The Role of Erythropoietin in Bovine Sperm Physiology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Samples
2.2. Motility Assessment
2.3. Viability Assessment
2.4. Determination of Total Antioxidant Capacity (TAC)
2.5. Quantification of the Superoxide Anion (O2−) Production (Nitroblue Tetrazolium Test-NBT)
2.6. Capacitation and Acrosome Reaction
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. EPO Preserved the Motility of Spermatozoa
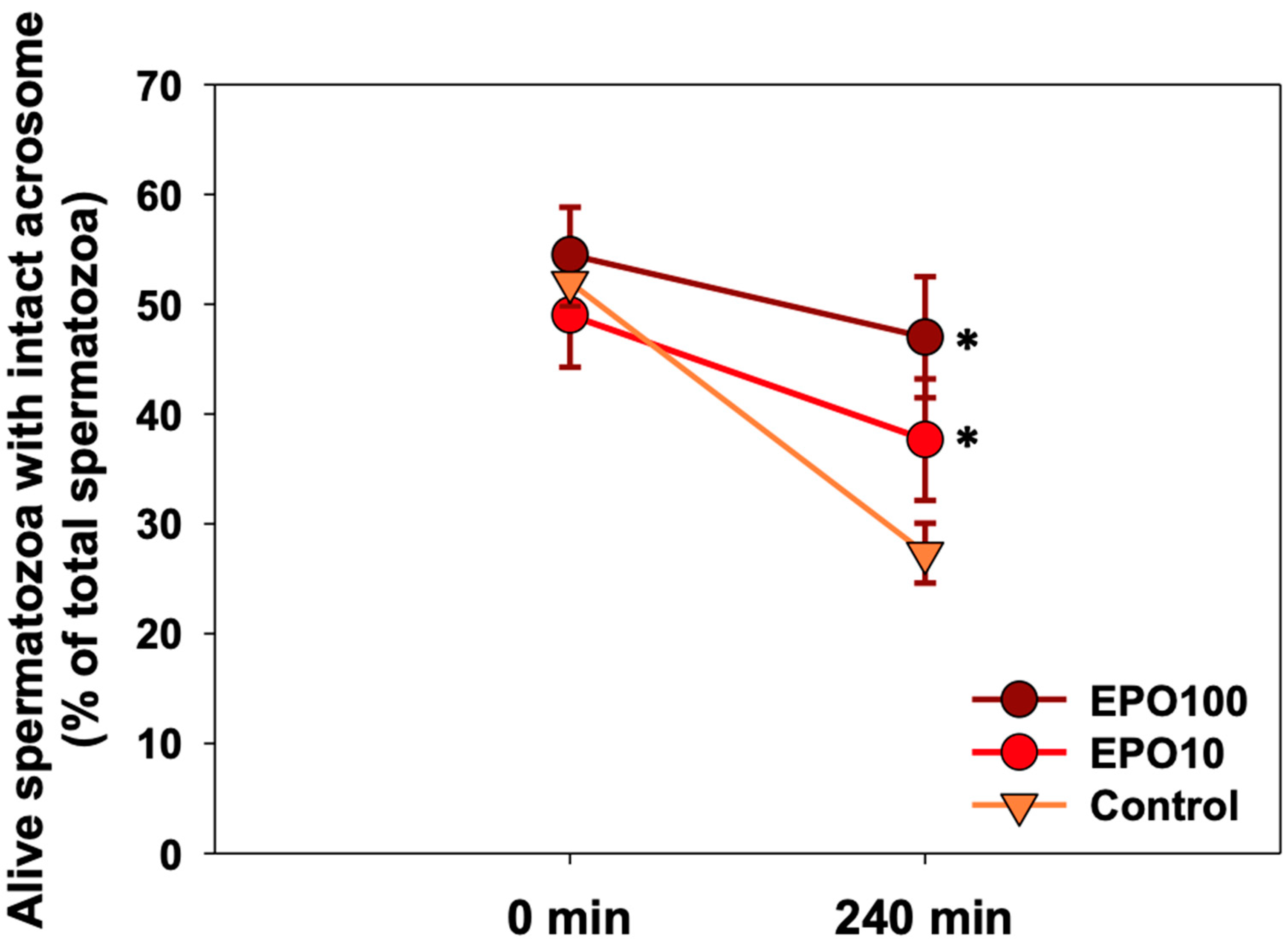
3.2. EPO Had a Positive Effect on Sperm Viability
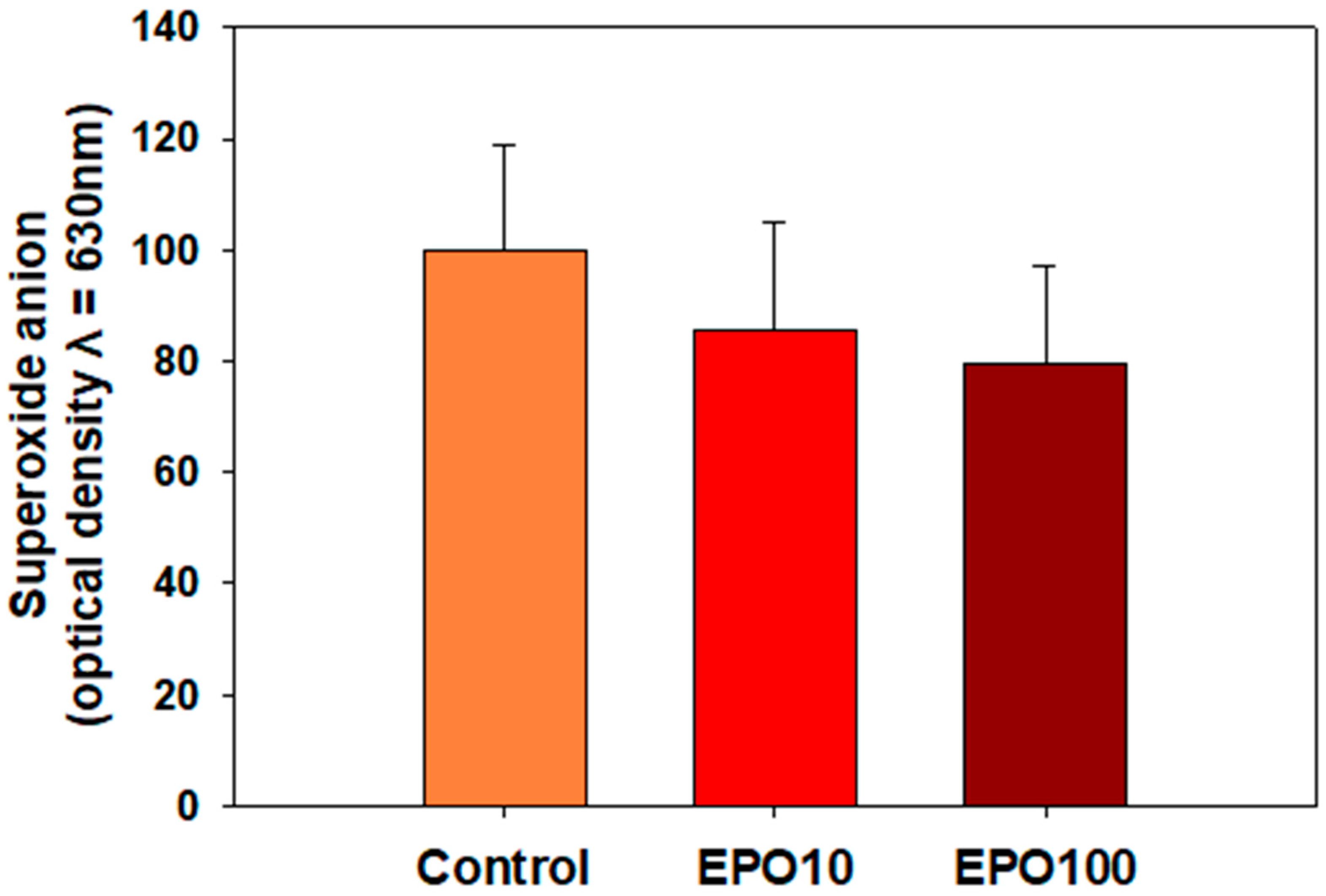
3.3. EPO Showed a Trend to Reduce O2−
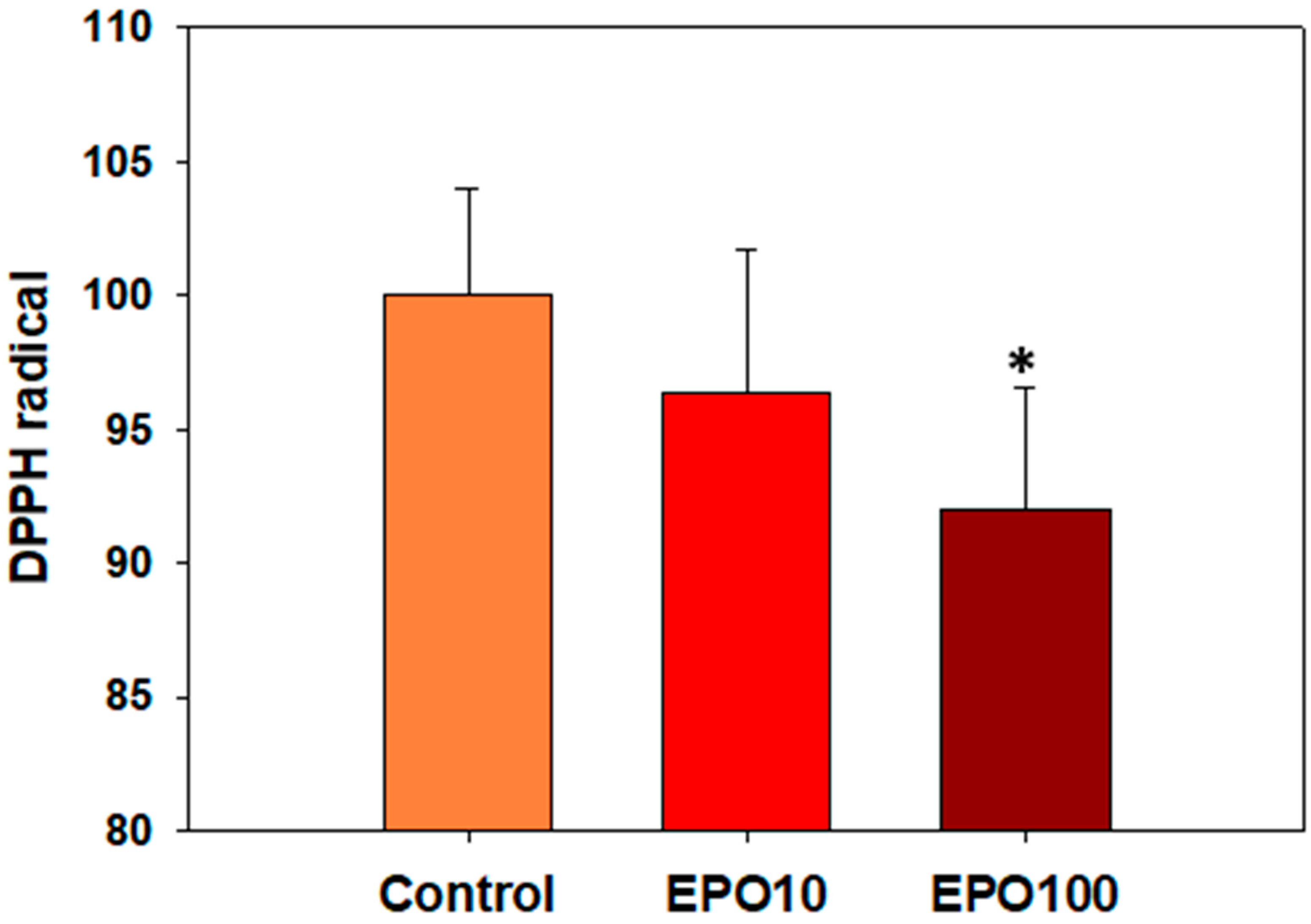
3.4. ΕΡO. Augmented the Total Antioxidant Capacity (TAC) of Spermatozoa
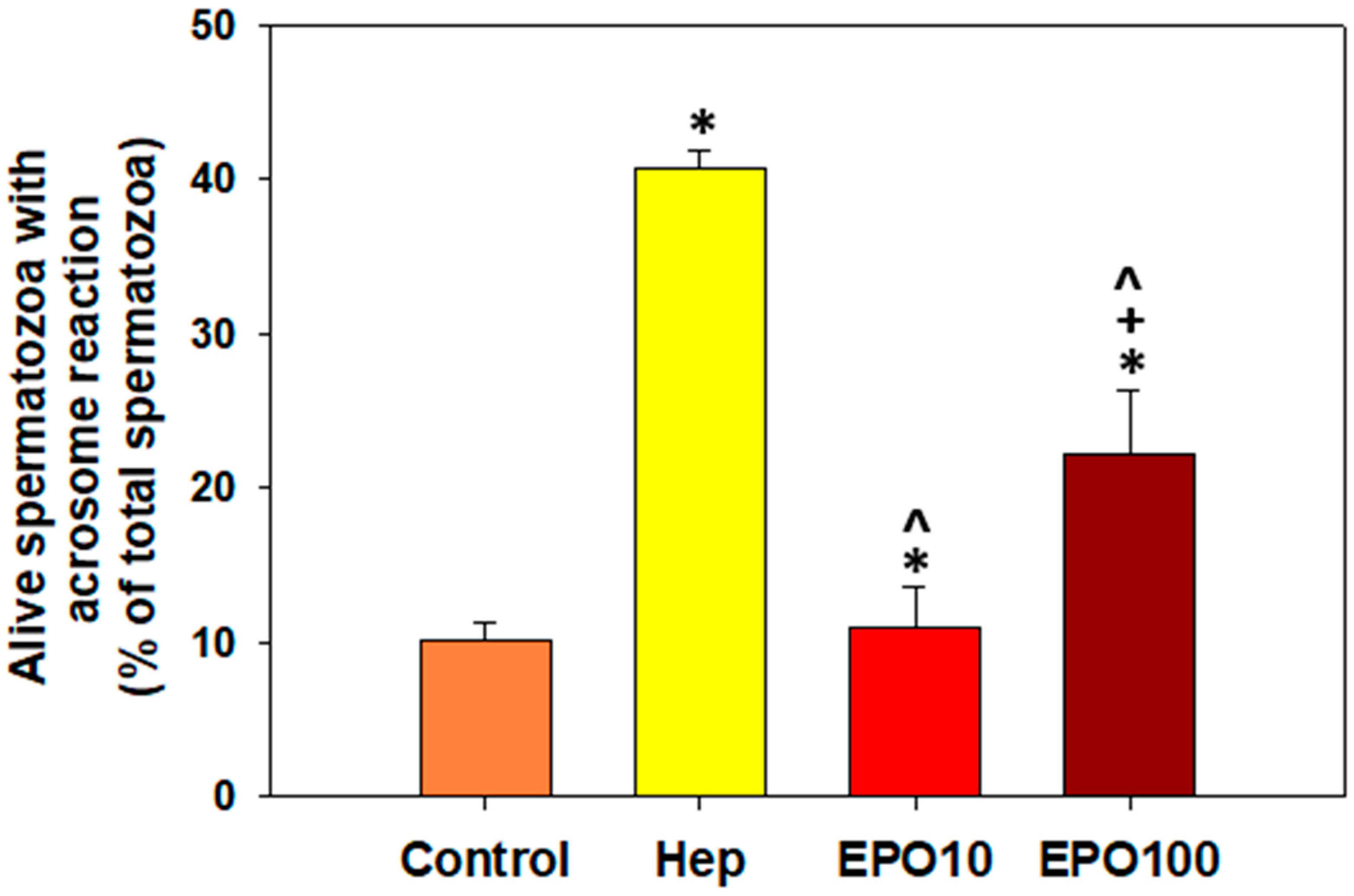
3.5. EPOInduced Capacitation and Acrosome Reaction in Spermatozoa Under Capacitating Conditions
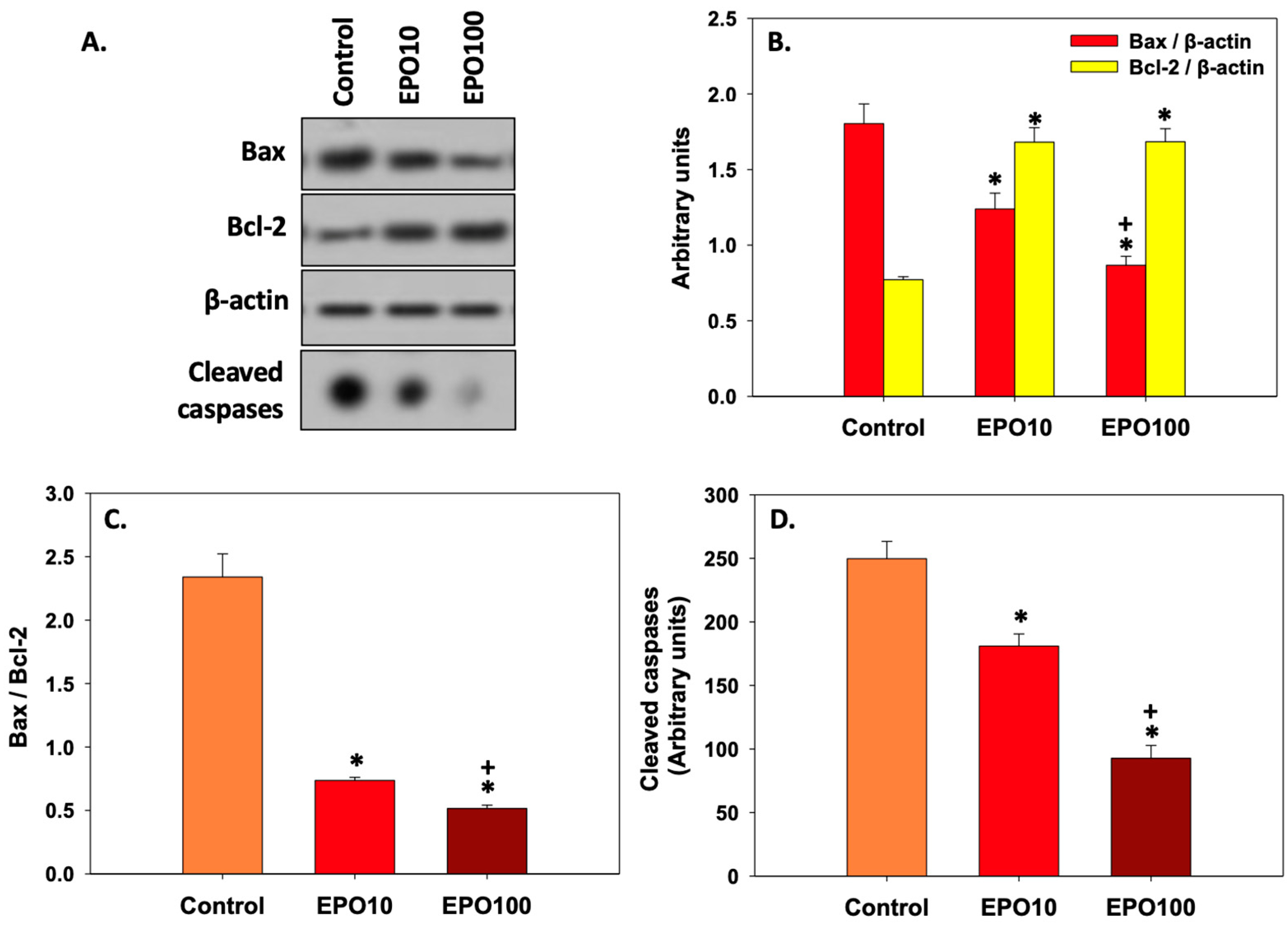
3.6. EPOInhibited Apoptosis in Spermatozoa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishibashi, T.; Koziol, J.A.; Burstein, S.A. Human recombinant erythropoietin promotes differentiation of murine megakaryocytes in vitro. J. Clin. Investig. 1987, 79, 286–289. [Google Scholar] [CrossRef]
