Effect of Sex on Intestinal Microbial Metabolites of Hainan Special Wild Boars
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Sample Collection
2.3. Sample Preparation and Metabolite Extraction
2.4. Metabolomic Analysis
2.5. Sample Preparation and SCFAs Detection
2.6. Data Processing and Statistical Analysis
2.7. Correlation Analysis between Metabolomics, SCFAs, and Microorganisms
3. Results
3.1. Metabolite Annotation and Principal Component Analysis
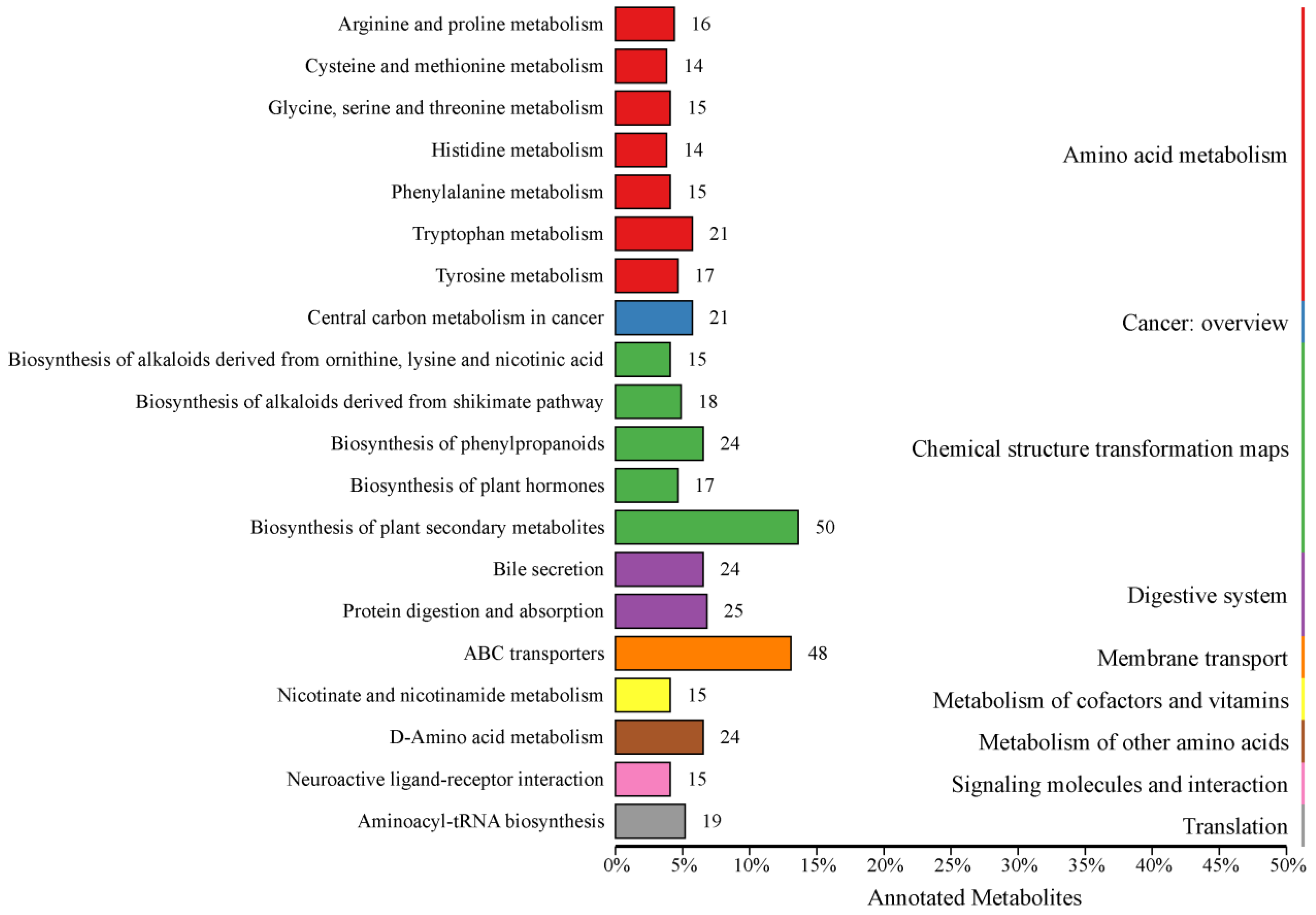
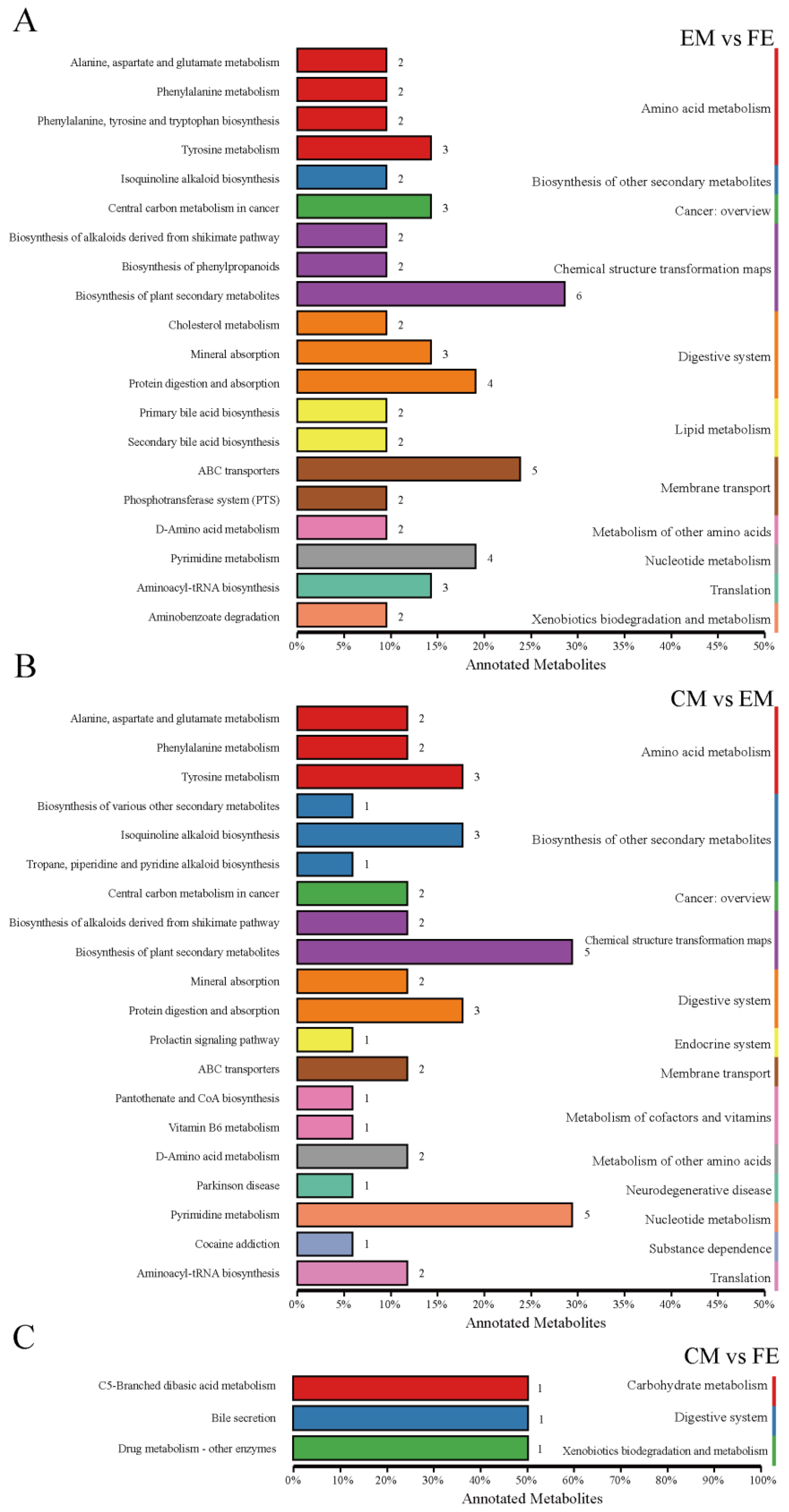
3.2. Analysis of Differential Metabolites and KEGG Functional Annotation
3.3. Metabolomic and Microbiome Correlation Analysis
3.4. Short-Chain Fatty Acid Analysis
3.5. Short-Chain Fatty Acid and Microbiome Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasquez, R.; Oh, J.K.; Song, J.H.; Kang, D.K. Gut microbiome-produced metabolites in pigs: A review on their biological functions and the influence of probiotics. J. Anim. Sci. Technol. 2022, 64, 671–695. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Audano, M.; Maldini, M.; De Fabiani, E.; Mitro, N.; Caruso, D. Gender-related metabolomics and lipidomics: From experimental animal models to clinical evidence. J. Proteom. 2018, 178, 82–91. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Trefflich, I.; Dietrich, S.; Braune, A.; Abraham, K.; Weikert, C. Short- and Branched-Chain Fatty Acids as Fecal Markers for Microbiota Activity in Vegans and Omnivores. Nutrients 2021, 13, 1808. [Google Scholar] [CrossRef]
- Letsinger, A.C.; Menon, R.; Iyer, A.R.; Vellers, H.L.; Granados, J.Z.; Jayaraman, A.; Lightfoot, J.T. A High Fat/High Sugar Diet Alters the Gastrointestinal Metabolome in a Sex Dependent Manner. Metabolites 2020, 10, 421. [Google Scholar] [CrossRef]
- Zhang, Y.; Barupal, D.K.; Fan, S.; Gao, B.; Zhu, C.; Flenniken, A.M.; McKerlie, C.; Nutter, L.M.J.; Lloyd, K.C.K.; Fiehn, O. Sexual Dimorphism of the Mouse Plasma Metabolome Is Associated with Phenotypes of 30 Gene Knockout Lines. Metabolites 2023, 13, 947. [Google Scholar] [CrossRef]
- Mittelstrass, K.; Ried, J.S.; Yu, Z.; Krumsiek, J.; Gieger, C.; Prehn, C.; Roemisch-Margl, W.; Polonikov, A.; Peters, A.; Theis, F.J.; et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011, 7, e1002215. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Turley, S.D.; Schwarz, M.; Spady, D.K.; Dietschy, J.M. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology 1998, 28, 1088–1094. [Google Scholar] [CrossRef]
