Dietary Carbohydrate Type. Adv. Biosci. Biotechnol. 2020, 11, 369–390. [Google Scholar] [CrossRef]
- Jarvis, G.N.; Moore, E.R.B. Lipid Metabolism and the Rumen Microbial Ecosystem. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2245–2257. [Google Scholar]
- de Oliveira, M.N.; Jewell, K.A.; Freitas, F.S.; Benjamin, L.A.; Tótola, M.R.; Borges, A.C.; Moraes, C.A.; Suen, G. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 2013, 164, 307–314. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.Z.; Han, J.L.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- Gharechahi, J.; Sarikhan, S.; Han, J.L.; Ding, X.Z.; Salekdeh, G.H. Functional and phylogenetic analyses of camel rumen microbiota associated with different lignocellulosic substrates. NPJ Biofilms Microbiomes 2022, 8, 46. [Google Scholar] [CrossRef]
- Pitta, D.W.; Pinchak, W.E.; Indugu, N.; Vecchiarelli, B.; Sinha, R.; Fulford, J.D. Metagenomic Analysis of the Rumen Microbiome of Steers with Wheat-Induced Frothy Bloat. Front. Microbiol. 2016, 7, 689. [Google Scholar] [CrossRef]
- Tong, F.; Wang, T.; Gao, N.L.; Liu, Z.; Cui, K.; Duan, Y.; Wu, S.; Luo, Y.; Li, Z.; Yang, C.; et al. The microbiome of the buffalo digestive tract. Nat. Commun. 2022, 13, 823. [Google Scholar] [CrossRef]
- Wanapat, M. Rumen Manipulation to Increase the Efficient Use of Local Feed Resources and Productivity of Ruminants in the Tropics. Asian-Aust. J. Anim. Sci. 2000, 13, 59–67. [Google Scholar]
- Falalyeyeva, T.; Chornenka, N.; Cherkasova, L.; Tsyryuk, O.; Molchek, N.; Kovalchuk, O.; Kyriachenko, Y.; Ostapchenko, L.; Kobyliak, N. Gut Microbiota Interactions with Obesity. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 201–219. [Google Scholar]
- Downes, J.; Sutcliffe, I.C.; Booth, V.; Wade, W.G. Prevotella maculosa sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 2007, 57, 2936–2939. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]
- Vastolo, A.; Matera, R.; Serrapica, F.; Cutrignelli, M.I.; Neglia, G.; Kiatti, D.D.; Calabrò, S. Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow. Fermentation 2022, 8, 351. [Google Scholar] [CrossRef]
- Wang, B.; Ma, M.P.; Diao, Q.Y.; Tu, Y. Saponin-Induced Shifts in the Rumen Microbiome and Metabolome of Young Cattle. Front. Microbiol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Betancur-Murillo, C.L.; Aguilar-Marín, S.B.; Jovel, J. Prevotella: A Key Player in Ruminal Metabolism. Microorganisms 2022, 11, 1. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.F.; Dai, T.Y.; Qi, X.; Wang, Y.; Zheng, T.Z.; Gao, X.; Zhang, Y.; Ai, Y.; Ma, L.; et al. Short-Chain Fatty Acids Produced by Ruminococcaceae Mediate α-Linolenic Acid Promote Intestinal Stem Cells Proliferation. Mol. Nutr. Food Res. 2022, 66, e2100408. [Google Scholar] [CrossRef]
- Hazlewood, G.; Dawson, R.M. Characteristics of a lipolytic and fatty acid-requiring Butyrivibrio sp. isolated from the ovine rumen. J. Gen. Microbiol. 1979, 112, 15–27. [Google Scholar] [CrossRef]
