Effects of Premortem Stress on Protein Expression, Steak Color, Oxidation, and Myofibrillar Fragmentation Index in the Longissimus Lumborum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
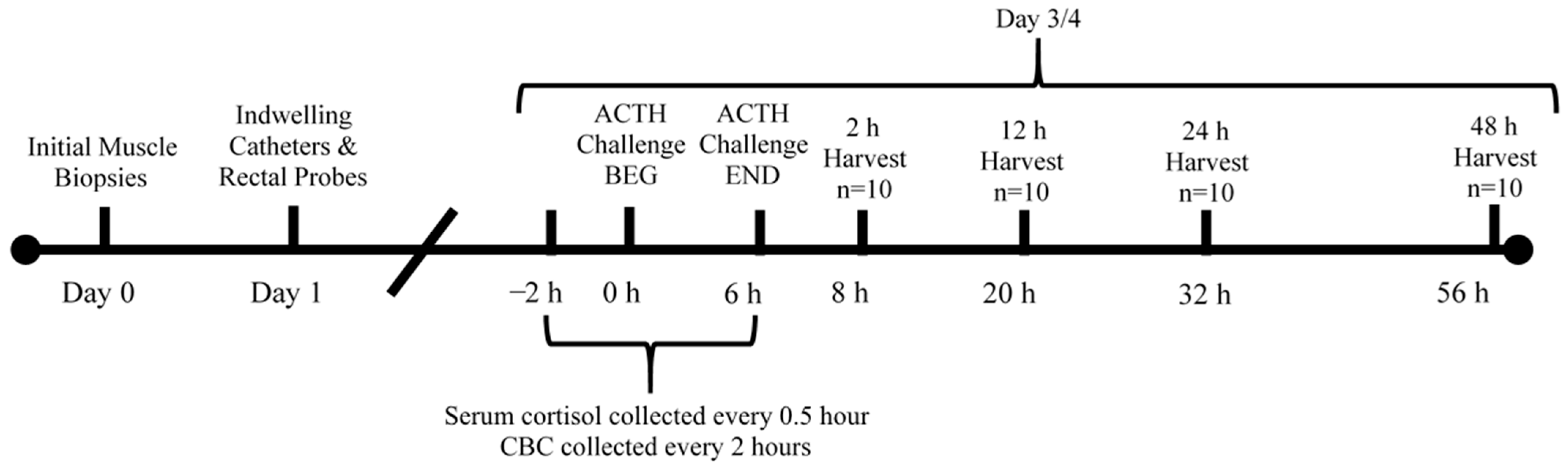
2.1. Initial Skeletal Muscle Samples
2.2. ACTH Injection
2.3. Blood Analyses
2.4. Sample Collection
2.5. Western Blotting
2.6. Myofibrillar Fragmentation Index
2.7. Color and pH
2.8. TBARSs
2.9. Statistical Analysis
3. Results
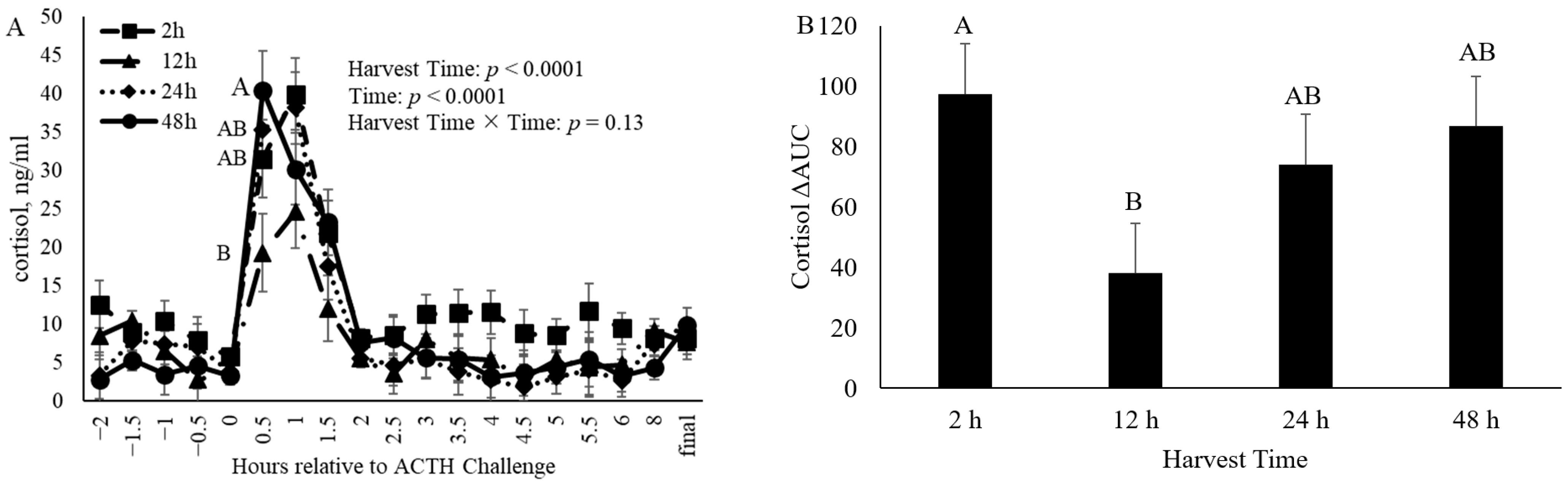
3.1. Cortisol
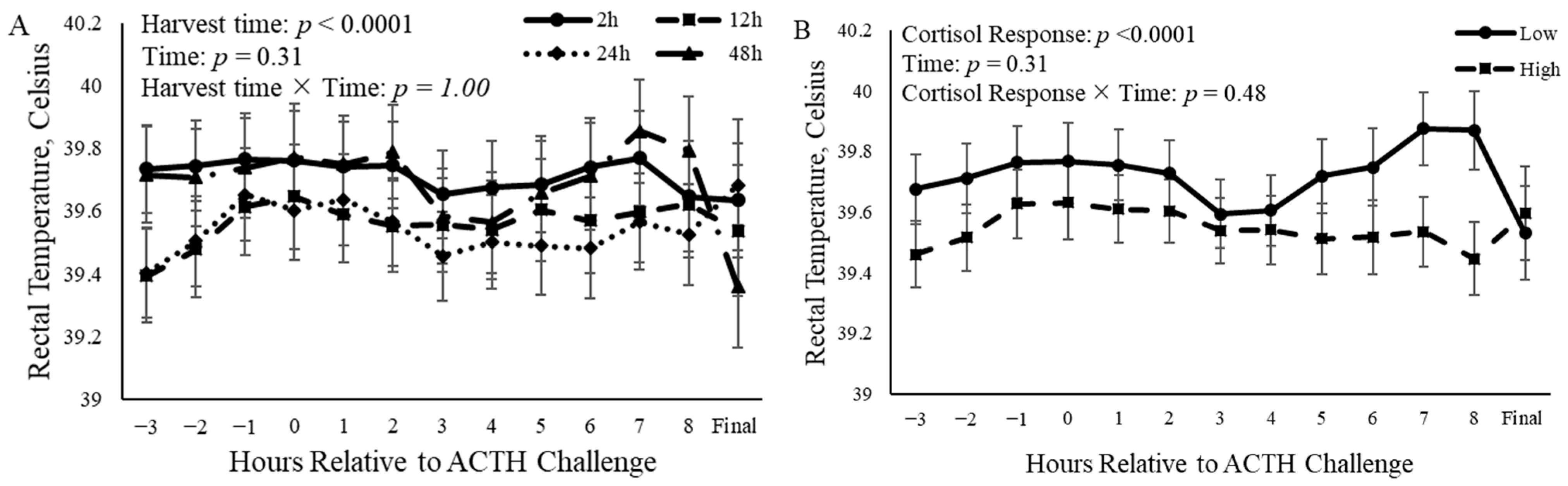
3.2. Rectal Temperature
3.3. Protein Abundance
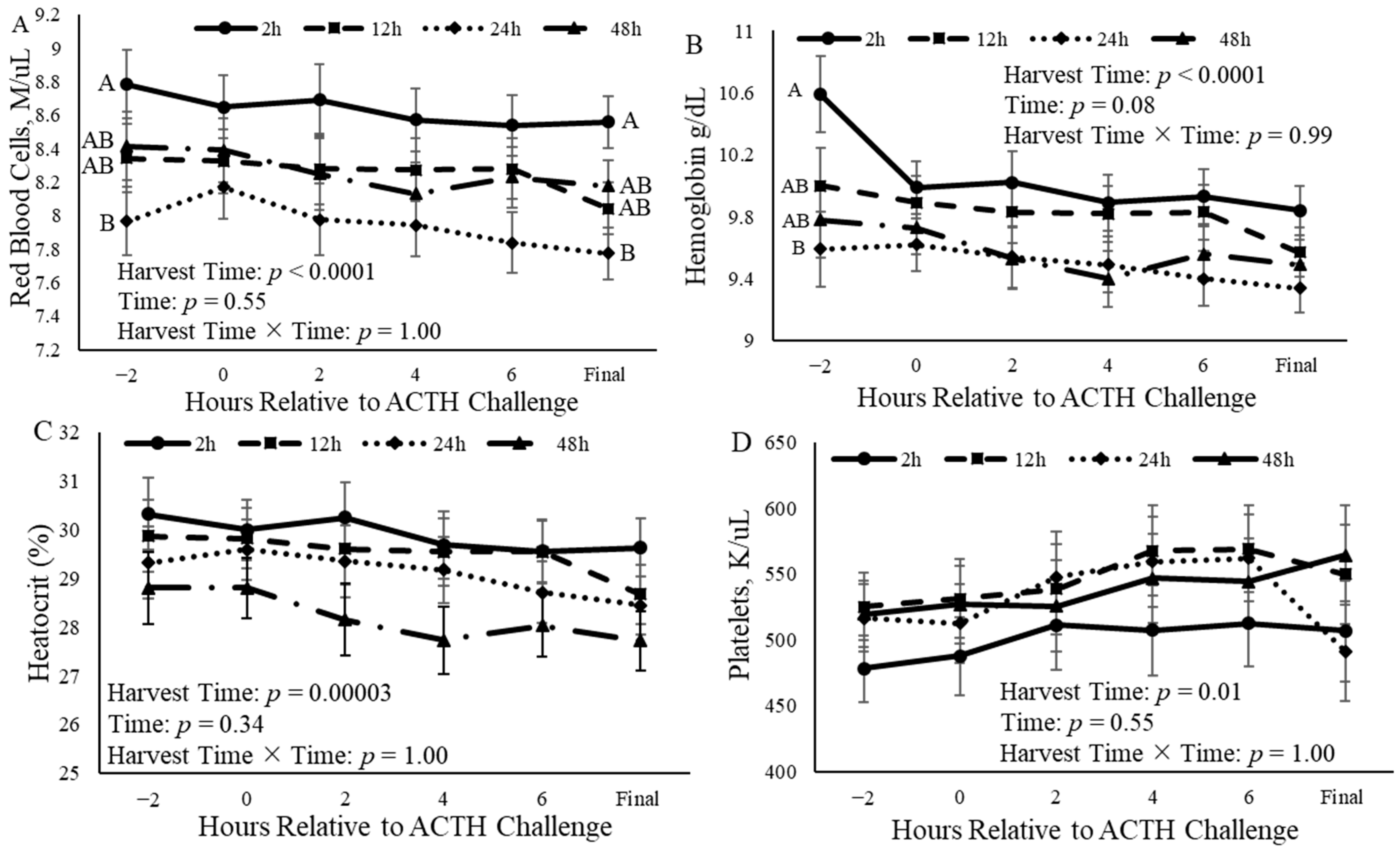
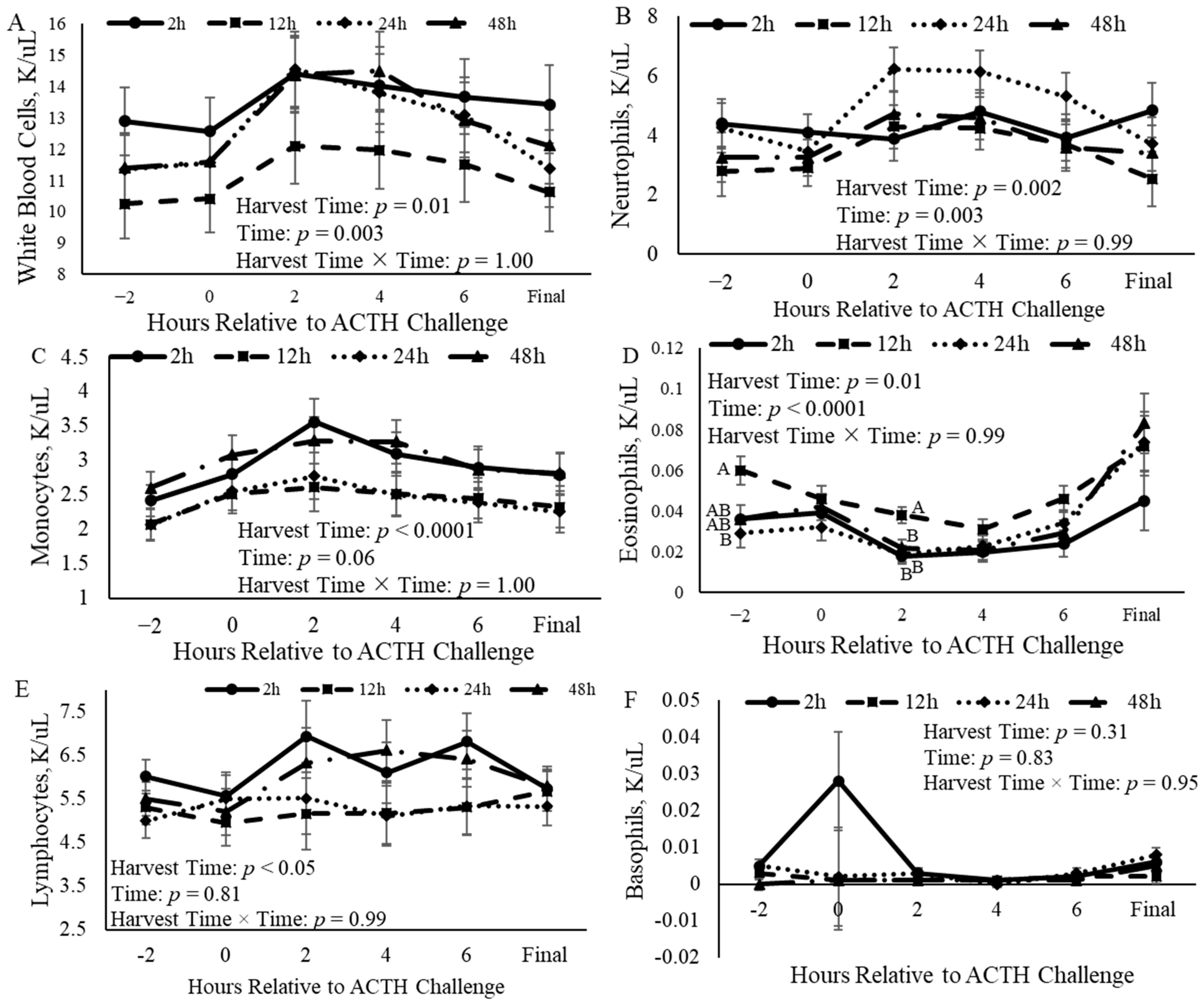
3.4. Complete Blood Counts
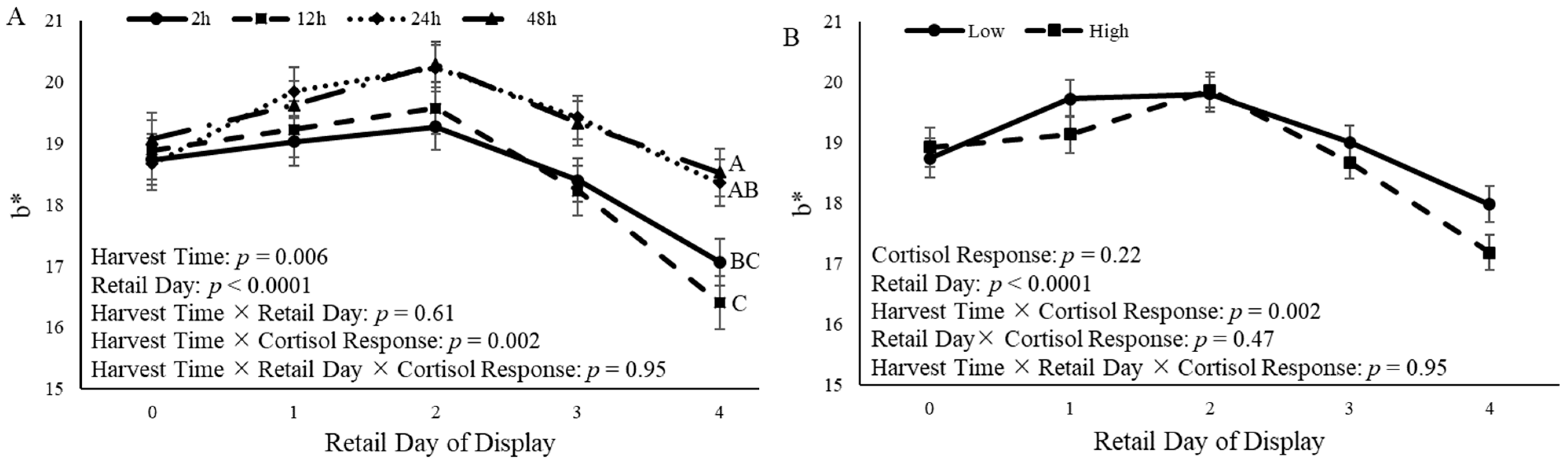
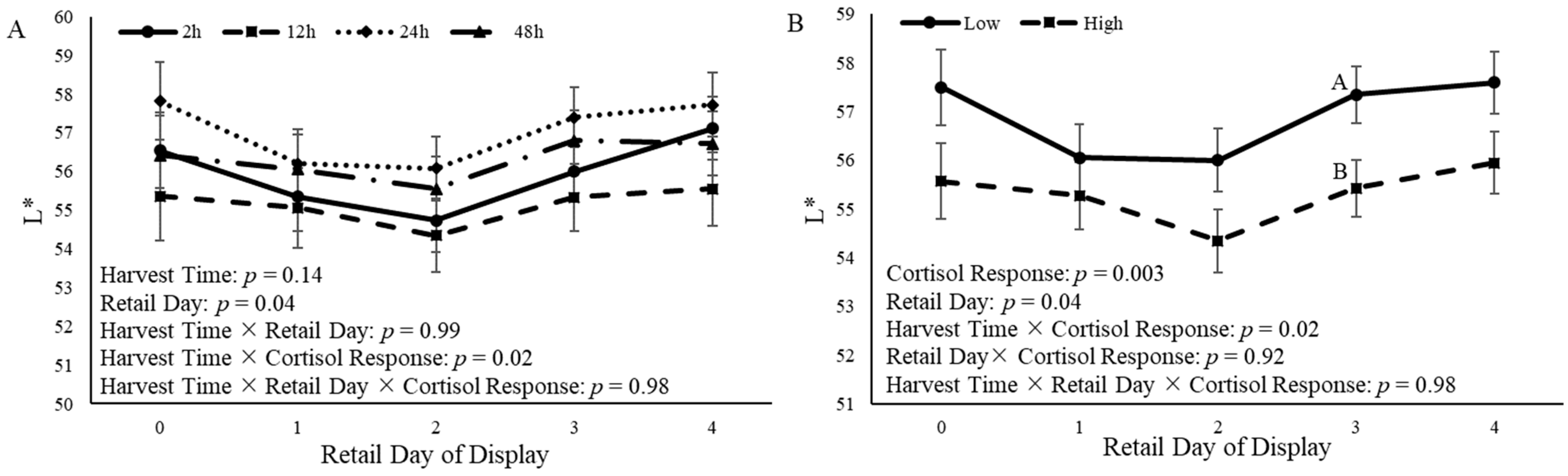
3.5. Steak Color
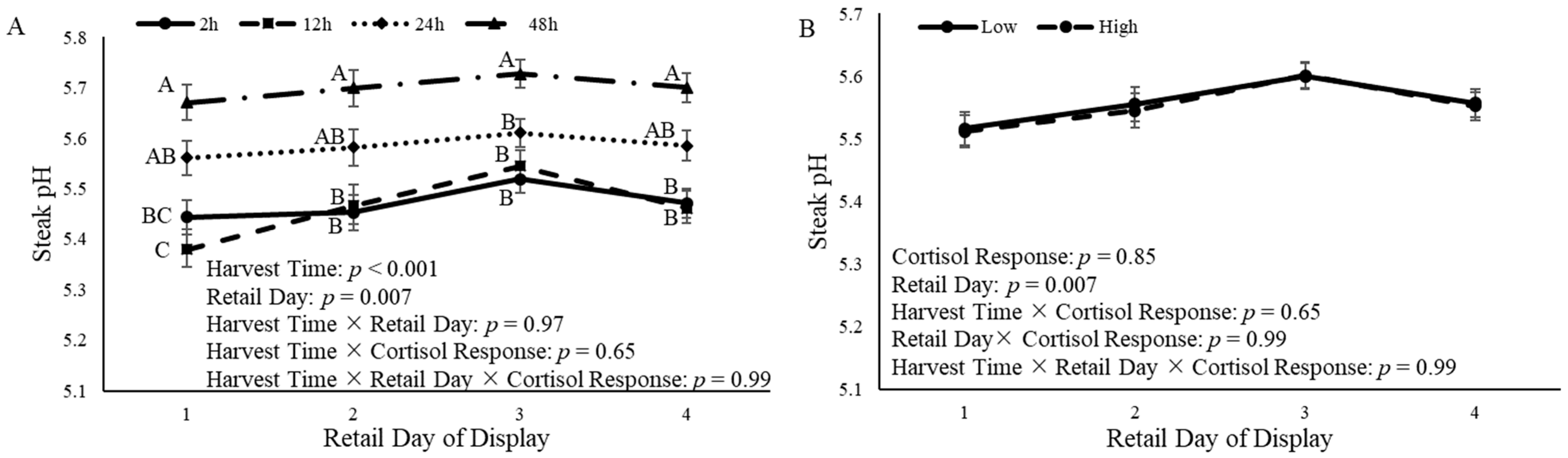
3.6. Steak pH
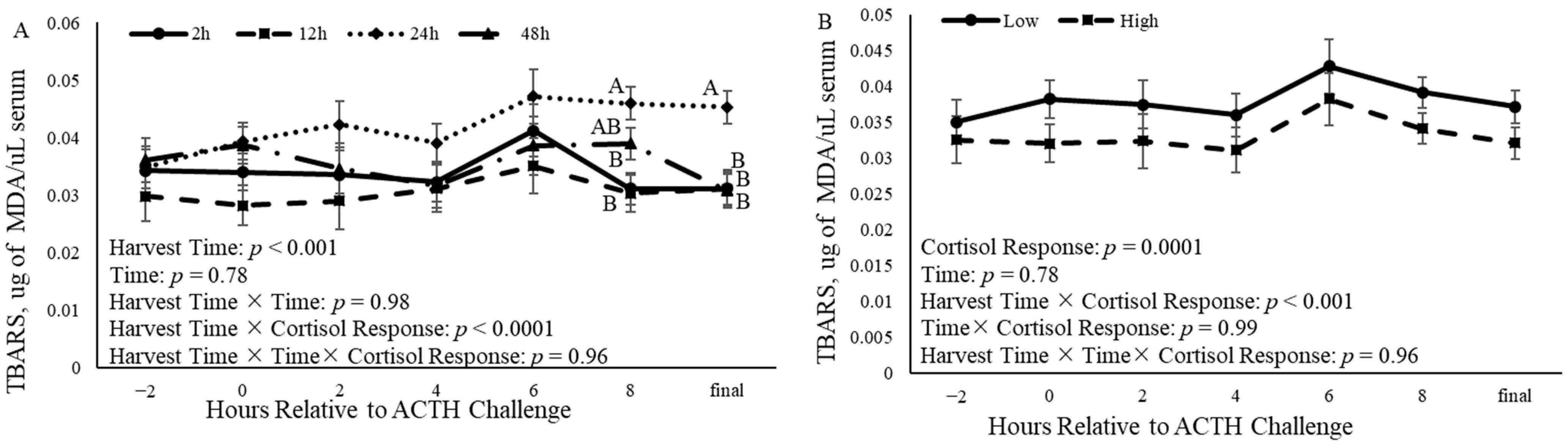
3.7. Oxidation
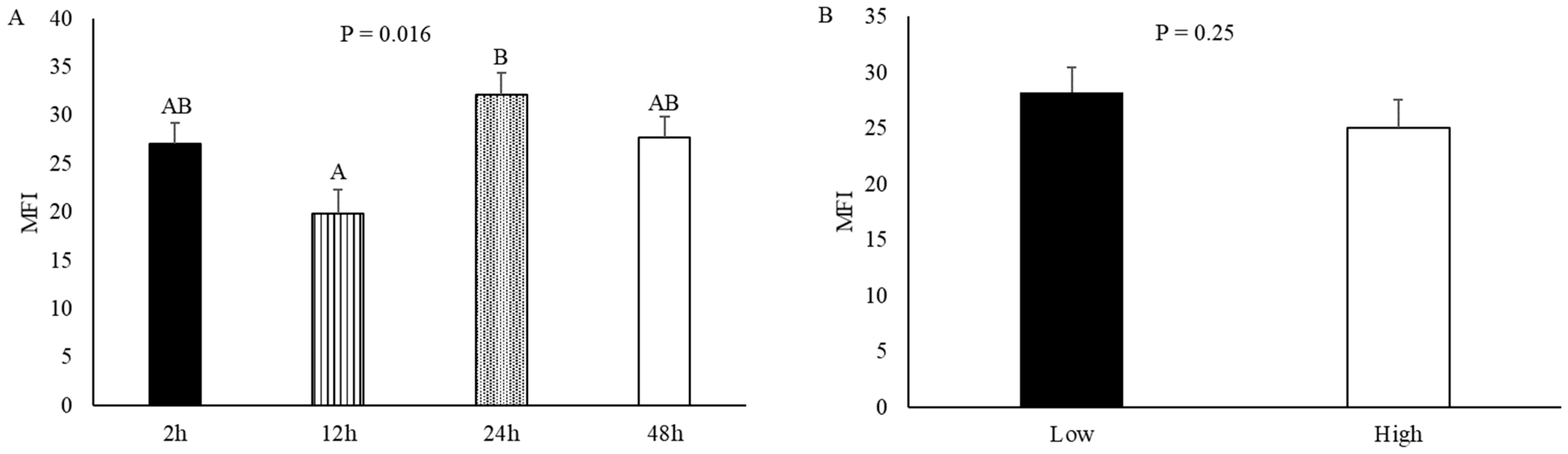
3.8. Myofibrillar Fragmentation Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koohmaraie, M.; Shackelford, S.D.; Wheeler, T.L. Biological Bases That Determine Beef Tenderness; British Society of Animal Science: Fife, UK, 2005. [Google Scholar]
- Mancini, R.A.; Hunt, M.C. Current Research in Meat Color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Hunt, M.C.; Mancini, R.A.; Nair, M.N.; Denzer, M.L.; Suman, S.P.; Mafi, G.G. Recent Updates in Meat Color Research: Integrating Traditional and High-Throughput Approaches. Meat Muscle Biol. 2020, 4. [Google Scholar] [CrossRef]
- Nair, M.N.; Suman, S.P.; Chatli, M.K.; Li, S.; Joseph, P.; Beach, C.M.; Rentfrow, G. Proteome Basis for Intramuscular Variation in Color Stability of Beef Semimembranosus. Meat Sci. 2016, 113, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tian, F.; Yu, Y.; Luo, J.; Mitra, A.; Zhan, F.; Hou, Y.; Liu, G.; Zan, L.; Updike, M.S.; et al. Functional Genomic Analysis of Variation on Beef Tenderness Induced by Acute Stress in Angus Cattle. Comp. Funct. Genom. 2012, 2012, e756284. [Google Scholar] [CrossRef]
- Briggs, R.K.; Christensen, R.C.; Quarnberg, S.M.; Legako, J.F.; Raymond, R.C.; MacNeil, M.D.; Thornton, K.J. Relationship Between Meat Quality, Carcass Characteristics, and Protein Abundance of HSPβ1, HSPA, and DJ1 in Beef Longissimus thoracis Pre-Rigor or After 14 Days’ Aging. Meat Muscle Biol. 2021, 5, 22. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.M.; Wiklund, E.; Young, O.A. Small Heat Shock Proteins and Their Role in Meat Tenderness: A Review. Meat Sci. 2014, 96, 26–40. [Google Scholar] [CrossRef]