- Xiang, X.; Backman, J.T.; Neuvonen, P.J.; Niemi, M. Gender, but not CYP7A1 or SLCO1B1 polymorphism, affects the fasting plasma concentrations of bile acids in human beings. Basic Clin. Pharmacol. Toxicol. 2012, 110, 245–252. [Google Scholar] [CrossRef]
- Frommherz, L.; Bub, A.; Hummel, E.; Rist, M.J.; Roth, A.; Watzl, B.; Kulling, S.E. Age-Related Changes of Plasma Bile Acid Concentrations in Healthy Adults--Results from the Cross-Sectional KarMeN Study. PLoS ONE 2016, 11, e0153959. [Google Scholar] [CrossRef]
- Fisher, M.M.; Yousef, I.M. Sex differences in the bile acid composition of human bile: Studies in patients with and without gallstones. Can. Med. Assoc. J. 1973, 109, 190–193. [Google Scholar]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; De la Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wen, Q.; Wang, Y.; Wang, Z.; Tan, Z.; Wu, K. Sex Differences in Intestinal Microbial Composition and Function of Hainan Special Wild Boar. Animals 2020, 10, 1553. [Google Scholar] [CrossRef]
- Feng, D.; Mao, K.; Yang, Y.; Hu, Y. Crop-livestock Integration for Sustainable Agriculture in China: The History of State Policy Goals, Reform Opportunities and Institutional Constraints. Front. Agric. Sci. Eng. 2023, 10, 518–529. [Google Scholar] [CrossRef]
- Fornós, M.; Sanz-Fernández, S.; Jiménez-Moreno, E.; Carrión, D.; Gasa, J.; Rodríguez-Estévez, V. The Feeding Behaviour Habits of Growing-Finishing Pigs and Its Effects on Growth Performance and Carcass Quality: A Review. Animals 2022, 12, 1128. [Google Scholar] [CrossRef]
- He, M.; Gao, J.; Wu, J.; Zhou, Y.; Fu, H.; Ke, S.; Yang, H.; Chen, C.; Huang, L. Host Gender and Androgen Levels Regulate Gut Bacterial Taxa in Pigs Leading to Sex-Biased Serum Metabolite Profiles. Front. Microbiol. 2019, 10, 1359. [Google Scholar] [CrossRef]
- Dadi, T.H.; Vahjen, W.; Zentek, J.; Melzig, M.F.; Granica, S.; Piwowarski, J.P. Lythrum salicaria L. herb and gut microbiota of healthy post-weaning piglets. Focus on prebiotic properties and formation of postbiotic metabolites in ex vivo cultures. J. Ethnopharmacol. 2020, 261, 113073. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wells, A.E.; Barrington, W.T.; Dearth, S.; Milind, N.; Carter, G.W.; Threadgill, D.W.; Campagna, S.R.; Voy, B.H. Independent and Interactive Effects of Genetic Background and Sex on Tissue Metabolomes of Adipose, Skeletal Muscle, and Liver in Mice. Metabolites 2022, 12, 337. [Google Scholar] [CrossRef]
