Abstract
Simple Summary
Parasites can be transmitted from animals to humans by consumption of undercooked meat. One of these parasites is Toxoplasma gondii, a unicellular parasite that could cause severe disease in immunocompromised patients and foetal problems during pregnancy. T. gondii can be found in wild boar meat and be a source of infection for humans, particularly hunters and their inner circle of acquaintances. We tested serum samples of 42 free-ranging wild boars from Thuringia in central Germany during the hunting season of 2017/2018 and were able to detect antibodies against T. gondii in 18 of these animals (37.50%). The explicit prevalence of T. gondii specific antibodies points towards a risk of toxoplasmosis for people consuming game meat, particularly when it is not properly cooked. The hunting community as well as authorities should be aware of such possible exposures and infections. Additional studies among free-ranging wild boars would help to obtain more prevalence data from other areas of Germany and to better estimate the actual risk of T. gondii infection among the hunting community and game meat consumers’ interface.
Abstract
Game meat is an important source of meat borne parasitic infections. Due to its omnivorous diet, the wild boar is an important host of zoonotic parasites such as Toxoplasma gondii. T. gondii can cause severe to fatal disease in immunosuppressed patients, as well as congenital disorders in foetus and neonates. Consumption of undercooked infected meat is a main source of T. gondii infection. Information about the risk of toxoplasmosis through game meat is scarce. We collected serum samples from 42 wild boars from the federal state of Thuringia (Germany) between December 2017 and February 2018. Identification of anti-T. gondii IgG antibodies was conducted using a commercial indirect ELISA kit. Seropositivity was confirmed in 18 of the 42 samples (37.50%). From these, the highest seroprevalence was found in adult animals. This study joins another single database from wild boars in Brandenburg. The necessity of a country-wide database regarding T. gondii prevalence in wild boar and other game meat is pivotal for a profound risk analysis with its consequential impact in future mean hygiene policies.
1. Introduction
Meat borne parasites are an often neglected source of zoonotic disease [1]. This disregard can be in part due to the current intensive livestock production methods, which are considered to have significantly reduced the presence of food borne parasites in the meat industry [2]. Intensive animal production sites could limit the access to potential hosts that facilitate the parasite’s completion of its life cycle. Game, conversely, is not submitted to the same anthropogenic stress and the main pressure for the parasite’s survival, including host infection, comes primarily from the ecological variations in the natural environment of its host(s) [3].
Game meat is, therefore, commonly found to be at higher risk of parasite contamination than livestock [4,5]. In Germany, its consumption amongst the general population is low in comparison with livestock meat. However, the intensive consumer, namely hunters, their family and acquaintance circle, consumes game meat around 60 times per year [4,6]. This is in part because it is considered a lean meat and source of proteins and other nutrients, like healthy fatty acids [7]. Game meat includes different species of wild animals, including cervids such as roe deer (Capreolus capreolus), lagomorphs like hares (Lepus spp.), or even omnivores like raccoons (Procyon lotor) [8].
An important omnivore regularly hunted for its meat is the wild boar (Sus scrofa). Wild boars are widely found in Eurasia and north Africa and have been introduced to the Americas and Oceania. They can adapt, not only to different ecosystems but also to the use of anthropogenic resources [9]. Their omnivore diet and adaptability make them a common host for several zoonotic meat borne parasites. Including helminths and their larval forms of importance in meat hygiene and public health such as Trichinella spp. [10], Taenia spp. [11], Echinococcus spp. [12], and Alaria alata [13].
