Disparate Effects of Stressors on Met-Enkephalin System Parameters and on Plasma Concentrations of Corticosterone in Young Female Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Sampling
2.3. Tissue Sampling
2.4. Experimental Design
2.5. Hormone Assays
2.6. Proenkephalin Gene (PENK) Expression
2.7. In Vitro Met-Enkephalin Release
2.8. Statistical Analysis
3. Results
3.1. Effects of Water Deprivation on Plasma Concentrations of Met-Enkephalin (Native and Total) and Corticosterone Together with Tissue Concentrations of Met-Enkephalin and PENK Expression
3.2. Effects of Water Deprivation In Vivo in Pullets in the Presence or Absence of Naltrexone In Vitro on Release of Met-Enkephalin In Vitro
3.3. Effects of Feed Deprivation on Plasma Concentrations of Met-Enkephalin and Corticosterone
3.4. Effects of Light Deprivation (Darkness) on Plasma and Tissue Concentrations of Met-Enkephalin Together with PENK Expression and Plasma Concentrations of Corticosterone
3.5. Effect of Crowding on Plasma and Tissue Concentrations of Met-Enkephalin and Plasma Concentrations of Corticosterone in Pullets
4. Discussion
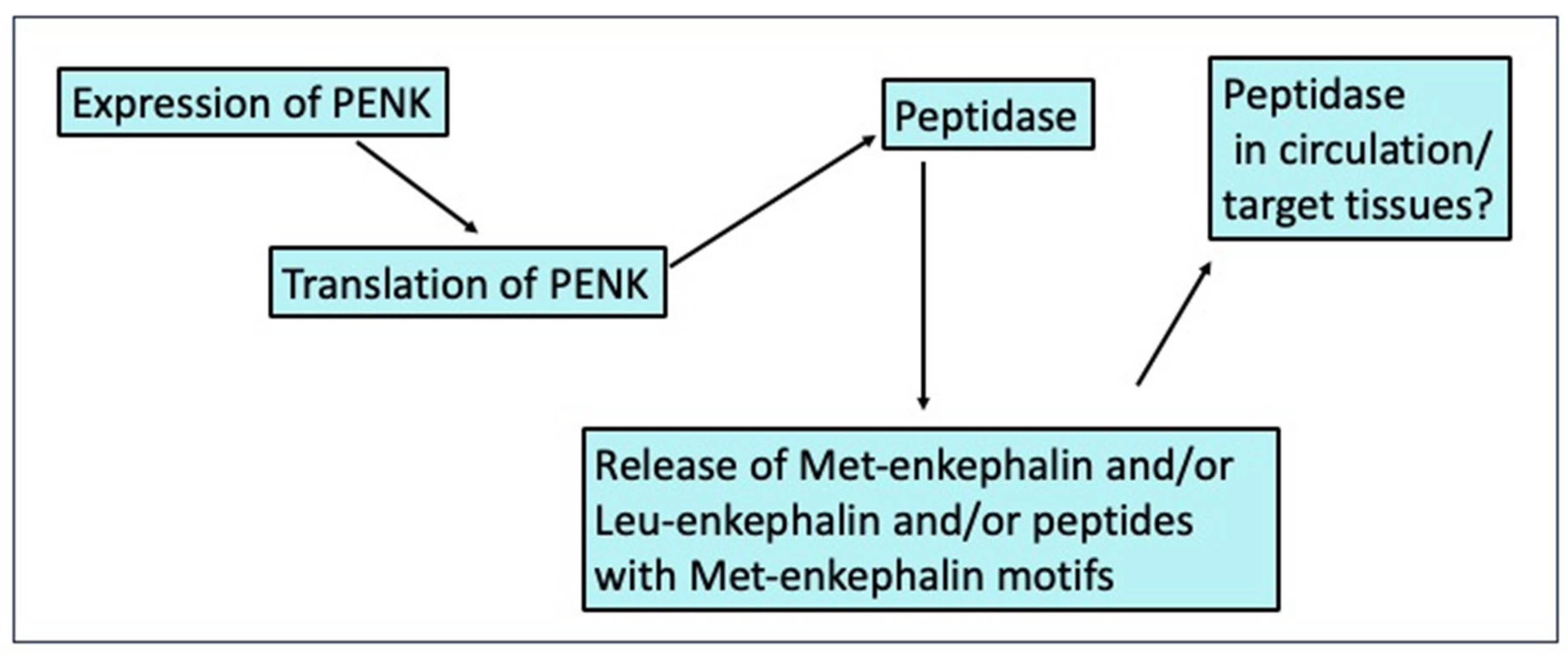
- Changed synthesis of pro-enkephalin at levels of expression and/or translation;
- Changed generation of Met-enkephalin and other PENK-derived peptides by peptidases;
- Changed release of Met-enkephalin and other PENK-derived peptides;
- Shifts in peptidases either generating or degrading Met-enkephalin and other PENK-derived peptide in the circulation and/or at target tissues.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scanes, C.G. Animal well-being and behavior: Biology of stress in poultry with emphasis on glucocorticoids and the neutrophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef]
- Levada, O.A.; Troyan, A.S. Poststroke depression biomarkers: A narrative review. Front. Neurol. 2018, 9, 577. [Google Scholar] [CrossRef]
- Kasatkina, M.Y.; Zhanin, I.S.; Gulyaeva, N.V. Ischemic stroke and depression biomarkers: Are there specific markers for post-stroke depression. Neurochem. J. 2020, 37, 318–327. [Google Scholar] [CrossRef]
- Potter, T.; Gange, N.; Whiteside, E.; Gyawali, P. Scoping review of molecular biomarkers associated with fatigue, stress, and depression in stroke survivors: A protocol. PLoS ONE 2023, 18, e0281238. [Google Scholar] [CrossRef]
- Sauriyal, D.S.; Jaggi, A.S.; Singh, N. Extending pharmacological spectrum of opioids beyond analgesia: Multifunctional aspects in different pathophysiological states. Neuropeptides 2021, 45, 175–188. [Google Scholar] [CrossRef]
- Aurich, J.E.; Dobrinski, I.; Hoppen, H.O.; Grunert, E. Beta-endorphin and met-enkephalin in plasma of cattle during pregnancy, parturition and the neonatal period. J. Reprod. Fertil. 1990, 89, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Calero, A.; Villanueva, H.; Giri, P.; Tischler, A.S.; Powers, J.F.; Evinger, M. Acute hypoxia induces enkephalin production and release in an adrenergic cell line model of neonatal chromaffin cell responses to hypoxic stress. Am. J. Perinatol. 2018, 35, 1100–1106. [Google Scholar] [CrossRef]