- Xing, T.; Gao, F.; Tume, R.; Zhou, G.H.; Xu, X.-L. Stress Effects on Meat Quality: A Mechanistic Perspective: Stress Effects on Meat Quality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.N.; SØrensen, J.G.; Loeschcke, V. Mild Heat Stress at a Young Age in Drosophila Melanogaster Leads to Increased Hsp70 Synthesis after Stress Exposure Later in Life. J. Genet. 2003, 82, 89–94. [Google Scholar] [CrossRef]
- Balan, P.; Kim, Y.H.B.; Blijenburg, R. Small Heat Shock Protein Degradation Could be an Indicator of the Extent of Myofibrillar Protein Degradation. Meat Sci. 2014, 97, 220–222. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large Potentials of Small Heat Shock Proteins. Physiol. Rev. 2011, 91, 1123–1159. [Google Scholar] [CrossRef]
- Joseph, P.; Suman, S.P.; Rentfrow, G.; Li, S.; Beach, C.M. Proteomics of Muscle-Specific Beef Color Stability. J. Agric. Food Chem. 2012, 60, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.P.; Joseph, P. Myoglobin Chemistry and Meat Color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Mitacek, R.M.; Abraham, A.; Mafi, G.G.; VanOverbeke, D.L.; DeSilva, U.; Ramanathan, R. Effects of Muscle-Specific Oxidative Stress on Cytochrome c Release and Oxidation–Reduction Potential Properties. J. Agric. Food Chem. 2017, 65, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Hopkins, D.L.; Giri, K.; Jacobs, J.L.; Plozza, T.; Lewandowski, P.; Bekhit, A. The Use of Oxidative Stress Biomarkers in Live Animals (in Vivo) to Predict Meat Quality Deterioration Postmortem (in Vitro) Caused by Changes in Muscle Biochemical Components. J. Anim. Sci. 2017, 95, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Chapter 8—Inflammatory and Oxidative Stress Markers in Skeletal Muscle of Obese Subjects. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 163–189. ISBN 978-0-12-812504-5. [Google Scholar]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Gagaoua, M.; Claudia Terlouw, E.M.; Boudjellal, A.; Picard, B. Coherent Correlation Networks among Protein Biomarkers of Beef Tenderness: What They Reveal. J. Proteom. 2015, 128, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Veiseth-Kent, E.; Grove, H.; Kuziora, P.; Aass, L.; Hildrum, K.I.; Hollung, K. Peroxiredoxin-6—A Potential Protein Marker for Meat Tenderness in Bovine Longissimus Thoracis Muscle. J. Anim. Sci. 2009, 87, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Laville, E.; Sayd, T.; Morzel, M.; Blinet, S.; Chambon, C.; Lepetit, J.; Renand, G.; Hocquette, J.F. Proteome Changes during Meat Aging in Tough and Tender Beef Suggest the Importance of Apoptosis and Protein Solubility for Beef Aging and Tenderization. J. Agric. Food Chem. 2009, 57, 10755–10764. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.-F.; Terlouw, C.E.M. Inverse Relationships between Biomarkers and Beef Tenderness According to Contractile and Metabolic Properties of the Muscle. J. Agric. Food Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef]
- Thornton, K.J.; Welch, C.M.; Davis, L.C.; Doumit, M.E.; Hill, R.A.; Murdoch, G.K. Bovine Sire Selection based on Maintenance Energy Affects Muscle Fiber Type and Meat Color of F1 Progeny. J. Anim. Sci. 2012, 90, 1617–1627. [Google Scholar] [CrossRef]
- Reuter, R.R.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Galyean, M.L. Technical Note: Development of a Self-Contained, Indwelling Rectal Temperature Probe for Cattle Research. J. Anim. Sci. 2010, 88, 3291–3295. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Reuter, R.R.; Chase, C.C.; Coleman, S.W.; Riley, D.G.; Spiers, D.E.; Arthington, J.D.; Galyean, M.L. Profile of the Bovine Acute-Phase Response Following an Intravenous Bolus-Dose Lipopolysaccharide Challenge. Innate Immun. 2009, 15, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.J.; Chapalamadugu, K.C.; Eldredge, E.M.; Murdoch, G.K. Analysis of Longissimus thoracis Protein Expression Associated with Variation in Carcass Quality Grade and Marbling of Beef Cattle Raised in the Pacific Northwestern United States. J. Agric. Food Chem. 2017, 65, 1434–1442. [Google Scholar] [CrossRef]
- Culler, R.D.; Parrish, F.C.; Smith, G.C.; Cross, H.R. Relationship of Myofibril Fragmentation Index to Certain Chemical, Physical and Sensory Characteristics of Bovine Longissimus Muscle. J. Food Sci. 1978, 43, 1177–1180. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Fleischer, S., Packer, L., Eds.; Biomembranes—Part C: Biological Oxidations; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Luqué, L.D.; Johnson, B.J.; Martin, J.N.; Miller, M.F.; Hodgen, J.M.; Hutcheson, J.P.; Nichols, W.T.; Streeter, M.N.; Yates, D.A.; Allen, D.M.; et al. Zilpaterol Hydrochloride Supplementation Has no Effect on the Shelf Life of Ground Beef. J. Anim. Sci. 2011, 89, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Geesink, G.H. Contribution of Postmortem Muscle Biochemistry to the Delivery of Consistent Meat Quality with Particular Focus on the Calpain