A major zoonotic parasite found in wild boar meat is Toxoplasma gondii, an apicomplexan protozoon with global distribution. Around a quarter of the global human population is seropositive to T. gondii [14], with the highest seroprevalence found in Africa (61.40%) while 29.60% of the population of Europe is reported to be seropositive [14]. T. gondii’s final host are all members of the Felidae family in which the parasite can produce oocysts that are shed in the faeces [15]. Intermediate hosts, namely all warm-blooded vertebrates, can become infected by ingestion of oocysts in water, food or contaminated surfaces. After entering the intermediate, T. gondii will colonize different organs and finally develop into an encysted resting stage called bradyzoite. Bradyzoites can further reach cats and other intermediate hosts (including humans) by predation of infected prey, or by consumption of undercooked meat in the case of humans and domestic carnivores [16]. Toxoplasmosis in humans is of particular importance for immunosuppressed and pregnant patients. In the former, it can be manifested as a neurological disease mainly due to the reactivation of encysted bradyzoites; also in immunocompromised patients, multiple organs can be compromised when the infection is acute [17]. During pregnancy, congenital problems for the foetus or neonate are seldom but can occur in the form of neurological disorders such as hydrocephalous or microcephalus [18].
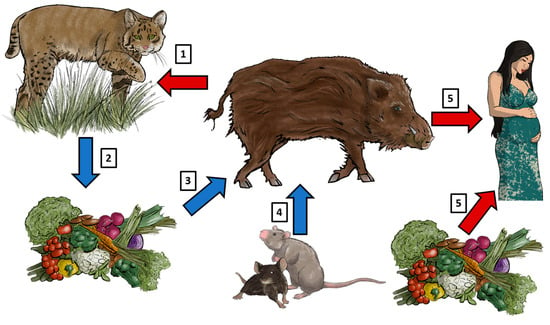
Wild boars can be infected through T. gondii in different routes (Figure 1). They can accidentally ingest sporulated oocysts through contaminated water, soil, or food sources [19,20]. Moreover, intake of bradyzoite cysts can happen by predation or ingestion of infected prey, for example, small rodents. Wild boars can also play a role in keeping the T. gondii cycle by acting as source of infection to the final host (e.g., Felis silvestris) [21] (Figure 1).
Figure 1.
The wild boar as host and source of T. gondii infection. (1) Wild boars can become infected by oral ingestion of sporulated oocysts which are shed by feline species (i.e., wild cats); likewise, predation of infected wild boars can become a source of infection for cats. (2) Vegetables, crops, soil, and water can become contaminated with sporulated T. gondii oocysts. (3) Consequently, wild boars will ingest these sources of food and water and thus become infected with T. gondii. (4) Another route of infection for wild boars is the ingestion of small mammals infected with T. gondii. (5) Finally, human consumption of undercooked meat from T. gondii-infected wild boars can cause congenital toxoplasmosis in pregnant patients; likewise, vegetables contaminated with oocysts can also be a source of infection for humans. Blue arrows: source of T. gondii infection for the wild boar. Red arrows: infected wild boars as source of infection for humans and felines. Illustrations: Aleida Rentería.
In Central Europe, T. gondii seroprevalence in wild boars is reported between 16.80% to 48.00% [22,23,24,25]. With the highest percentage of seropositive animals found in Poland (48.00%) [25]. In Germany, a prevalence of T. gondii antibodies in wild boars has been reported only in animals from the Federal state of Brandenburg [22,26]. The objective of this investigation is to expand the current knowledge by reporting the seroprevalence of T. gondii in free-ranging wild boars from the Federal State of Thuringia, in central Germany.
2. Materials and Methods
2.1. Area of Study and Sample Collection
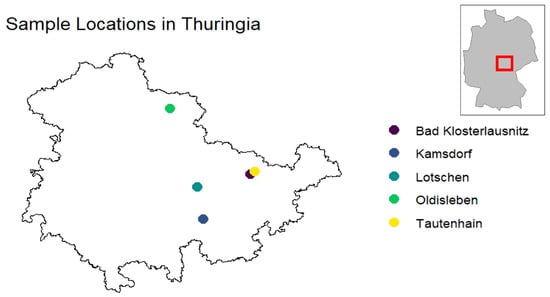
Legally hunted animals were collected in the Federal State of Thuringia, in central Germany, from December 2017 to February 2018. The animals were from 5 municipalities in the eastern (Bad Klosterlausnitz, Lotschen, Tautenhain), southern (Kamsdorf), and the northern part of the state (Oldisleben) (Figure 2). The landscape of these areas consists of mixed forests and rural regions with human recreational activities. Cardiac blood was collected from the animals immediately after they were shot during hunts and stored at 4 °C until further investigation. Centrifugation was performed within 48 h (h) at 1600× g and 4 °C for 10 min. Subsequently, the serum samples were stored at −80 °C.