- Duque-Díaz, E.; Alvarez-Ojeda, O.; Coveñas, R. Enkephalins and ACTH in the mammalian nervous system. Vitam. Horm. 2019, 111, 147–193. [Google Scholar] [CrossRef]
- Sobocanec, S.; Kusić, B.; Sverko, V.; Balog, T.; Marotti, T. Methionine-enkephalin modulated regulation of oxidant/antioxidant status in liver of CBA mice. Biogerontology 2006, 7, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, B.; Qu, N.; Zhang, S.; Bai, X.; Handley, M.; Shan, F. Research progress of opioid growth factor in immune-related diseases and cancer diseases. Int. Immunopharmacol. 2021, 99, 107713. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovac, J.; Marotti, T. Gender-related differences in murine T- and B-lymphocyte proliferative ability in response to in vivo [Met5] enkephalin administration. Eur. J. Pharmacol. 2000, 392, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Applegren, R.R.; Davis, J.M.; Cheung, H.T. Modulation of lymphocyte motility by beta-endorphin and met-enkephalin. Immunopharmacology 1989, 17, 81–89. [Google Scholar] [CrossRef]
- Martin-Kleiner, I.; Osmak, M.; Gabrilovac, J. Regulation of NK cell activity and the level of the intracellular cAMP in human peripheral blood lymphocytes by Met-enkephalin. Res. Exp. Med. 1992, 192, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, S.; Eriksson, F.; Kavelaars, A.; Zijlstra, J.; van de Pol, M.; Kuis, W.; Heijnen, C.J. Role of endogenous pro-enkephalin A-derived peptides in human T cell proliferation and monocyte IL-6 production. J. Neuroimmunol. 1998, 84, 53–60. [Google Scholar] [CrossRef]
- Cabot, P.J.; Carter, L.; Schäfer, M.; Stein, C. Methionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory pain. Pain 2001, 93, 207–212. [Google Scholar] [CrossRef]
- Hua, S. Neuroimmune interaction in the regulation of peripheral opioid-mediated analgesia in inflammation. Front. Immunol. 2016, 7, 293. [Google Scholar] [CrossRef]
- Ayala, F.J.; Rzhetsky, A.; Ayalam, F.J. Origin of the metazoan phyla: Molecular clocks confirm paleontological estimates. Proc. Natl. Acad. Sci. USA 1998, 95, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Sha, A.; Chen, H.; Zhang, Y. Expression profile and immunomodulatory roles of methionine-enkephalin and delta opioid receptor in Octopus ocellatus. Fish Shellfish. Immunol. 2024, 150, 109637. [Google Scholar] [CrossRef]
- Lightman, S.L.; Young, W.S. Changes in hypothalamic preproenkephalin A mRNA following stress and opiate withdrawal. Nature 1987, 328, 643–645. [Google Scholar] [CrossRef]
- Marotti, T.; Rocić, B.; Gabrilovac, J.; Haberstok, H. Interaction of Met-enkephalin and corticosteroids in immunomodulation. Int. J. Immunopharmacol. 1992, 14, 621–627. [Google Scholar] [CrossRef]