- Pisoni, S.; Marrachelli, V.G.; Morales, J.M.; Maestrini, S.; Di Blasio, A.M.; Monleón, D. Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals. Metabolites 2022, 12, 419. [Google Scholar] [CrossRef]
- Trefan, L.; Doeschl-Wilson, A.; Rooke, J.A.; Terlouw, C.; Bünger, L. Meta-analysis of effects of gender in combination with carcass weight and breed on pork quality. J. Anim. Sci. 2013, 91, 1480–1492. [Google Scholar] [CrossRef]
- Yao, Y.C.; Cai, Z.W.; Zhao, C.J.; Wu, K.L.; Wu, C.X.; Han, W.P.; Xu, N.Y. Influence of castration-induced sex hormone deficiency on serum lipid levels and the genes expression in male pigs. Horm. Metab. Res. 2011, 43, 674–680. [Google Scholar] [CrossRef]
- Bovo, S.; Mazzoni, G.; Calò, D.G.; Galimberti, G.; Fanelli, F.; Mezzullo, M.; Schiavo, G.; Scotti, E.; Manisi, A.; Samoré, A.B.; et al. Deconstructing the pig sex metabolome: Targeted metabolomics in heavy pigs revealed sexual dimorphisms in plasma biomarkers and metabolic pathways. J. Anim. Sci. 2015, 93, 5681–5693. [Google Scholar] [CrossRef]
- Dorries, K.M.; Adkins-Regan, E.; Halpern, B.P. Olfactory sensitivity to the pheromone, androstenone, is sexually dimorphic in the pig. Physiol. Behav. 1995, 57, 255–259. [Google Scholar] [CrossRef]
- Rao, Z.X.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Gebhardt, J.T. Effects of Various Feed Additives on Finishing Pig Growth Performance and Carcass Characteristics: A Review. Animals 2023, 13, 200. [Google Scholar] [CrossRef]
- Wu, G.; Ott, T.L.; Knabe, D.A.; Bazer, F.W. Amino acid composition of the fetal pig. J. Nutr. 1999, 129, 1031–1038. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, X.; Mao, X.; Wu, G.; Qiao, S. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J. Nutr. 2010, 140, 981–986. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Seok, W.J.; Kim, I.H. Organic Acids Mixture as a Dietary Additive for Pigs-A Review. Animals 2020, 10, 952. [Google Scholar] [CrossRef]
- Xu, E.; Chen, C.; Fu, J.; Zhu, L.; Shu, J.; Jin, M.; Wang, Y.; Zong, X. Dietary fatty acids in gut health: Absorption, metabolism and function. Anim. Nutr. 2021, 7, 1337–1344. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Yang, Z.; Xie, Y.; Mo, Z. The Effects of Cholesterol Metabolism on Follicular Development and Ovarian Function. Curr. Mol. Med. 2019, 19, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yuan, J.; Li, J.; Li, H.; Zhang, J.; Tang, J.; Ni, Y.; Huang, T.; Wang, F.; Zhao, F.; et al. Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: A 6-month randomized controlled-feeding trial. Clin. Nutr. 2020, 39, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Golonka, R.M.; Yeoh, B.S.; Saha, P.; Tian, Y.; Chiang, J.Y.L.; Patterson, A.D.; Gewirtz, A.T.; Joe, B.; Vijay-Kumar, M. Sex Dimorphic Effects of Bile Acid Metabolism in Liver Cancer in Mice. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Kister, B.; Viehof, A.; Rolle-Kampczyk, U.; Schwentker, A.; Treichel, N.S.; Jennings, S.A.V.; Wirtz, T.H.; Blank, L.M.; Hornef, M.W.; von Bergen, M.; et al. A physiologically based model of bile acid metabolism in mice. iScience 2023, 26, 107922. [Google Scholar] [CrossRef]
- Christoffersen, B.O.; Gade, L.P.; Golozoubova, V.; Svendsen, O.; Raun, K. Influence of castration-induced testosterone and estradiol deficiency on obesity and glucose metabolism in male Göttingen minipigs. Steroids 2010, 75, 676–684. [Google Scholar] [CrossRef]
- Matsubara, T.; Li, F.; Gonzalez, F.J. FXR signaling in the enterohepatic system. Mol. Cell. Endocrinol. 2013, 368, 17–29. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef]
- Baptissart, M.; Vega, A.; Martinot, E.; Pommier, A.J.; Houten, S.M.; Marceau, G.; de Haze, A.; Baron, S.; Schoonjans, K.; Lobaccaro, J.M.; et al. Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology 2014, 60, 1054–1065. [Google Scholar] [CrossRef]
- Baptissart, M.; Martinot, E.; Vega, A.; Sédes, L.; Rouaisnel, B.; de Haze, A.; Baron, S.; Schoonjans, K.; Caira, F.; Volle, D.H. Bile acid-FXRα pathways regulate male sexual maturation in mice. Oncotarget 2016, 7, 19468–19482. [Google Scholar] [CrossRef]
- Yu, G.; Xu, C.; Zhang, D.; Ju, F.; Ni, Y. MetOrigin: Discriminating the origins of microbial metabolites for integrative analysis of the gut microbiome and metabolome. iMeta 2022, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, G.; Prandini, A. Dietary Supplementation of Inorganic, Organic, and Fatty Acids in Pig: A Review. Animals 2020, 10, 1740. [Google Scholar] [CrossRef]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Johnstone, R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002, 1, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Mishiro, T.; Kusunoki, R.; Otani, A.; Ansary, M.M.; Tongu, M.; Harashima, N.; Yamada, T.; Sato, S.; Amano, Y.; Itoh, K.; et al. Butyric acid attenuates intestinal inflammation in murine DSS-induced colitis model via milk fat globule-EGF factor 8. Lab. Investig. A J. Tech. Methods Pathol. 2013, 93, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.I.; Marzorati, M.; Grootaert, C.; Baran, M.; Van Craeyveld, V.; Courtin, C.M.; Broekaert, W.F.; Delcour, J.A.; Verstraete, W.; Van de Wiele, T. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb. Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef]