System. Meat Sci. 2006, 74, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ouali, A.; Herrera-Mendez, C.H.; Coulis, G.; Becila, S.; Boudjellal, A.; Aubry, L.; Sentandreu, M.A. Revisiting the Conversion of Muscle into Meat and the Underlying Mechanisms. Meat Sci. 2006, 74, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.; Gannon, J.; O’Connell, K.; Ohlendieck, K. Aging Skeletal Muscle Shows a Drastic Increase in the Small Heat Shock Proteins αB-Crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol. 2007, 86, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Pulford, D.J.; Fraga Vazquez, S.; Frost, D.F.; Fraser-Smith, E.; Dobbie, P.; Rosenvold, K. The Intracellular Distribution of Small Heat Shock Proteins in Post-Mortem Beef Is Determined by Ultimate pH. Meat Sci. 2008, 79, 623–630. [Google Scholar] [CrossRef]
- Giannetto, C.; Fazio, F.; Casella, S.; Marafioti, S.; Giudice, E.; Piccione, G. Acute Phase Protein Response during Road Transportation and Lairage at a Slaughterhouse in Feedlot Beef Cattle. J. Vet. Med. Sci. 2011, 73, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, H.; Kariya, Y. Effects of Peripheral Blood Polymorphonuclear Leukocyte Function and Blood Components in Japanese Black Steers Administered ACTH in a Cold Environment. J. Vet. Med. Sci. 1999, 61, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Aengwanich, W.; Kongbuntad, W.; Boonsorn, T. Effects of Shade on Physiological Changes, Oxidative Stress, and Total Antioxidant Power in Thai Brahman Cattle. Int. J. Biometeorol. 2011, 55, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lomborg, S.R.; Nielsen, L.R.; Heegaard, P.M.H.; Jacobsen, S. Acute Phase Proteins in Cattle after Exposure to Complex Stress. Vet. Res. Commun. 2008, 32, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Stockman, C.A.; Collins, T.; Barnes, A.L.; Miller, D.; Wickham, S.L.; Beatty, D.T.; Blache, D.; Wemelsfelder, F.; Fleming, P.A. Qualitative Behavioural Assessment and Quantitative Physiological Measurement of Cattle Naïve and Habituated to Road Transport. Anim. Prod. Sci. 2011, 51, 240–249. [Google Scholar] [CrossRef]
- Båge, R.; Forsberg, M.; Gustafsson, H.; Larsson, B.; Rodríguez-Martínez, H. Effect of ACTH-Challenge on Progesterone and Cortisol Levels in Ovariectomised Repeat Breeder Heifers. Anim. Reprod. Sci. 2000, 63, 65–76. [Google Scholar] [CrossRef]
- Schwinn, A.-C.; Sauer, F.J.; Gerber, V.; Bruckmaier, R.M.; Gross, J.J. Free and Bound Cortisol in Plasma and Saliva during ACTH Challenge in Dairy Cows and Horses. J. Anim. Sci. 2018, 96, 76–84. [Google Scholar] [CrossRef]