- Pierzchała-Koziec, K.; Van Loon, G.R. Effects of nicotine on the concentration of native and cryptic Met- and Leu-enkephalin in peripheral tissues. J. Physiol. Pharmacol. 1994, 45, 319–330. [Google Scholar] [PubMed]
- Pierzchala, K.; Houdi, A.A.; Van Loon, G.R. Nicotine-induced alterations in brain regional concentrations of native and cryptic met- and leu-enkephalin. Peptides 1987, 8, 1035–1043. [Google Scholar] [CrossRef]
- Denning, G.M.; Ackermann, L.W.; Barna, T.J.; Armstrong, J.G.; Stoll, L.L.; Weintraub, N.L.; Dickson, E.W. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 2008, 29, 83–92. [Google Scholar] [CrossRef]
- Van Loon, G.R.; Pierzchała, K.; Houdi, A.A. Nicotine-induced alterations in peripheral tissue concentrations of native and cryptic met- and leu-enkephalin. Neuropeptides 1991, 19, 35–41. [Google Scholar] [CrossRef]
- Barron, B.A.; Pierzchala, K.; Loon, G.R. Source of stress-induced increase in plasma Met-enkephalin in rats: Contribution of adrenal medulla and/or sympathetic nerves. J. Neuroendocrinol. 1990, 2, 381–388. [Google Scholar] [CrossRef]
- Walsh, J.P.; Rao, A.; Thompson, R.C.; Clarke, I.J. Proenkephalin and opioid mu-receptor mRNA expression in ovine hypothalamus across the estrous cycle. Neuroendocrinology 2001, 73, 26–36. [Google Scholar] [CrossRef]
- Staszkiewicz, J.; Skowronski, M.T.; Siawrys, G.; Kaminski, T.; Krazinski, B.E.; Plonka, K.J.; Wylot, B.; Przala, J.; Okrasa, S. Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine luteal cells. Acta Vet. Hung. 2007, 55, 435–449. [Google Scholar] [CrossRef]
- Rosenberger, J.; Petrovics, G.; Buzas, B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J. Neurochem. 2001, 79, 35–44. [Google Scholar] [CrossRef]
- Slominska, T.; Zmijewski, M.A.; Zbytek, B.; Brozyna, A.A.; Granese, J.; Pisarchik, A.; Szczesniewski, A.; Tobin, D.J. Regulated proenkephalin expression in human skin and cultured skin cells. J. Investig. Dermatol. 2011, 131, 613–622. [Google Scholar] [CrossRef]
- Keshet, E.; Polakiewicz, R.D.; Itin, A.; Ornoy, A.; Rosen, H. Proenkephalin A is expressed in mesodermal lineages during organogenesis. EMBO J. 1989, 8, 2917–2923. [Google Scholar] [CrossRef]
- Marengo, F.D.; Cárdenas, A.M. How does the stimulus define exocytosis in adrenal chromaffin cells? Pflug. Arch. 2018, 470, 155–167. [Google Scholar] [CrossRef]
- Bu, G.; Cui, L.; Lv, C.; Lin, D.; Huang, L.; Li, Z.; Li, J.; Zeng, X.; Wang, Y. Opioid peptides and their receptors in chickens: Structure, functionality, and tissue distribution. Peptides 2020, 128, 170307. [Google Scholar] [CrossRef] [PubMed]
- Pierzchala-Koziec, K.; Scanes, C.G. Avian opioid peptides: Evolutionary considerations, hormonal and functional roles and a challenge to address critical questions. Front. Physiol. 2023, 14, 1164031. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.L.; Taniguchi, T.N.; Jones, B.N.; Stern, A.S.; Shively, J.E.; Hullihan, J.; Kimura, S.; Stein, S.; Udenfriend, S. A highly potent 3200-dalton adrenal opioid peptide that contains both a [Met]- and [Leu]enkephalin sequence. Proc. Natl. Acad. Sci. USA 1981, 78, 3265–3268. [Google Scholar] [CrossRef]