2.2. Serological Testing
Antibodies against T. gondii were detected in serum using the ID Screen® Toxoplasmosis Indirect Multi-species (ID.Vet Innovative diagnostics, Grabels, France) according to the manufacturer’s instructions. Optical densities (OD450mm) were measured using a microplate reader 800 TS (Biotek Instruments, Winooski, VT, USA). The results were presented as S/P ratios using the following formula provided by the kit’s manufacturer:
S/P % = [(ODsample − ODnegative control)/(ODpositive control − ODnegative control)] × 100
The interpretation of the results was also conducted according to the manufacturer’s guidelines whereby samples with an S/P ratio ≤ 40% were considered negative, samples with an S/P ratio higher than 40% but lower than 50% were registered as doubtful, and samples with an S/P ratio ≥ 50% were positive.
2.3. Statistical Analysis
Data were presented as percentages (%) with their respective confidence intervals (95% CI). Seroprevalence was compared with wild boars’ collection area (municipalities) and age, using the Fisher’s exact tests (α = 0.05). Statistical analysis and data visualisation were performed in R (version 4.3.2, Vienna, Austria) [27] using the RStudio environment (Boston, MA, USA) [28].
3. Results
In total, sera from 42 wild boars were collected. Most of the animals were from Bad Klosterlautsnitz (n = 20) and only a single serum sample was from Tautenhain. The distribution of sample origins to the individual locations is shown in Table 1. Twenty-nine of the samples were juveniles (animals younger than 1 year of age), six animals were yearlings (between 1 and 2 years old), and seven were adults (older than 2 years).
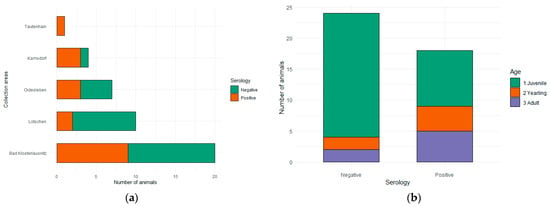
Overall, T. gondii antibodies (Immunoglobulin G, IgG) were successfully detected in 18 serum samples (37.50%, CI: 25.19–51.67%). In Bad Klosterlausnitz, 45.00% of the samples were positive (CI: 25.81–51.67%). The rest of the municipalities showed prevalences between 20.00% and 75.00%, with the exception of Tautenhain, where the only sample collected was positive (Table 1, Figure 3a). Seroprevalences per age were as follows: 31.03% (CI: 17.14–49.37%) in juveniles, 66.67% (CI: 29.57–90.75%) in yearlings, and 71.49% (CI: 35.24–92.44%) in adults (Table 1, Figure 3b). Seropositivity did not vary significantly across state districts (p = 0.25) or ages (p = 0.08) (Table 1). A summary of the results can be found in Table 1.
4. Discussion
We report for the first time the seroprevalence of T. gondii in free-ranging wild boars from Thuringia in central Germany. To the authors’ knowledge, two studies from the same research group in the north eastern part of the country precede our efforts [22,26]. In one of these studies, Bier et al. [22] reported seroprevalences of 24.14% and 25.00% in wild boars from the Federal State of Brandenburg, eastern Germany, during the hunting seasons of 2017/2018 and 2018/2019, respectively. We found a higher seroprevalence (37.50%) in the Thuringia animals during the same time point, namely the hunting season of 2017/2018; however, our sampled population is smaller than the one investigated in Brandenburg. A larger sample size, if possible, should be considered for future investigations in the area.