- Verkerk, G.A.; Macmillan, K.L.; McLeay, L.M. Adrenal Cortex Response to Adrenocorticotropic Hormone in Dairy Cattle. Domest. Anim. Endocrinol. 1994, 11, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, S.M.; Carroll, J.A.; Keisler, D.H.; Sartin, J.L.; Elsasser, T.H.; Buntyn, J.O.; Broadway, P.R.; Schmidt, T.B. Evaluation of the Endocrine response of cattle during the relocation process. Livest. Sci. 2013, 151, 203–212. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Willard, S.T.; Vann, R.C.; Welsh, T.H.; Randel, R.D. Relationships between Temperament and Transportation with Rectal Temperature and Serum Concentrations of Cortisol and Epinephrine in Bulls. Livest. Sci. 2010, 129, 166–172. [Google Scholar] [CrossRef]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of Heat Stress on Health and Performance of Dairy Animals: A Review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Kirbach, B.; Golenhofen, N. Reaction of Small Heat-Shock Proteins to Different Kinds of Cellular Stress in Cultured Rat Hippocampal Neurons. Cell Stress. Chaperones 2014, 19, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.V.; Stafuzza, N.B.; Lima, S.B.G.P.N.P.; Negrão, J.A.; Paz, C.C.P. Differential Expression of Heat Shock Protein Genes Associated with Heat Stress in Nelore and Caracu Beef Cattle. Livest. Sci. 2019, 230, 103839. [Google Scholar] [CrossRef]
- Deng, L.; Yang, G.; Zhang, C.; Zhang, Z.; Xu, L.; He, C. Variation in Expression of HSPs and Their Corresponding mRNA Transcripts in the Peripheral Blood Lymphocytes of Beef Cattle by Transportation Durations. J. Anim. Vet. Adv. 2013, 12, 612–617. [Google Scholar]
- Whipple, G.; Koohmaraie, M.; Dikeman, M.E.; Crouse, J.; Hunt, M.C.; Klemm, R. Evaluation of Attributes that Affect Longissimus Muscle Tenderness in Bos Taurus and Bos Indicus Cattle. J. Anim. Sci. 1990, 68, 2716–2728. [Google Scholar] [CrossRef]
- del Campo, M.; Brito, G.; Soares de Lima, J.; Hernández, P.; Montossi, F. Finishing Diet, Temperament and Lairage Time Effects on Carcass and Meat Quality Traits in Steers. Meat Sci. 2010, 86, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.A.d.S.; Ramos, P.M.; da Luz e Silva, S.; Martello, L.S.; Pereira, A.S.C.; Delgado, E.F. Divergent Temperaments Are Associated with Beef Tenderness and the Inhibitory Activity of Calpastatin. Meat Sci. 2017, 134, 61–67. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Koohmaraie, M.; Whipple, G.; Wheeler, T.L.; Miller, M.F.; Crouse, J.D.; Reagan, J.O. Predictors of Beef Tenderness: Development and Verification. J. Food Sci. 1991, 56, 1130–1135. [Google Scholar] [CrossRef]