- Koury, M.J.; Bondurant, M.C. The molecular mechanism of erythropoietin action. Eur. J. Biochem. 1992, 210, 649–663. [Google Scholar] [CrossRef]
- Lombardero, M.; Kovacs, K.; Scheithauer, B.W. Erythropoietin: A hormone with multiple functions. Pathobiology 2011, 78, 41–53. [Google Scholar] [CrossRef]
- Magnanti, M.; Gandini, O.; Giuliani, L.; Gazzaniga, P.; Marti, H.H.; Gradilone, A.; Frati, L.; Aglianò, A.M.; Gassmann, M. Erythropoietin expression in primary rat Sertoli and peritubular myoid cells. Blood 2001, 98, 2872–2874. [Google Scholar] [CrossRef]
- Sawyer, S.T.; Penta, K. Association of JAK2 and STAT5 with erythropoietin receptors. Role of receptor phosphorylation in erythropoietin signal transduction. J. Biol. Chem. 1996, 271, 32430–32437. [Google Scholar] [CrossRef]
- Silva, M.; Benito, A.; Sanz, C.; Prosper, F.; Ekhterae, D.; Nuñez, G.; Fernandez-Luna, J.L. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J. Biol. Chem. 1999, 274, 22165–22169. [Google Scholar] [CrossRef]
- Oztürk, E.; Demirbilek, S.; Köroğlu, A.; But, A.; Begeç, Z.O.; Gülec, M.; Akyol, O.; Ersoy, M.O. Propofol and erythropoietin antioxidant properties in rat brain injured tissue. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 81–86. [Google Scholar] [CrossRef]