- Scanes, C.G.; Pierzchała-Koziec, K.; Gajewska, A. Effects of restraint stress on circulating corticosterone and met enkephalin in chickens: Induction of shifts in insulin secretion and carbohydrate metabolism. Animals 2024, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Pierzchala-Koziec, K.; Kępys, B.; Oeltgen, P.; Scanes, C.G. Developmental changes in the pituitary-adrenocortical axis and plasma enkephalin concentration in response to isolation stress in growing lambs. Folia Biol. 2018, 66, 53–61. [Google Scholar] [CrossRef]
- Pierzchała, K.; Van Loon, G.R. Plasma native and peptidase-derivable Met-enkephalin responses to restraint stress in rats. Adaptation to repeated restraint. J. Clin. Investig. 1990, 85, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Klandorf, H. Reduced adrenocortical function and increased thyroid function in fasted and refed chickens. J. Endocrinol. 1983, 98, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Klandorf, H.; Pinchasov, Y. Visual and metabolic stimuli cause adrenocortical suppression in fasted chickens during refeeding. Neuroendocrinology 1983, 37, 59–63. [Google Scholar] [CrossRef]
- Weber, H.; Kocsis, J.F.; Lauterio, T.J.; Carsia, R.V. Dietary protein restriction stress and adrenocortical function: Evidence for transient and long-term induction of enhanced cellular function. Endocrinology 1990, 127, 3138–3150. [Google Scholar] [CrossRef]
- Derkho, M.A.; Sajfutdinova, L.N.; Strizhikov, V.K.; Strizhikova, S.V.; Ponomaryova, T.A. The role of corticosterone in the regulation of the cellular composition of chicken blood during the stress reaction. E3S Web Conf. 2021, 282, 03003. [Google Scholar] [CrossRef]
- Scanes, C.G.; Pierzchała-Koziec, K. Morphine influences circulating and tissue concentrations of met-enkephalin and proenkephalin (PENK) expression and plasma concentrations of corticosterone in chickens. Poult. Sci. 2024, 103, 103712. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, P.; Någren, K.; Lundberg, P.O.; Muhr, C.; Terenius, L.; Lundqvist, H.; Lärkfors, L.; Långström, B. Kinetics of four 11C-labelled enkephalin peptides in the brain, pituitary and plasma of rhesus monkeys. Regul. Pept. 1986, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]


| Study | Duration | n | Treatments Groups | Plasma Concentrations | Tissue Concentrations of Met-Enkephalin | Tissue PENK Expression |
|---|---|---|---|---|---|---|
| 1. Water deprivation | 24 h | 6 | 1. Control 2. Water withheld | Corticosterone Met-enkephalin peptides containing Met-enkephalin motifs | Hypothalamus, anterior pituitary gland, adrenal glands A | Hypothalamus, anterior pituitary gland, adrenal glands |
| 2. Feed deprivation | 24 h | 6 | 1. Control 2. Feed deprivation | Corticosterone Met-enkephalin peptides containing Met-enkephalin motifs | - | - |