- Farah, C.S.; Reinach, F.C. The Troponin Complex and Regulation of Muscle Contraction. FASEB J. 1995, 9, 755–767. [Google Scholar] [CrossRef]
- Carlson, K.B.; Prusa, K.J.; Fedler, C.A.; Steadham, E.M.; Outhouse, A.C.; King, D.A.; Huff-Lonergan, E.; Lonergan, S.M. Postmortem Protein Degradation Is a Key Contributor to Fresh Pork Loin Tenderness. J. Anim. Sci. 2017, 95, 1574–1586. [Google Scholar] [CrossRef]
- Franco, D.; Mato, A.; Salgado, F.J.; López-Pedrouso, M.; Carrera, M.; Bravo, S.; Parrado, M.; Gallardo, J.M.; Zapata, C. Tackling Proteome Changes in the Longissimus Thoracis Bovine Muscle in Response to Pre-Slaughter Stress. J. Proteom. 2015, 122, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hayes, N.S.; Schwartz, C.A.; Phelps, K.J.; Borowicz, P.; Maddock-Carlin, K.R.; Maddock, R.J. The Relationship between Pre-Harvest Stress and the Carcass Characteristics of Beef Heifers That Qualified for Kosher Designation. Meat Sci. 2015, 100, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Chirase, N.K.; Greene, L.W.; Purdy, C.W.; Loan, R.W.; Auvermann, B.W.; Parker, D.B.; Walborg, E.F.; Stevenson, D.E.; Xu, Y.; Klaunig, J.E. Effect of Transport Stress on Respiratory Disease, Serum Antioxidant Status, and Serum Concentrations of Lipid Peroxidation Biomarkers in Beef Cattle. Am. J. Vet. Res. 2004, 65, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Urban-Chmiel, R.; Kankofer, M.; Mikucki, P.; Puchalski, A. Evaluation of Plasma Cortisol and TBARS Levels in Calves after Short—Term Transportation. Rev. Méd. Vét. 2006, 157, 30. [Google Scholar]
- Grewal, A.; Ahuja, C.S.; Singha, S.P.S.; Chaudhary, K.C. Status of Lipid Peroxidation, Some Antioxidant Enzymes and Erythrocytic Fragility of Crossbred Cattle Naturally Infected with Theileria annulata. Vet. Res. Commun. 2005, 29, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Influence of Early Postmortem Protein Oxidation on Beef Quality. J. Anim. Sci. 2004, 82, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.M.; Shaw, F.D.; Stark, J.L. Effect of Reduced Lairage Duration on Beef Quality. Aust. J. Exp. Agric. 2007, 47, 770–773. [Google Scholar] [CrossRef]