- Cung, T.; Wang, H.; Hartnett, M.E. The Effects of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase and Erythropoietin, and Their Interactions in Angiogenesis: Implications in Retinopathy of Prematurity. Cells 2022, 11, 1951. [Google Scholar] [CrossRef]
- Metallinou, C.; Staneloudi, C.; Nikolettos, K.; Asimakopoulos, B. NGF, EPO, and IGF-1 in the Male Reproductive System. J. Clin. Med. 2024, 13, 2918. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yanase, H.; Iwanaga, T.; Sasaki, R.; Nagao, M. Epididymis is a novel site of erythropoietin production in mouse reproductive organs. Biochem. Biophys. Res. Commun. 2002, 296, 145–151. [Google Scholar] [CrossRef]
- Yasuda, Y.; Fujita, Y.; Musha, T.; Tanaka, H.; Shiokawa, S.; Nakamatsu, K.; Mori, S.; Matsuo, T.; Nakamura, Y. Expression of erythropoietin in human female reproductive organs. Ital. J. Anat. Embryol. 2001, 106, 215–222. [Google Scholar]
- Temma, K.; Shimoya, K.; Hashimoto, K.; Zhang, Q.; Koyama, M.; Murata, Y. Detection of erythropoietin in human seminal plasma. Fertil. Steril. 2004, 81, 798–801. [Google Scholar] [CrossRef]