- Hong, Y.H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.C.; Feng, D.D.; Chen, C.; Lee, H.G.; et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Mølbak, L.; Thomsen, L.E.; Jensen, T.K.; Bach Knudsen, K.E.; Boye, M. Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J. Appl. Microbiol. 2007, 103, 1853–1867. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Wei, H.; Chen, Y.; Shang, H. Study on the Diversity and Function of Gut Microbiota in Pigs Following Long-Term Antibiotic and Antibiotic-Free Breeding. Curr. Microbiol. 2020, 77, 4114–4128. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Z.; Wang, S.; Cao, L.; Zhou, L.; Sun, A.; Zhong, Z.; Nabben, M. Microbial-Driven Butyrate Regulates Jejunal Homeostasis in Piglets During the Weaning Stage. Front. Microbiol. 2018, 9, 3335. [Google Scholar] [CrossRef]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related With Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Czech, A.; Kusior, G.; Szczotka-Bochniarz, A.; Klebaniuk, R. The effect of feeding system and sex on the performance and selected gastrointestinal features of fattening pigs. Pol. J. Vet. Sci. 2018, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Andretta, I.; Pomar, C.; Kipper, M.; Hauschild, L.; Rivest, J. Feeding behavior of growing-finishing pigs reared under precision feeding strategies. J. Anim. Sci. 2016, 94, 3042–3050. [Google Scholar] [CrossRef] [PubMed]

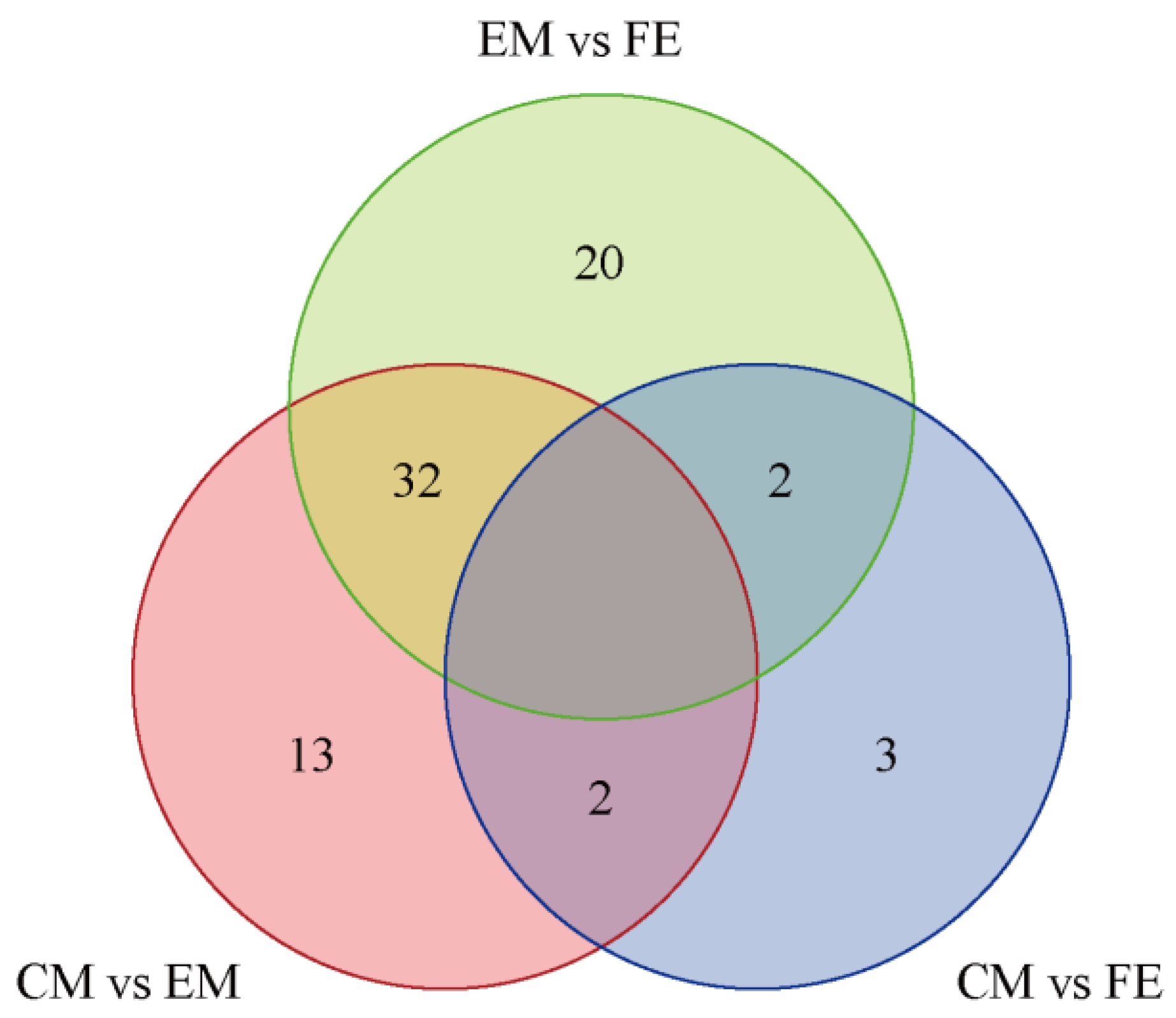



| Metabolite Name | EM vs. FE | CM vs. EM | CM vs. FE |
|---|---|---|---|
| Butabarbital | 1 | 0 | 1 |
| 4,6-Diamino-5-formamidopyrimidine | 0 | 1 | 0 |
| Glycochenodeoxycholate | 1 | 0 | 0 |
| N-.alpha.-Acetyl-L-arginine | 1 | 0 | 0 |
| Actinonin | 1 | 0 | 1 |
| 5-Hydroxyhexanoic acid | 1 | 0 | 0 |
| Methacholine | 1 | 0 | 0 |
| Cortisol 21-acetate | 1 | 1 | 0 |
| Inosine | 1 | 0 | 0 |
| L-Alanine | 1 | 1 | 0 |
| 3,3′,4,5′-Tetrahydroxy-trans-stilbene | 1 | 0 | 0 |
| Docosatrienoic Acid | 1 | 1 | 0 |
| 1-O-Octadecyl-sn-glyceryl-3-phosphorylcholine | 1 | 0 | 0 |
| p-Acetamidophenol (Acetaminophen, Tylenol) | 1 | 1 | 0 |
| Ser-Leu | 1 | 0 | 0 |
| N2-Acetyl-L-ornithine | 0 | 1 | 0 |
| 3-Hydroxyanthranilic acid | 1 | 0 | 0 |
| Stearoylcarnitine | 0 | 1 | 0 |
| Cytidine | 1 | 1 | 0 |
| DL-lactate | 1 | 1 | 0 |
| Uracil | 1 | 1 | 0 |
| 5-Methylcytosine | 0 | 1 | 0 |
| 3-Methylglutaric acid | 1 | 0 | 0 |