- Teke, B.; Akdag, F.; Ekiz, B.; Ugurlu, M. Effects of Different Lairage Times after Long Distance Transportation on Carcass and Meat Quality Characteristics of Hungarian Simmental Bulls. Meat Sci. 2014, 96, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Lizondo, G.; Knowles, T. Effects of Journey and Lairage Time on Steers Transported to Slaughter in Chile. Vet. Rec. 2003, 152, 361–364. [Google Scholar] [CrossRef]
- Carrasco-García, A.A.; Pardío-Sedas, V.T.; León-Banda, G.G.; Ahuja-Aguirre, C.; Paredes-Ramos, P.; Hernández-Cruz, B.C.; Murillo, V.V. Effect of Stress during Slaughter on Carcass Characteristics and Meat Quality in Tropical Beef Cattle. Asian-Australas. J. Anim. Sci. 2020, 33, 1656–1665. [Google Scholar] [CrossRef]
- Kiyimba, F.; Hartson, S.D.; Rogers, J.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Changes in Glycolytic and Mitochondrial Protein Profiles Regulates Postmortem Muscle Acidification and Oxygen Consumption in Dark-Cutting Beef. J. Proteom. 2021, 232, 104016. [Google Scholar] [CrossRef] [PubMed]
- Strappini, A.C.; Frankena, K.; Metz, J.H.M.; Gallo, B.; Kemp, B. Prevalence and Risk Factors for Bruises in Chilean Bovine Carcasses. Meat Sci. 2010, 86, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; Bekhit, A.E. Causes and Contributing Factors to “Dark Cutting” Meat: Current Trends and Future Directions: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 400–430. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D.; Ferguson, D.M.; Cottrell, J.J.; Knee, B.W.; Warner, R.D.; Ferguson, D.M.; Cottrell, J.J.; Knee, B.W. Acute Stress Induced by the Preslaughter Use of Electric Prodders Causes Tougher Beef Meat. Aust. J. Exp. Agric. 2007, 47, 782–788. [Google Scholar] [CrossRef]
- King, D.A.; Schuehle Pfeiffer, C.E.; Randel, R.D.; Welsh, T.H.; Oliphint, R.A.; Baird, B.E.; Curley, K.O.; Vann, R.C.; Hale, D.S.; Savell, J.W. Influence of Animal Temperament and Stress Responsiveness on the Carcass Quality and Beef Tenderness of Feedlot Cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef]
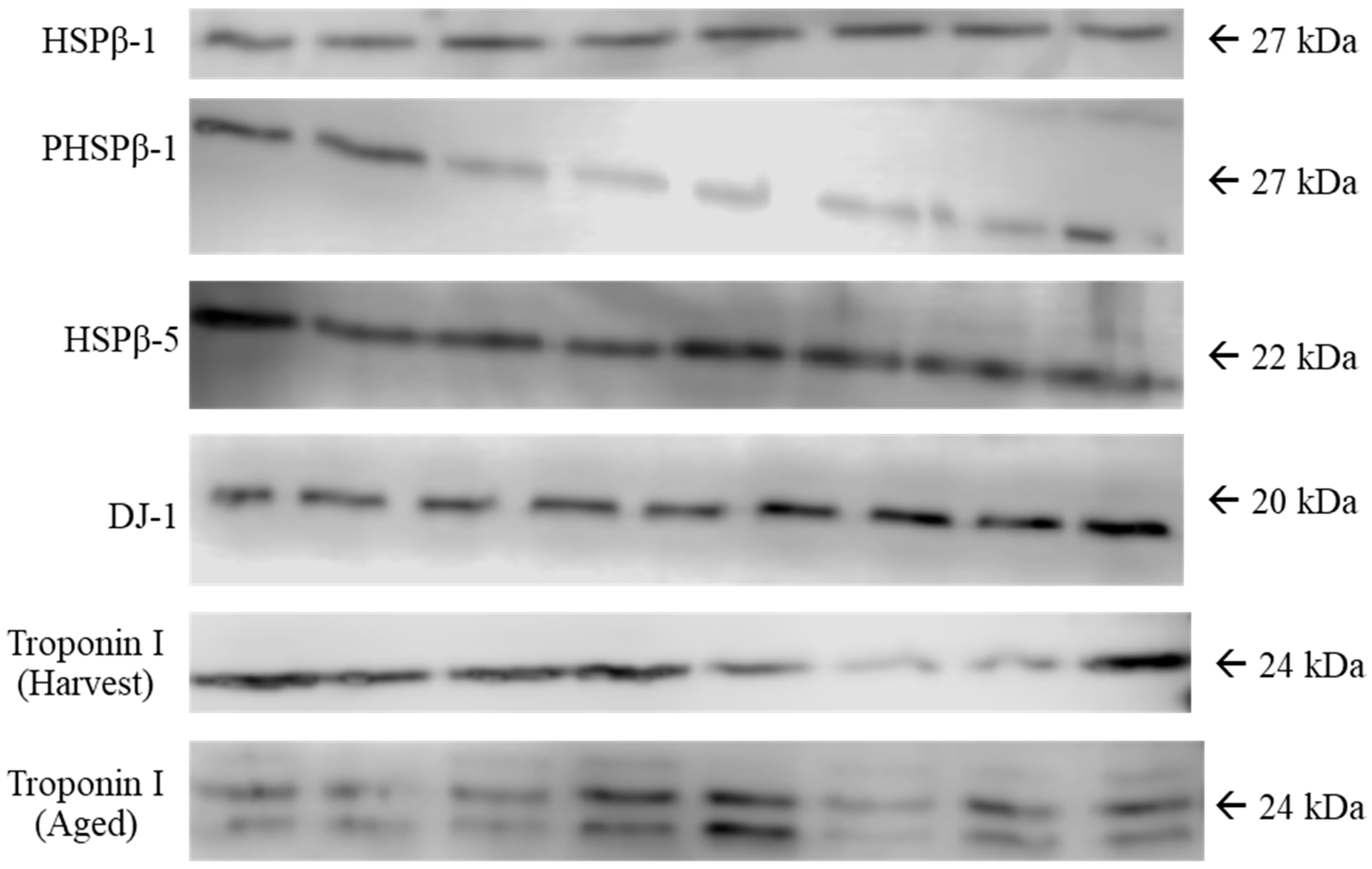



| Protein Name a | Antibody Company | Host | Product Number | Primary Concentration | Secondary Concentration |
|---|---|---|---|---|---|
| HSPβ-1 | Invitrogen b | rabbit | PA1-25494 | 1:500 | 1:1000 |
| PHSPβ-1 | Invitrogen b | rabbit | PA5-23340 | 1:2000 | 1:1000 |
| HSPβ-5 | Invitrogen b | mouse | MAS-27708 | 1:4000 | 1:7500 |
| DJ1 | Abcam c | rabbit | ab18257 | 1:1000 | 1:1000 |
| Troponin | Invitrogen b | rabbit | PA5-42108 | 1:500 | 1:1000 |
| Harvest Time Relative to End of ACTH Challenge a | ||||||
|---|---|---|---|---|---|---|
| 2 h b | 12 h b | 24 h b | 48 h b | SEM c | p-Value x | |