Spatial and temporal variations in parasite prevalence are not a rarity, even within the same country. This is particularly true for multi-host parasites like T. gondii [29]. One example of this can be found in Italy. Ranucci et al. [30] reported a seroprevalence of 14.00% in collected wild boars from the central part of the country. During the same time frame, 16.19% of the sampled wild boars in northern Italy were seropositive to T. gondii [31]. Around 10 years later in 2023, Villa et al. [32] reported a 53.10% seroprevalence in wild boars collected also in the north of Italy. Interestingly, another recent study from the Emilia-Romagna region (also northern Italy) reported 22.60% of seropositive animals [33].
In Germany, a less dramatic variation was found in Brandenburg. After Bier et al. showed the seroprevalences previously mentioned (24.14% and 25.00%) during the hunting seasons of 2017/2018 and 2018/2019 [22], the same group reported a seropositivity of 14.30% in wild boars collected in the exact same areas from Brandenburg during the following hunting seasons of 2019/2020 and 2020/2021 [26]. In our study, we only had access to samples from a single hunting season. However, given the previous record of temporal differences in T. gondii seropositivity in wild boars from Brandenburg, an expansion of the current dataset across later hunting seasons in Thuringia should be pursued.
In humans, the overall Toxoplasma seropositivity in Germany is higher than the global mean with around half of the adult population showing antibodies against T. gondii [34]. Moreover, a difference between the eastern and western part of Germany seems evident, with the eastern area showing a significantly higher number of seropositive inhabitants than the western region [35]. The federal state of Thuringia is located in the eastern part of Germany. The collection of samples in a previous study in Germany took place in the federal state of Brandenburg [22,26], which is also situated in the eastern part of the country.
In Germany, one of the main factors associated with T. gondii infection in humans is high meat consumption [35]. Current meat hygiene regulations in Europe neglect T. gondii meat infection [4]. Moreover, they focus on the detection of macroscopic findings such as Cysticerci in tissue, nematode larvae or young Fasciola spp. or Ascaris suum larvae migration lesions in some organs such as the liver. Additional tests include the official Trichinella meat inspection which can help to identify not only Trichinella spp. larvae but also Alaria alata mesocercariae [4]. However, none of these techniques can be used for the diagnosis of T. gondii. Approaches such as PCR or real-time polymerase chain reaction (PCR) or serological tests, for example, ELISA, can identify T. gondii antibodies in meat [26]. However, their use in routine meat hygiene means is cost and time intensive and requires trained personnel. Instead of this, simple common practices such as proper cooking of meat between 60 °C and 70 °C or freezing the game at −20 °C for at least 3 days can inactivate the parasite, and thus make the meat safe for human consumption [36].
In order to increase the efforts to educate the public regarding the proper way to handle and cook meat to inactivate T. gondii, efforts need to be first directed to elucidate the prevalence of T. gondii in game. The information currently available is insufficient. We hope that our study can attract more investigations like ours and, thus, increase the database available to accurately evaluate the risk game meat consumption possesses for the general population. This could also help to enhance strategies and policies to inform the consumer about the urgency of properly handling game meat in order to inactivate T. gondii.
5. Conclusions
The present study is a presentation of T. gondii seropositivity in free-ranging wild boars from Thuringia, central Germany. The seroprevalence in this small database is higher than the previously reported data in another region in the country. Nevertheless, the reduced sample size of our study should be taken into consideration before drawing strong conclusions. We highlight the importance of further studies with a larger number of animals and a country-wide investigation aimed to explicate the prevalence, and its possible variations, of T. gondii in wild boars across Germany. With this, a risk analysis program could be developed. This is of particular importance for sectors of the population who regularly consume wild boar meat: hunters, their inner circle and other game meat enthusiasts. We also hope to bring T. gondii into the spotlight as a present but sometimes neglected meat borne parasite.