- Tug, N.; Kilic, U.; Karateke, A.; Yilmaz, B.; Ugur, M.; Kilic, E. Erythropoietin receptor-like immunostaining on human spermatozoa. Reprod. Biomed. Online 2010, 21, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Mioni, R.; Gottardello, F.; Bordon, P.; Montini, G.; Foresta, C. Evidence for specific binding and stimulatory effects of recombinant human erythropoietin on isolated adult rat Leydig cells. Acta Endocrinol. 1992, 127, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Akman, O.; Ozkanlar, Y.; Oruc, E.; Ulas, N.; Ziypak, T.; Lehimcioglu, N.C.; Turkeli, M.; Ucar, O. Erythropoietin Hormone and ACE Inhibitor Protect the Sperm Parameters of Adult Male Rats Against Doxorubicin Toxicity. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 805–812. [Google Scholar] [CrossRef]
- Asimakopoulos, B.; Tiptiri-Kourpeti, A.; Metalinou, C. Erythropoitein Increases In Vitro Motility and Vitality of Human Spermatozoa. In Vivo 2021, 35, 2669–2673. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Shim, M.R.; Oh, M.Y.; Kim, K.W.; Lee, H.C.; Yang, B.C.; Chung, H.K.; Kim, J.H.; Lee, H.T.; Hwang, I.S.; et al. Proteins associated with reproductive disorders in testes of human erythropoietin gene-harboring transgenic boars. Theriogenology 2012, 78, 1020–1029. [Google Scholar] [CrossRef]