| 3. Light deprivation (darkness) | 24 h | 5 | 1.Control 2. Light deprivation | Corticosterone Met-enkephalin peptides containing Met-enkephalin motifs | Hypothalamus, anterior pituitary gland, adrenal glands | Hypothalamus, anterior pituitary gland, adrenal glands |
| 4. Space deprivation (crowding) | 30 min | 5 | 1. Control 2. Crowding 3. Naltrexone 4. Crowding and naltrexone | Corticosterone Met-enkephalin | Hypothalamus, anterior pituitary gland, adrenal glands | Hypothalamus, anterior pituitary gland, adrenal glands |
| Tissue and Parameter | Control | Water Withheld (24 h) |
|---|---|---|
| Plasma Concentrations | ||
| Native Met-enkephalin (pmoles L−1) | 50.2 ± 7.9 | 68.7 ± 6.2 |
| Total peptides containing Met-enkephalin motifs (pmoles Met-enkephalin equivalents L−1) | 705 ± 69 | 570 ± 49 |
| Corticosterone (nmoles L−1) | 11.1 ± 2.0 | 38.5 ± 2.6 *** |
| Tissue concentration of Native Met-enkephalin (pmoles g−1) | ||
| Hypothalamus | 371 ± 29 | 285 ± 31 |
| Anterior pituitary gland | 822 ± 63 | 402 ± 29 *** |
| Adrenal gland | 95 ± 10 | 68 ± 6 * |
| PENK expression as % of control | ||
| Hypothalamus | 100 ± 1.0 | 102 ± 1.0 |
| Anterior pituitary gland | 100 ± 2.1 | 530 ± 38 *** |
| Adrenal gland | 100 ± 2.0 | 328 ± 9 *** |
| Tissue | In Vivo Treatment | Relative Met-Enkephalin Release (% of Tissue Content per 20 min) | |
|---|---|---|---|
| In vitro treatment | |||
| Control | Naltrexone | ||
| Hypothalamus as a % of tissue content per 20 min | |||
| No treatment (control) | 0.86 ± 0.05 b | 0.78 ± 0.04 b | |
| Water withheld | 0.66 ± 0.05 a | 1.05 ± 0.05 c | |
| Anterior pituitary gland as a % of tissue content per 20 min | |||
| No treatment (control) | 0.017 ± 0.002 b | 0.012 ± 0.001 a | |
| Water withheld | 0.014 ± 0.001 a | 0.18 ± 0.002 b | |
| Adrenal gland as a % of tissue content per 20 min | |||
| No treatment (control) | 2.56 ± 0.23 b | 1.69 ± 0.16 a | |
| Water withheld | 2.54 ± 0.20 b | 3.72 ± 0.34 c | |
| Control | Feed Deprivation | Re-Feed for 2 h | |
|---|---|---|---|
| Native plasma concentrations of Met-enkephalin (pmoles L−1) | 56.0 ± 5.7 b | 32 ± 4.3 a | 63.0 ± 7.7 b |
| Total plasma concentration of peptides containing Met-enkephalin motifs (pmoles Met-enkephalin equivalents L−1) | 660 ± 49 | 720 ± 49 | 750 ± 59 |
| Plasma concentrations of corticosterone (nmoles L−1) | 16.0 ± 1.3 a | 37.4 ± 4.3 b | 34.8 ± 3.9 b |
| Control | Dark Stress | |
|---|---|---|
| Plasma Concentrations of Mean ± SEM (n = 5) | ||
| Native Met-enkephalin (pmole L−1) | 31.0 ± 0.88 | 38.6 ± 0.90 *** |
| Total peptides containing Met-enkephalin motifs (pmole L−1) | 462 ± 10.7 | 560 ± 15.5 *** |
| Corticosterone | 17.4 ± 0.51 | 21.3 ± 0.80 ** |
| Native tissue concentrations of Met-enkephalin (pmole g−1) mean ± SEM (n = 5) | ||
| Hypothalamus | 411 ± 4.01 | 196 ± 2.05 *** |
| Anterior pituitary gland | 826 ± 4.91 | 1496 ± 35 ** |
| Adrenal gland | 112 ± 4.14 | 55.8 ± 0.86 *** |