- Storry, J.E.; Rook, J.A. Effect in the cow of intraruminal infusions of volatile fatty acids and of lactic acid on the secretion of the component fatty acids of the milk fat and on the composition of blood. Biochem. J. 1965, 96, 210–217. [Google Scholar] [CrossRef]
- Lyons, T.; Bielak, A.; Doyle, E.; Kuhla, B. Variations in methane yield and microbial community profiles in the rumen of dairy cows as they pass through stages of first lactation. J. Dairy Sci. 2018, 101, 5102–5114. [Google Scholar] [CrossRef]
- Arif, M.; Sarwar, M.; Mehr Un, N.; Hayat, Z.; Muhammad, Y. Effect of Supplementary sodium nitrate and sulphur on methane production and growth rates in sheep and goats fed forage based diet low in true protein. J. Anim. Plant Sci. 2016, 26, 69–78. [Google Scholar]
- Cui, L.; Zhang, X.; Cheng, R.; Ansari, A.R.; Elokil, A.A.; Hu, Y.; Chen, Y.; Nafady, A.A.; Liu, H. Sex differences in growth performance are related to cecal microbiota in chicken. Microb. Pathog. 2021, 150, 104710. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, G.; Men, H.; Wang, W.; Li, S. The Response of Fecal Microbiota and Host Metabolome in Dairy Cows Following Rumen Fluid Transplantation. Front. Microbiol. 2022, 13, 940158. [Google Scholar] [CrossRef]




| Ingredients | Dry Matter, % |
|---|---|
| Corn | 8.95 |
| Soybean meal | 2.88 |
| Beer residue | 26.56 |
| White wine lees | 7.50 |
| Pineapple peel | 13.96 |
| Elephant grass silage | 18.75 |
| Sodium cyclamate | 5.00 |
| Peanut vine | 9.00 |
| Stevia | 2.50 |
| Wheat bran | 3.46 |
| NaCl | 0.23 |
| NaHCO3 | 0.40 |
| CaHPO4 | 0.25 |
| CaCO3 | 0.30 |
| Additive 1 | 0.07 |
| Premix 2 | 0.19 |
| Chemical components, % | |
| Dry matter (DM) | 35.86 |
| Crude protein (CP) | 18.21 |
| Ether extract (EE) | 3.97 |
| Neutral detergent fiber (NDF) | 38.51 |
| Acid detergent fiber (ADF) | 22.05 |
| Crude ash (ASH) | 8.24 |
| Breed | Average Dry Matter Intake (kg/d) | Milk Yield (kg/d) | Milk Protein (%) | Milk Fat (%) | Lactose (%) | Total Solid (TS, %) | Solids Non-Fat (SNF, %) |
|---|---|---|---|---|---|---|---|
| Nili-Ravi buffalo | 10.07 ± 0.37 | 8.60 ± 1.29 | 4.45 ± 0.46 | 8.26 ± 1.23 | 5.22 ± 0.15 | 19.12 ± 1.73 | 10.00 ± 0.51 |
| Murrah buffalo | 10.26 ± 0.29 | 7.88 ± 1.40 | 4.69 ± 0.33 | 9.13 ± 1.09 | 5.12 ± 0.28 | 20.27 ± 1.52 | 10.23 ± 0.50 |
| p-value | 0.607 | 0.272 | 0.165 | 0.091 | 0.336 | 0.112 | 0.314 |
| Fatty Acid | Nili-Ravi Buffalo | Murrah Buffalo | p-Value |
|---|---|---|---|
| C11:0 | 1.27 ± 0.22 | 0.99 ± 0.26 | 0.032 |
| C12:0 | 217.97 ± 126.77 | 334.11 ± 152.71 | 0.065 |
| C13:0 | 8.77 ± 5.37 | 12.13 ± 6.72 | 0.209 |
| C14:1 | 86.79 ± 53.89 | 121.57 ± 68.52 | 0.199 |
| C14:0 | 1679.54 ± 785.02 | 1975.84 ± 841.19 | 0.402 |
| C15:1 | 10.62 ± 5.95 | 13.61 ± 9.32 | 0.427 |
| C15:0 | 206.13 ± 115.63 | 229.97 ± 117.18 | 0.636 |
| C16:1 | 25.16 ± 22.86 | 23.18 ± 20.62 | 0.858 |
| C16:0 | 3577.65 ± 1106.44 | 3589.67 ± 1086.30 | 0.980 |