- Collares, T.F.; Campos, V.F.; Urtiaga, G.; Leon, P.M.; Amaral, M.G.; Hartleben, C.P.; McBride, A.J.; Dellagostin, O.A.; Deschamps, J.C.; Seixas, F.K.; et al. Erythropoietin non-viral gene therapy does not affect motility, viability, morphology or concentration of rabbit sperm. Animal 2013, 7, 778–783. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhang, J.; Xiong, Q. Suppressive effect erythropoietin on oxidative stress by targeting AMPK/Nox4/ROS pathway in renal ischemia reperfusion injury. Transpl. Ιmmunol. 2022, 72, 101537. [Google Scholar] [CrossRef] [PubMed]
- Mignotte, B.; Vayssiere, J.L. Mitochondria and apoptosis. Eur. J. Biochem. 1998, 252, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004, 71, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Mason, M.C.; Govindaraju, A.; Belser, L.; Kaya, A.; Stokes, J.; Rowe, D.; Memili, E. Interrelationships between apoptosis and fertility in bull sperm. J. Reprod. Dev. 2013, 59, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior. Stresses 2023, 3, 687–700. [Google Scholar] [CrossRef]
- Parrish, J.J.; Susko-Parrish, J.; Winer, M.A.; First, N.L. Capacitation of bovine sperm by heparin. Biol. Reprod. 1988, 38, 1171–1180. [Google Scholar] [CrossRef]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef]