Author Contributions
Conceptualization, Z.R.-S., K.H.; methodology, Z.R.-S., P.D., K.H.; formal analysis, Z.R.-S.; investigation, Z.R.-S., P.D., K.H.; resources, Z.R.-S., K.H.; data curation, Z.R.-S., K.H.; writing—original draft preparation, Z.R.-S.; writing—review and editing, Z.R.-S., K.H., P.D., T.W.V.; visualization, Z.R.-S.; funding acquisition, Z.R.-S., K.H., T.W.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by a grant from the Leipzig veterinary junior scientist support program financed by the “Freundeskreis Tiermedizin”, the Faculty of Veterinary Medicine, and by Ceva Santé Animale (Project No. 25180944 to Z.R.-S.). Article processing charges were covered thanks to the support of the Open-Access Publishing Fund Program from Leipzig University. The funding institutions had no role in this study.
Institutional Review Board Statement
All the hunted animals were shot by licensed hunters. The samples were obtained as secondary use from animals that were shot as part of regular hunting activities. The sampling itself had no influence on the killing of the individual animals. The hunts were carried out in compliance with the German Hunting Act and all other applicable legal standards. Compliance with these regulations and the validity of the hunting licences of all participating hunters was checked by employees of the Thuringian Ministry of Forestry, who organized the hunts.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are very grateful with Anna Obiegala (Institute of Animal Hygiene and Public Health, Leipzig University) for her insights during the statistical analysis. Many thanks also to S. Gawlowska (Institute of Parasitology, Leipzig University) for her excellent technical assistance. We are also thankful with A. Rentería for the illustrations in Figure 1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gabriël, S.; Dorny, P.; Saelens, G.; Dermauw, V. Foodborne Parasites and Their Complex Life Cycles Challenging Food Safety in Different Food Chains. Foods 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.R. Intensive Swine Production and Pork Safety. Foodborne Pathog. Dis. 2011, 8, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.; Barber, I.; Boag, B.; Ellison, A.R.; Morgan, E.R.; Murray, K.; Pascoe, E.L.; Sait, S.M.; Wilson, A.J.; Booth, M. Global Change, Parasite Transmission and Disease Control: Lessons from Ecology. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160088. [Google Scholar] [CrossRef] [PubMed]
- Bundesinstitut für Risikobewertung. Game Meat: Health Assessment of Human-Pathogenic Parasites: BfR Opinion No 045/2018 of 21 December 2018; BfR-Stellungnahmen; Bundesinstitut für Risikobewertung: Berlin, Germany, 2018; Volume 2018, no. 045. [Google Scholar] [CrossRef]
- Olsen, A.; Berg, R.; Tagel, M.; Must, K.; Deksne, G.; Enemark, H.L.; Alban, L.; Johansen, M.V.; Nielsen, H.V.; Sandberg, M.; et al. Seroprevalence of Toxoplasma gondii in Domestic Pigs, Sheep, Cattle, Wild Boars, and Moose in the Nordic-Baltic Region: A Systematic Review and Meta-Analysis. Parasite Epidemiol. Control 2019, 5, e00100. [Google Scholar] [CrossRef] [PubMed]