| L-Leucine | 1 | 0 | 0 |
| L-Asparagine | 1 | 1 | 0 |
| 1-Palmitoyl-sn-glycero-3-phosphocholine | 1 | 1 | 0 |
| Procaine | 0 | 1 | 0 |
| sn-Glycerol 1-phosphate | 0 | 0 | 1 |
| L-Dopa | 1 | 1 | 0 |
| Tyramine | 0 | 1 | 0 |
| N6-Methyladenine | 1 | 1 | 0 |
| 1-Stearoyl-sn-glycerol 3-phosphocholine | 1 | 1 | 0 |
| 2-Methyl-3-hydroxybutyric acid | 1 | 1 | 0 |
| trans-3-Coumaric acid | 0 | 1 | 0 |
| Thymine | 1 | 1 | 0 |
| 6-Mercaptopurine | 0 | 0 | 1 |
| 2-Hydroxyadenine | 0 | 1 | 0 |
| Hydroxyisocaproic acid | 1 | 1 | 0 |
| Phenylpyruvate | 1 | 1 | 0 |
| Glycolithocholic acid | 1 | 0 | 0 |
| Trehalose | 1 | 0 | 0 |
| Acetohydroxamic acid | 0 | 1 | 0 |
| 5-Amino-4-carbamoylimidazole (AICA) | 0 | 1 | 0 |
| Oxyquinoline | 1 | 1 | 0 |
| D(−)-beta-hydroxy butyric acid | 1 | 1 | 0 |
| N-Tigloylglycine | 0 | 0 | 1 |
| Acetylglycine | 1 | 1 | 0 |
| Itaconic acid | 0 | 1 | 1 |
| L-Ascorbic acid | 1 | 1 | 0 |
| 7-Oxocholesterol | 1 | 1 | 0 |
| Acetyl-DL-Leucine | 1 | 1 | 0 |
| 3-Dehydroshikimic acid | 1 | 0 | 0 |
| Pyridoxine | 0 | 1 | 0 |
| PS(16:0/16:0) | 1 | 1 | 0 |
| Deoxygalactonojirimycin | 0 | 1 | 0 |
| 3,3-Dimethylglutaric acid | 1 | 0 | 0 |
| Gaboxadol | 0 | 1 | 0 |
| D-Fructose | 1 | 1 | 0 |
| Val-Gln | 0 | 1 | 1 |
| Glycodeoxycholic acid | 1 | 0 | 0 |
| Phenol | 1 | 0 | 0 |
| N-Acetyl-L-phenylalanine | 1 | 1 | 0 |
| 4-Hydroxycinnamic acid | 1 | 1 | 0 |
| Hydroxyphenyllactic acid | 1 | 1 | 0 |
| Scytalone | 1 | 1 | 0 |
| Glycocholic Acid | 1 | 0 | 0 |
| Pseudouridine | 1 | 1 | 0 |
| 2-Dehydro-3-deoxy-D-gluconate | 1 | 1 | 0 |
| 1-Methylxanthine | 1 | 0 | 0 |
| Androsterone sulfate | 1 | 1 | 0 |
| 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine | 1 | 1 | 0 |
| Dihydroxyfumarate | 1 | 0 | 0 |
| Metabolite Name | log2(Fold Change) | p-Value | Regulated |
|---|---|---|---|
| EM vs. FE | |||
| 1-Palmitoyl-sn-glycero-3-phosphocholine | 1.352 | <0.001 | up |
| p-Acetamidophenol (Acetaminophen, Tylenol) | 1.251 | <0.001 | up |
| Trehalose | 1.252 | <0.001 | up |
| Glycochenodeoxycholate | 1.973 | 0.001 | up |
| 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine | 1.366 | 0.001 | up |
| Cytidine | 1.193 | 0.001 | up |
| Docosatrienoic Acid | 1.651 | 0.002 | up |
| Glycocholic Acid | 1.213 | 0.002 | up |
| 3-Hydroxyanthranilic acid | 1.013 | 0.002 | up |
| 1-Stearoyl-sn-glycerol 3-phosphocholine | 1.149 | 0.004 | up |
| N-.alpha.-Acetyl-L-arginine | 1.258 | 0.005 | up |
| 1-O-Octadecyl-sn-glyceryl-3-phosphorylcholine | 1.120 | 0.005 | up |
| 7-Oxocholesterol | 1.194 | 0.005 | up |