- Patel, M.; Gandotra, V.K.; Cheema, R.S.; Bansal, A.K.; Kumar, A. Seminal Plasma Heparin Binding Proteins Improve Semen Quality by Reducing Oxidative Stress during Cryopreservation of Cattle Bull Semen. Asian-Australas. J. Anim. Sci. 2016, 29, 1247–1255. [Google Scholar] [CrossRef]
- Galantino-Homer, H.L.; Visconti, P.E.; Kopf, G.S. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol. Reprod. 1997, 56, 707–719. [Google Scholar] [CrossRef] [PubMed]
| Min | Group | Rapid (%) | Medium (%) | Slow (%) | Total Motile (%) | Static (%) | Progressive Motile (%) |
|---|---|---|---|---|---|---|---|
| 0 | Control | 43.1±2.75 | 21.46 ± 2.65 | 5.26 ± 2.65 | 69.82 ± 7.80 | 30.18 ± 7.80 | 21.05 ± 3.29 |
| EPO 10 | 45.48 ± 2.77 | 25.68 ± 4.66 | 8.1 ± 6.04 | 79.26 ± 13.40 | 20.74 ± 12.23 | 20.51 ± 3.69 | |
| EPO 100 | 41.7 ± 3.16 | 22.44 ± 6.96 | 15.57 ± 15.87 | 79.67 ± 7.88 | 20.33 ± 3.75 | 20.8 ± 3.75 | |
| 240 | Control | 21.7 ± 3.16 | 22.44 ± 6.96 | 15.57 ± 15.87 | 59.71 ± 6.30 | 40.29 ± 6.30 | 20.8 ± 3.75 |
| EPO 10 | 28.36 ± 4.45 | 25.00 ± 10.02 | 8.65 ± 3.15 | 62.01 ± 11.51 | 37.99 ± 11.53 | 24.05 ± 3.07 | |
| EPO 100 | 37.17 ± 2.3 * | 19.67 ± 6.77 | 6.55 ± 2.79 | 63.39 ± 6.71 | 36.61 ± 6.71 | 24.70 ± 3.45 |
| Min | Group | VCL (μm/s) | VSL(μm/s) | VAP (μm/s) | ALH |
|---|---|---|---|---|---|