| Tissue concentrations of total peptides containing Met-enkephalin motifs (pmole g−1) mean ± SEM (n = 5) | ||
| Hypothalamus | 3673 ± 71.5 | 1673 ± 41.5 ** |
| Anterior pituitary gland | 7623 ± 110 | 14,507 ± 223 *** |
| Adrenal gland | 726 ± 21.5 | 382 ± 8.5 *** |
| PENK expression (as % of controls) (n = 3) | ||
| Hypothalamus | 100 ± 6.9 | 97.1 ± 3.5 |
| Anterior pituitary gland | 100 ± 3.2 | 230 ± 9.5 *** |
| Adrenal gland | 100 ± 5.6 | 61.0 ± 5.6 ** |
| Control | Crowding for 30 min | Control | Crowding | |
|---|---|---|---|---|
| Sham | Naltrexone pretreatment | |||
| Plasma concentrations | ||||
| Native Met-enkephalin (pmoles L−1) | 37.7 ± 8.8 a,b | 52.2 ± 6.3 b | 45.9 ± 7.3 b | 30.9 ± 5.0 a |
| Corticosterone (nmoles L−1) | 13.4 ± 1.8 a | 24.0 ± 4.4 b | 16.7 ± 1.8 a | 24.7 ± 3.7 b |
| Tissue concentrations of native Met-enkephalin (pmoles g−1) | ||||
| Hypothalamus | 403 ± 44 b | 164 ± 26 a | 359 ± 45.7 b | 342 ± 53.6 b |
| Anterior pituitary gland | 930 ± 139 a,b | 1632 ± 261 b | 1044 ± 148 a,b | 600 ± 73 a |
| Adrenal gland | 118 ± 13 c | 54 ± 7 a | 108 ± 16 b,c | 73.1 ± 12 a,b |
| PENK expression as % of control | ||||
| Hypothalamus | 100 ± 4.8 a | 47.0 ± 2.3 c | 54.3 ± 2.8 b | 49.7 ± 1.9 b,c |
| Anterior pituitary gland | 100 ± 7.9 b | 244 ± 9.6 c | 105 ± 6.7 b | 39.6 ± 3.3 a |
| Adrenal gland | 100 ± 3.8 a | 62.5 ± 3.8 b | 114 ± 7.3 a | 66.7 ± 4.8 b |
| Deprivation | Plasma Concentration of Met-Enkephalin | Plasma Concentration of Corticosterone | Tissue Concentration of Met-Enkephalin | PENK Expression |
|---|---|---|---|---|
| Females | ||||
| Hypothalamus | ||||
| Water T | ↑? | ↑↑ | → | → |
| Food U | ↑↑ | ↑↑ | NA | NA |
| Darkness V | ↑ | ↑ | ↓↓ | → |
| Crowding W | ↑? | ↑ | ↓↓ | ↓↓ |
| Anterior pituitary gland | ||||
| Water T | ↑? | ↑↑ | ↑↑ | ↑↑↑ |
| Darkness V | ↑ | ↑ | ↑↑ | ↑↑ |
| Crowding W | ↑? | ↑ | ↑↑ | ↑↑ |
| Restraint X | ↑↑ | ↑↑ | NA | ↑↑ |
| Morphine Y | ↓↓ | ↑↑ | → | ↓↓ |
| Adrenal gland | ||||
| Water T | ↑? | ↑↑ | ↓↓ | ↑↑ |
| Darkness V | ↑ | ↑ | ↓↓ | ↓↓ |
| Crowding W | ↑? | ↑ | ↓↓ | ↓↓ |
| Restraint X | ↑↑ | ↑↑ | NA | ↑↑ |
| Morphine Y | ↓↓ | ↑↑ | → | ↓↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanes, C.G.; Pierzchala-Koziec, K. Disparate Effects of Stressors on Met-Enkephalin System Parameters and on Plasma Concentrations of Corticosterone in Young Female Chickens. Animals 2024, 14, 2201. https://doi.org/10.3390/ani14152201
Scanes CG, Pierzchala-Koziec K. Disparate Effects of Stressors on Met-Enkephalin System Parameters and on Plasma Concentrations of Corticosterone in Young Female Chickens. Animals. 2024; 14(15):2201. https://doi.org/10.3390/ani14152201
Chicago/Turabian StyleScanes, Colin Guy, and Krystyna Pierzchala-Koziec. 2024. "Disparate Effects of Stressors on Met-Enkephalin System Parameters and on Plasma Concentrations of Corticosterone in Young Female Chickens" Animals 14, no. 15: 2201. https://doi.org/10.3390/ani14152201