| D-Fructose | 1.057 | 0.007 | up |
| Glycodeoxycholic acid | 2.216 | 0.008 | up |
| Glycolithocholic acid | 2.000 | 0.014 | up |
| PS(16:0/16:0) | 1.059 | 0.016 | up |
| Ser-Leu | 1.088 | 0.040 | up |
| Scytalone | −1.594 | 0.002 | down |
| 3-Methylglutaric acid | −1.168 | 0.002 | down |
| Inosine | −1.139 | 0.002 | down |
| Phenylpyruvate | −1.350 | 0.002 | down |
| L-Alanine | −1.481 | 0.003 | down |
| Androsterone sulfate | −3.303 | 0.005 | down |
| Uracil | −1.999 | 0.009 | down |
| Hydroxyisocaproic acid | −1.368 | 0.010 | down |
| 3,3-Dimethylglutaric acid | −1.628 | 0.011 | down |
| L-Ascorbic acid | −1.754 | 0.012 | down |
| N6-Methyladenine | −2.191 | 0.012 | down |
| Oxyquinoline | −1.066 | 0.014 | down |
| DL-lactate | −2.033 | 0.015 | down |
| Thymine | −1.461 | 0.015 | down |
| Actinonin | −1.112 | 0.015 | down |
| Acetyl-DL-Leucine | −2.012 | 0.016 | down |
| D(−)-beta-hydroxy butyric acid | −2.028 | 0.017 | down |
| N-Acetyl-L-phenylalanine | −1.615 | 0.021 | down |
| Acetylglycine | −1.234 | 0.023 | down |
| Hydroxyphenyllactic acid | −2.060 | 0.024 | down |
| Dihydroxyfumarate | −1.060 | 0.024 | down |
| 2-Methyl-3-hydroxybutyric acid | −1.721 | 0.024 | down |
| 3,3′,4,5′-Tetrahydroxy-trans-stilbene | −2.141 | 0.025 | down |
| 5-Hydroxyhexanoic acid | −1.017 | 0.029 | down |
| 4-Hydroxycinnamic acid | −1.384 | 0.029 | down |
| 2-Dehydro-3-deoxy-D-gluconate | −2.691 | 0.029 | down |
| L-Leucine | −1.405 | 0.030 | down |
| Butabarbital | −1.298 | 0.032 | down |
| Cortisol 21-acetate | −4.483 | 0.032 | down |
| 3-Dehydroshikimic acid | −1.347 | 0.035 | down |
| L-Asparagine | −1.695 | 0.036 | down |
| L-Dopa | −1.049 | 0.038 | down |
| Methacholine | −1.009 | 0.040 | down |
| 1-Methylxanthine | −1.042 | 0.043 | down |
| Pseudouridine | −2.022 | 0.043 | down |
| Phenol | −1.349 | 0.046 | down |
| CM vs. EM | |||
| Val-Gln | 1.131 | 0.002 | up |
| L-Alanine | 1.627 | 0.002 | up |
| Phenylpyruvate | 1.286 | 0.003 | up |
| Scytalone | 1.468 | 0.003 | up |
| Procaine | 1.414 | 0.004 | up |
| trans-3-Coumaric acid | 1.018 | 0.006 | up |
| Androsterone sulfate | 2.677 | 0.008 | up |
| 2-Hydroxyadenine | 1.709 | 0.009 | up |
| Hydroxyisocaproic acid | 1.360 | 0.010 | up |
| Thymine | 1.661 | 0.010 | up |
| DL-lactate | 2.219 | 0.012 | up |
| Acetyl-DL-Leucine | 2.240 | 0.012 | up |
| N6-Methyladenine | 2.128 | 0.013 | up |
| Oxyquinoline | 1.049 | 0.014 | up |
| L-Ascorbic acid | 1.570 | 0.016 | up |
| D(−)-beta-hydroxy butyric acid | 2.091 | 0.016 | up |