| 0 | Control | 59.43 ± 5.78 | 19.7 ± 2.95 | 33.36 ± 2.73 | 3.00 ± 0.34 |
| EPO 10 | 58.76 ± 4.76 | 19.08 ± 1.30 | 32.66 ± 1.73 | 3.05 ± 0.33 | |
| EPO 100 | 59.06 ± 5.07 | 18.85 ± 2.65 | 32.96 ± 4.24 | 3.26 ± 0.53 | |
| 240 | Control | 53.75 ± 10.51 | 24.78 ± 7.73 | 34.92 ± 7.10 | 2.42 ± 0.22 |
| EPO 10 | 52.51 ± 7.13 | 23.46 ± 4.61 | 27.81 ± 10.29 | 2.95 ± 0.38 | |
| EPO 100 | 56.95 ± 8.77 | 30.55 ± 6.74 | 40.28 ± 7.11 | 2.28 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapanidou, V.G.; Asimakopoulos, B.; Lialiaris, T.; Lavrentiadou, S.N.; Feidantsis, K.; Kourousekos, G.; Tsantarliotou, M.P. The Role of Erythropoietin in Bovine Sperm Physiology. Animals 2024, 14, 2175. https://doi.org/10.3390/ani14152175
Sapanidou VG, Asimakopoulos B, Lialiaris T, Lavrentiadou SN, Feidantsis K, Kourousekos G, Tsantarliotou MP. The Role of Erythropoietin in Bovine Sperm Physiology. Animals. 2024; 14(15):2175. https://doi.org/10.3390/ani14152175
Chicago/Turabian StyleSapanidou, Vasiliki G., Byron Asimakopoulos, Theodoros Lialiaris, Sophia N. Lavrentiadou, Konstantinos Feidantsis, Georgios Kourousekos, and Maria P. Tsantarliotou. 2024. "The Role of Erythropoietin in Bovine Sperm Physiology" Animals 14, no. 15: 2175. https://doi.org/10.3390/ani14152175