| C17:1 | 285.85 ± 152.71 | 376.87 ± 240.38 | 0.301 |
| C17:0 | 33.75 ± 18.06 | 49.56 ± 38.85 | 0.234 |
| C18:3n6 | 2.36 ± 1.08 | 2.37 ± 0.81 | 0.981 |
| C18:2 | 113.92 ± 65.01 | 136.07 ± 81.09 | 0.487 |
| C18:3 | 2.50 ± 0.90 | 4.07 ± 1.82 | 0.044 |
| C18:2c9t11 | 266.25 ± 110.75 | 438.66 ± 212.54 | 0.037 |
| C18:1 | 1572.06 ± 620.68 | 955.94 ± 352.19 | 0.038 |
| C18:2c12t10 | 231.28 ± 150.47 | 219.87 ± 175.58 | 0.872 |
| C18:1t | 433.39 ± 278.39 | 585.40 ± 333.98 | 0.259 |
| C18:0 | 1171.43 ± 196.01 | 899.13 ± 158.18 | 0.027 |
| C20:4n6 | 72.02 ± 33.91 | 81.09 ± 43.99 | 0.617 |
| C20:5n3 | 1975.78 ± 765.05 | 1934.19 ± 675.14 | 0.894 |
| C22:3c11,14,17 | 18.26 ± 6.34 | 25.75 ± 10.12 | 0.124 |
| C20:3 | 2.34 ± 1.55 | 3.90 ± 2.76 | 0.117 |
| C20:1c11 | 13.57 ± 8.74 | 14.14 ± 7.64 | 0.873 |
| C20:2c11,14 | 29.85 ± 25.49 | 30.79 ± 19.7 | 0.924 |
| C20:0 | 2.45 ± 1.66 | 2.35 ± 1.23 | 0.871 |
| C21:0 | 64.65 ± 42.81 | 65.90 ± 37.56 | 0.943 |
| C22:6 | 4.73 ± 8.76 | 1.96 ± 1.40 | 0.313 |
| C22:5n-3 | 5.82 ± 4.12 | 6.00 ± 3.54 | 0.911 |
| C22:5n-6 | 1.16 ± 0.56 | 2.05 ± 1.27 | 0.045 |
| C22:1 | 1.80 ± 0.95 | 3.39 ± 3.34 | 0.144 |
| C22:1t | 5.24 ± 11.64 | 1.85 ± 0.89 | 0.347 |
| C22:0 | 14.86 ± 11.24 | 16.22 ± 9.96 | 0.768 |
| C23:0 | 7.86 ± 5.83 | 9.44 ± 6.00 | 0.536 |
| C24:1 | 1.20 ± 0.47 | 1.27 ± 0.44 | 0.733 |
| C24:0 | 8.00 ± 6.07 | 10.05 ± 6.56 | 0.457 |
| Saturated fatty acid (SFA) | 6374.57 ± 2309.23 | 6770.82 ± 2541.65 | 0.706 |
| Unsaturated fatty acid (UFA) | 5731.45 ± 2208.76 | 5329.84 ± 1373.62 | 0.614 |
| Monounsaturated fatty acid (MUFA) | 2298.65 ± 842.15 | 1688.92 ± 768.98 | 0.090 |
| Polyunsaturated fatty acid (PUFA) | 3432.80 ± 1659.16 | 3640.92 ± 960.35 | 0.722 |
| SFA/UFA | 1.20 ± 0.39 | 1.27 ± 0.35 | 0.623 |
| Total fatty acid | 12,106.02 ± 3985.09 | 12,100.66 ± 3635.53 | 0.997 |
| Items | Nili-Ravi Buffalo | Murrah Buffalo | p-Value |
|---|---|---|---|
| pH | 6.79 ± 0.18 | 6.41 ± 0.3 | 0.001 |
| Ammonia nitrogen (NH3-N, mg/100 mL) | 6.02 ± 1.85 | 9.34 ± 3.67 | 0.013 |
| Microbial protein (MCP, mg/mL) | 0.12 ± 0.03 | 0.13 ± 0.02 | 0.637 |
| Acetate (mmol/L) | 13.96 ± 1.89 | 16.38 ± 2.74 | 0.027 |
| Propionate (mmol/L) | 6.63 ± 1.07 | 8.58 ± 2.09 | 0.011 |
| Isobutyrate (mmol/L) | 0.15 ± 0.05 | 0.16 ± 0.06 | 0.523 |
| Butyrate (mmol/L) | 4.91 ± 0.87 | 5.61 ± 1.34 | 0.158 |
| Isovalera (mmol/L) | 0.29 ± 0.08 | 0.38 ± 0.12 | 0.049 |
| Valerate (mmol/L) | 0.60 ± 0.13 | 0.73 ± 0.15 | 0.033 |
| Total volatile fatty acid (TVFA, mmol/L) | 26.55 ± 3.92 | 31.31 ± 6.60 | 0.051 |
| Acetate/Propionate | 2.11 ± 0.12 | 1.88 ± 0.21 | 0.003 |
| Ruminal Fatty Acid | Nili-Ravi | Murrah | p-Value |
|---|---|---|---|
| C11:0 | 0.12 ± 0.09 | 0.14 ± 0.09 | 0.646 |
| C12:0 | 2.05 ± 0.61 | 1.39 ± 0.51 | 0.024 |
| C13:0 | 0.22 ± 0.09 | 0.15 ± 0.09 | 0.060 |
| C14:0 | 2.81 ± 0.71 | 3.12 ± 0.96 | 0.425 |
| C15:1 | 0.58 ± 0.46 | 0.25 ± 0.14 | 0.164 |
| C15:0 | 2.16 ± 0.69 | 3.52 ± 0.92 | 0.005 |