- Hedman, H.D.; Varga, C.; Duquette, J.; Novakofski, J.; Mateus-Pinilla, N.E. Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America. Vet. Sci. 2020, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Valencak, T.G.; Gamsjäger, L.; Ohrnberger, S.; Culbert, N.J.; Ruf, T. Healthy N-6/n-3 Fatty Acid Composition from Five European Game Meat Species Remains after Cooking. BMC Res. Notes 2015, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.; Hamedy, A.; Kornacka-Stackonis, A.; Langner, T.; Birka, S.; Koethe, M. Toxoplasma gondii in Raccoons (Procyon lotor) in Germany: A Serosurvey Based on Meat Juice. Parasitol. Res. 2022, 121, 3417–3425. [Google Scholar] [CrossRef]
- Stillfried, M.; Gras, P.; Busch, M.; Börner, K.; Kramer-Schadt, S.; Ortmann, S. Wild inside: Urban Wild Boar Select Natural, Not Anthropogenic Food Resources. PLoS ONE 2017, 12, e0175127. [Google Scholar] [CrossRef]
- Sgroi, G.; D’Alessio, N.; Marucci, G.; Pacifico, L.; Buono, F.; Deak, G.; Anastasio, A.; Interisano, M.; Fraulo, P.; Pesce, A.; et al. Trichinella britovi in Wild Boar Meat from Italy, 2015–2021: A Citizen Science Approach to Surveillance. One Health 2023, 16, 100480. [Google Scholar] [CrossRef]
- Sgroi, G.; Varcasia, A.; D’Alessio, N.; Varuzza, P.; Buono, F.; Amoroso, M.G.; Boufana, B.; Otranto, D.; Fioretti, A.; Veneziano, V. Taenia hydatigena Cysticercosis in Wild Boar (Sus scrofa) from Southern Italy: An Epidemiological and Molecular Survey. Parasitology 2020, 147, 1636–1642. [Google Scholar] [CrossRef]
- Sgroi, G.; Varcasia, A.; Dessi, G.; D’Alessio, N.; Tamponi, C.; Saarma, U.; Laurimäe, T.; Kinkar, L.; Santoro, M.; Caputo, V.; et al. Cystic Echinococcosis in Wild Boars (Sus scrofa) from Southern Italy: Epidemiological Survey and Molecular Characterization. Int. J. Parasitol. Parasites Wildl. 2019, 9, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Solís, Z.; Kołodziej-Sobocińska, M.; Riehn, K. Alaria Spp. Mesocercariae in Eurasian Badger (Meles meles) and Wild Boar (Sus scrofa) from the Białowieża Forest, North-Eastern Poland. Parasitol. Res. 2018, 117, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global Status of Toxoplasma gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar] [PubMed]
- Dubey, J.P. Duration of Immunity to Shedding of Toxoplasma gondii Oocysts by Cats. J. Parasitol. 1995, 81, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The Life-Cycle of Toxoplasma gondii Reviewed Using Animations. Parasit. Vectors 2020, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-D.; Liu, H.-H.; Ma, Z.-X.; Ma, H.-Y.; Li, Z.-Y.; Yang, Z.-B.; Zhu, X.-Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 2017, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.O.; Faschingbauer, F.; Kagan, K.O.; Groß, U.; Enders, M.; Kehl, S. AGG Section Maternal Diseases Toxoplasma gondii Infection in Pregnancy—Recommendations of the Working Group on Obstetrics and Prenatal Medicine (AGG—Section on Maternal Disorders). Geburtshilfe Frauenheilkd. 2023, 83, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; De Wit, L.A.; VanWormer, E.; Villena, I. Environmental Transmission of Toxoplasma gondii: Oocysts in Water, Soil and Food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Sousa, S.; Castro, A.; Da Costa, J.M.C. Detection of Toxoplasma gondii Oocysts in Fresh Vegetables and Berry Fruits. Parasit. Vectors 2020, 13, 180. [Google Scholar] [CrossRef]
- Apostolico, F.; Vercillo, F.; La Porta, G.; Ragni, B. Long-Term Changes in Diet and Trophic Niche of the European Wildcat (Felis silvestris silvestris) in Italy. Mammal Res. 2016, 61, 109–119. [Google Scholar] [CrossRef]