| Harvest | ||||||
| HSPβ1 d | 2.26 | 6.10 | 4.15 | 1.13 | 1.54 | 0.22 |
| P-HSPβ1 e | 122.83 xy | −86.06 x | 45.73 xy | 380.38 y | 107.03 | 0.03 |
| HSPβ5 f | −0.20 | 2.27 | 7.66 | −0.41 | 3.75 | 0.67 |
| DJ1 g | 6.44 xy | −17.76 x | 73.25 y | 16.10 xy | 20.10 | 0.002 |
| Troponin h | −24.52 | 127.26 | 995.10 | 162.52 | 320.74 | 0.22 |
| 14 d Aged | ||||||
| HSPβ1 d | −0.02 | 5.48 | 2.05 | 1.94 | 1.10 | 0.03 |
| P-HSPβ1 e | 0.26 x | 0.97 y | 0.54 xy | 0.16 x | 0.15 | 0.001 |
| HSPβ5 f | −0.73 | 11.05 | 25.66 | −2.31 | 9.82 | 0.43 |
| DJ-1 g | 2.55 x | −5.18 x | 54.57 y | 12.32 x | 7.72 | <0.0001 |
| Troponin— Entire Band i | 1.07 xy | 0.19 x | 2.98 xy | 3.37 y | 0.73 | 0.01 |
| Troponin— Lower Band j | 0.31 x | 0.21 x | 0.48 xy | 1.34 y | 0.24 | 0.02 |
| Cortisol Response a | ||||
|---|---|---|---|---|
| Low b | High b | SEM c | p-Value x | |
| Harvest | ||||
| HSPβ1 d | 2.29 | 4.14 | 1.15 | 0.29 |
| P-HSPβ1 e | 210.32 | 21.12 | 80.04 | 0.15 |
| HSPβ5 f | 0.52 | 4.14 | 2.80 | 0.40 |
| DJ1 g | 38.77 | 0.24 | 15.03 | 0.07 |
| Troponin h | 188.44 | 441.74 | 239.86 | 0.49 |
| 14 d Aged | ||||
| HSPβ1 d | 1.52 | 3.20 | 1.67 | 0.19 |
| P-HSPβ1 e | 0.45 | 0.51 | 0.11 | 0.81 |
| HSPβ5 f | 2.14 | 14.69 | 7.34 | 0.25 |
| DJ-1 g | 23.49 | 8.64 | 5.99 | 0.08 |
| Troponin— Entire Band i | 2.14 | 1.66 | 0.54 | 0.52 |
| Troponin— Lower Band j | 0.56 | 0.61 | 0.18 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briggs, R.K.; Legako, J.F.; Broadway, P.R.; Carroll, J.A.; Burdick Sanchez, N.C.; Ineck, N.E.; Smith, Z.K.; Ramanathan, R.; Thornton, K.J. Effects of Premortem Stress on Protein Expression, Steak Color, Oxidation, and Myofibrillar Fragmentation Index in the Longissimus Lumborum. Animals 2024, 14, 2170. https://doi.org/10.3390/ani14152170
Briggs RK, Legako JF, Broadway PR, Carroll JA, Burdick Sanchez NC, Ineck NE, Smith ZK, Ramanathan R, Thornton KJ. Effects of Premortem Stress on Protein Expression, Steak Color, Oxidation, and Myofibrillar Fragmentation Index in the Longissimus Lumborum. Animals. 2024; 14(15):2170. https://doi.org/10.3390/ani14152170
Chicago/Turabian StyleBriggs, Reganne K., Jerrad F. Legako, Paul R. Broadway, Jeff A. Carroll, Nicole C. Burdick Sanchez, Nikole E. Ineck, Zachary K. Smith, Ranjith Ramanathan, and Kara J. Thornton. 2024. "Effects of Premortem Stress on Protein Expression, Steak Color, Oxidation, and Myofibrillar Fragmentation Index in the Longissimus Lumborum" Animals 14, no. 15: 2170. https://doi.org/10.3390/ani14152170