| Acetylglycine | 1.353 | 0.017 | up |
| Hydroxyphenyllactic acid | 2.276 | 0.020 | up |
| 2-Methyl-3-hydroxybutyric acid | 1.866 | 0.020 | up |
| Deoxygalactonojirimycin | 1.028 | 0.021 | up |
| L-Asparagine | 2.027 | 0.024 | up |
| Acetohydroxamic acid | 1.187 | 0.025 | up |
| Tyramine | 1.023 | 0.026 | up |
| 4-Hydroxycinnamic acid | 1.377 | 0.028 | up |
| 2-Dehydro-3-deoxy-D-gluconate | 2.690 | 0.030 | up |
| Uracil | 1.398 | 0.030 | up |
| Pseudouridine | 2.395 | 0.032 | up |
| 5-Methylcytosine | 1.789 | 0.033 | up |
| Cortisol 21-acetate | 4.326 | 0.033 | up |
| L-Dopa | 1.091 | 0.034 | up |
| Pyridoxine | 1.023 | 0.034 | up |
| 5-Amino-4-carbamoylimidazole (AICA) | 1.201 | 0.039 | up |
| N-Acetyl-L-phenylalanine | 1.196 | 0.047 | up |
| N2-Acetyl-L-ornithine | 1.419 | 0.048 | up |
| Gaboxadol | 1.807 | 0.049 | up |
| 1-Stearoyl-sn-glycerol 3-phosphocholine | −1.187 | 0.007 | down |
| p-Acetamidophenol (Acetaminophen, Tylenol) | −1.082 | 0.008 | down |
| 4,6-Diamino-5-formamidopyrimidine | −1.082 | 0.009 | down |
| Cytidine | −1.003 | 0.012 | down |
| 1-Palmitoyl-sn-glycero-3-phosphocholine | −1.392 | 0.015 | down |
| D-Fructose | −1.457 | 0.018 | down |
| PS(16:0/16:0) | −1.175 | 0.019 | down |
| 7-Oxocholesterol | −1.075 | 0.020 | down |
| Itaconic acid | −1.694 | 0.020 | down |
| 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine | −1.260 | 0.021 | down |
| Docosatrienoic Acid | −2.023 | 0.025 | down |
| Stearoylcarnitine | −2.210 | 0.027 | down |
| CM vs. FE | |||
| Val-Gln | 1.193 | 0.012 | up |
| Actinonin | −1.427 | 0.014 | down |
| Itaconic acid | −1.752 | 0.018 | down |
| N-Tigloylglycine | −1.774 | 0.018 | down |
| 6-Mercaptopurine | −1.579 | 0.033 | down |
| Butabarbital | −1.725 | 0.041 | down |
| sn-Glycerol 1-phosphate | −1.067 | 0.042 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wen, Q.; Wu, H.; Peng, W.; Cai, K.; Tan, Z.; Na, W.; Wu, K. Effect of Sex on Intestinal Microbial Metabolites of Hainan Special Wild Boars. Animals 2024, 14, 2164. https://doi.org/10.3390/ani14152164
Wang X, Wen Q, Wu H, Peng W, Cai K, Tan Z, Na W, Wu K. Effect of Sex on Intestinal Microbial Metabolites of Hainan Special Wild Boars. Animals. 2024; 14(15):2164. https://doi.org/10.3390/ani14152164
Chicago/Turabian StyleWang, Xiaozhe, Qiong Wen, Hongfen Wu, Wenchuan Peng, Keqi Cai, Zhen Tan, Wei Na, and Kebang Wu. 2024. "Effect of Sex on Intestinal Microbial Metabolites of Hainan Special Wild Boars" Animals 14, no. 15: 2164. https://doi.org/10.3390/ani14152164