- Bier, N.S.; Stollberg, K.; Mayer-Scholl, A.; Johne, A.; Nöckler, K.; Richter, M. Seroprevalence of Toxoplasma gondii in Wild Boar and Deer in Brandenburg, Germany. Zoonoses Public Health 2020, 67, 601–606. [Google Scholar] [CrossRef]
- Opsteegh, M.; Swart, A.; Fonville, M.; Dekkers, L.; Van Der Giessen, J. Age-Related Toxoplasma gondii Seroprevalence in Dutch Wild Boar Inconsistent with Lifelong Persistence of Antibodies. PLoS ONE 2011, 6, e16240. [Google Scholar] [CrossRef]
- Kornacka, A.; Moskwa, B.; Werner, A.; Nowosad, P.; Jankowska, W.; Cybulska, A.; Majewska, A.C. The Seroprevalence of Toxoplasma gondii in Wild Boars from Three Voivodeships in Poland, MAT Analyses. Acta Parasitol. 2020, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, M.; Pyziel, A.; Wiśniewski, J.; Steiner-Bogdaszewska, Ż.; Klich, D.; Anusz, K. Prevalence of Toxoplasma gondii Antibodies in Wild Boar (Sus scrofa) from Strzałowo Forest Division, Warmia and Mazury Region, Poland. Ann. Agric. Environ. Med. 2021, 28, 237–242. [Google Scholar] [CrossRef]
- Stollberg, K.C.; Schares, G.; Mayer-Scholl, A.; Hrushetska, I.; Diescher, S.; Johne, A.; Richter, M.H.; Bier, N.S. Comparison of Direct and Indirect Toxoplasma gondii Detection and Genotyping in Game: Relationship and Challenges. Microorganisms 2021, 9, 1663. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2023. [Google Scholar]
- Wilson, A.G.; Lapen, D.R.; Provencher, J.F.; Wilson, S. The Role of Species Ecology in Predicting Toxoplasma gondii Prevalence in Wild and Domesticated Mammals Globally. PLoS Pathog. 2024, 20, e1011908. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, D.; Veronesi, F.; Moretti, A.; Branciari, R.; Miraglia, D.; Manfredi, M.T.; Piergili Fioretti, D. Seroprevalence of Toxoplasma gondii in Wild Boars (Sus scrofa) from Central Italy. Parasite 2013, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferroglio, E.; Bosio, F.; Trisciuoglio, A.; Zanet, S. Toxoplasma gondii in Sympatric Wild Herbivores and Carnivores: Epidemiology of Infection in the Western Alps. Parasit. Vectors 2014, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.; Allievi, C.; Gazzonis, A.L.; Ventura, G.; Gradassi, M.; Zanzani, S.A.; Manfredi, M.T. Serological Prevalence of Toxoplasma gondii, Neospora caninum, and Sarcoptes scabiei var. suis in Wild Boars (Sus scrofa) Hunted in a Highly Anthropized Area in Italy. Animals 2023, 13, 1730. [Google Scholar] [CrossRef]
- Dini, F.M.; Musto, C.; De Nigris, V.M.; Bellinello, E.; Sampieri, M.; Merialdi, G.; Barca, L.; Delogu, M.; Galuppi, R. Sero-Epidemiological Investigation on Toxoplasma gondii Infection in Apennine Wolf (Canis lupus italicus) and Wild Boar (Sus scrofa) in Italy. BMC Vet. Res. 2024, 20, 62. [Google Scholar] [CrossRef]
- Wilking, H.; Thamm, M.; Stark, K.; Aebischer, T.; Seeber, F. Prevalence, Incidence Estimations and Risk Factors of Toxoplasma gondii Infection in Germany: A Representative, Cross-Sectional, Serological Study. Sci. Rep. 2016, 6, 22551. [Google Scholar] [CrossRef] [PubMed]
- Seeber, F. Past and Present Seroprevalence and Disease Burden Estimates of Toxoplasma gondii Infections in Germany: An Appreciation of the Role of Serodiagnostics. Int. J. Med. Microbiol. 2023, 313, 151592. [Google Scholar] [CrossRef] [PubMed]
- Mirza Alizadeh, A.; Jazaeri, S.; Shemshadi, B.; Hashempour-Baltork, F.; Sarlak, Z.; Pilevar, Z.; Hosseini, H. A Review on Inactivation Methods of Toxoplasma gondii in Foods. Pathog. Glob. Health 2018, 112, 306–319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).