| C16:1 | 1.03 ± 0.17 | 0.91 ± 0.28 | 0.275 |
| C16:0 | 100.94 ± 3.42 | 100.24 ± 17.50 | 0.927 |
| C17:1 | 0.43 ± 0.06 | 0.33 ± 0.06 | 0.001 |
| C17: 0 | 0.52 ± 0.21 | 0.83 ± 0.19 | 0.006 |
| C18:3n6 | 0.53 ± 0.21 | 0.65 ± 0.32 | 0.213 |
| C18:2 | 0.46 ± 0.20 | 1.33 ± 0.39 | 0.001 |
| C18:3 | 2.12 ± 0.70 | 1.01 ± 0.55 | 0.008 |
| C18:2c9,t11 | 6.41 ± 0.98 | 9.00 ± 2.77 | 0.034 |
| C18:1 | 7.02 ± 2.35 | 7.65 ± 1.49 | 0.521 |
| C18:2t10,c12 | 3.07 ± 0.74 | 4.88 ± 1.39 | 0.006 |
| C18:1t | 0.19 ± 0.13 | 17.60 ± 2.48 | 0.001 |
| C18:0 | 6.91 ± 3.71 | 1.85 ± 0.72 | 0.028 |
| C20:4n6 | 0.32 ± 0.17 | 0.65 ± 0.35 | 0.019 |
| C20:5n-3 | 114.09 ± 15.25 | 137.58 ± 33.31 | 0.099 |
| C22:3 | 0.31 ± 0.07 | 0.14 ± 0.05 | 0.001 |
| C20:3 | 0.29 ± 0.12 | 0.17 ± 0.08 | 0.028 |
| C20:1 | 0.21 ± 0.09 | 0.15 ± 0.09 | 0.152 |
| C20:2 | 0.32 ± 0.13 | 0.64 ± 0.31 | 0.013 |
| C20:0 | 0.25 ± 0.08 | 0.16 ± 0.08 | 0.017 |
| C21:0 | 1.35 ± 0.30 | 1.93 ± 0.73 | 0.037 |
| C22:6 | 0.17 ± 0.09 | 0.13 ± 0.08 | 0.362 |
| C22:5n-3 | 0.20 ± 0.11 | 0.23 ± 0.09 | 0.422 |
| C22:5n-6 | 0.19 ± 0.08 | 0.17 ± 0.10 | 0.607 |
| C22:1 | 0.20 ± 0.15 | 0.20 ± 0.08 | 0.875 |
| C22:1t | 0.22 ± 0.12 | 0.17 ± 0.11 | 0.316 |
| C22:0 | 0.19 ± 0.07 | 0.28 ± 0.14 | 0.074 |
| C23:0 | 0.15 ± 0.12 | 0.13 ± 0.07 | 0.651 |
| C24:1 | 0.21 ± 0.12 | 0.16 ± 0.08 | 0.308 |
| C24:0 | 0.22 ± 0.11 | 0.36 ± 0.18 | 0.037 |
| Omega-3 1 | 120.89 ± 39.57 | 163.46 ± 51.90 | 0.041 |
| Omega-6 2 | 1.14 ± 0.48 | 1.88 ± 0.95 | 0.030 |
| Omega-9 3 | 9.82 ± 6.70 | 19.53 ± 8.26 | 0.010 |
| Saturated fatty acid (SFA) | 98.21 ± 28.80 | 120.83 ± 36.21 | 0.119 |
| Unsaturated fatty acid (UFA) | 146.27 ± 47.28 | 200.74 ± 63.65 | 0.033 |
| Monounsaturated fatty acid (MUFA) | 14.63 ± 8.72 | 25.88 ± 12.08 | 0.020 |
| Polyunsaturated fatty acid (PUFA) | 131.63 ± 41.27 | 174.86 ± 54.44 | 0.051 |
| SFA/UFA | 0.68 ± 0.06 | 0.61 ± 0.05 | 0.001 |
| Total fatty acid | 244.48 ± 75.28 | 321.57 ± 99.03 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Guo, Y.; Yang, C.; Song, Z.; Luo, X. Differences in Milk Fatty Acids Profile of Two Breeds of Water Buffaloes Explained by Their Gastrointestinal Microbiota. Animals 2024, 14, 2146. https://doi.org/10.3390/ani14152146
Zhao Y, Guo Y, Yang C, Song Z, Luo X. Differences in Milk Fatty Acids Profile of Two Breeds of Water Buffaloes Explained by Their Gastrointestinal Microbiota. Animals. 2024; 14(15):2146. https://doi.org/10.3390/ani14152146
Chicago/Turabian StyleZhao, Yameng, Yanxia Guo, Chengjian Yang, Ziyi Song, and Xianqing Luo. 2024. "Differences in Milk Fatty Acids Profile of Two Breeds of Water Buffaloes Explained by Their Gastrointestinal Microbiota" Animals 14, no. 15: 2146. https://doi.org/10.3390/ani14152146
APA StyleZhao, Y., Guo, Y., Yang, C., Song, Z., & Luo, X. (2024). Differences in Milk Fatty Acids Profile of Two Breeds of Water Buffaloes Explained by Their Gastrointestinal Microbiota. Animals, 14(15), 2146. https://doi.org/10.3390/ani14152146





