A Critical Functional Missense Mutation (T117M) in Sheep MC4R Gene Significantly Leads to Gain-of-Function
Abstract
:Simple Summary
Abstract
1. Introduction
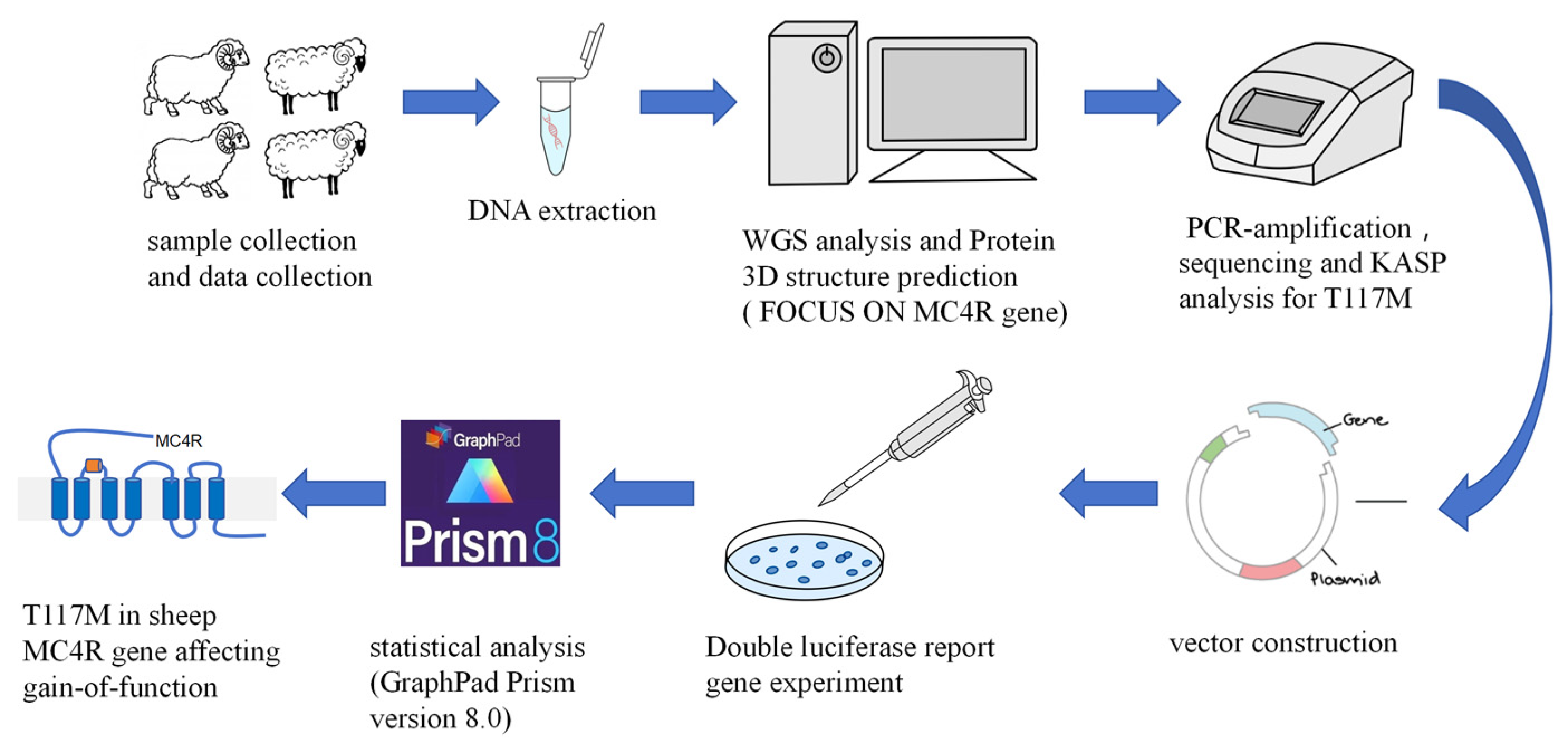
2. Materials and Methods
2.1. Ethics Statement
2.2. Frequency of Mutation Sites Detected by Whole-Genome Sequencing
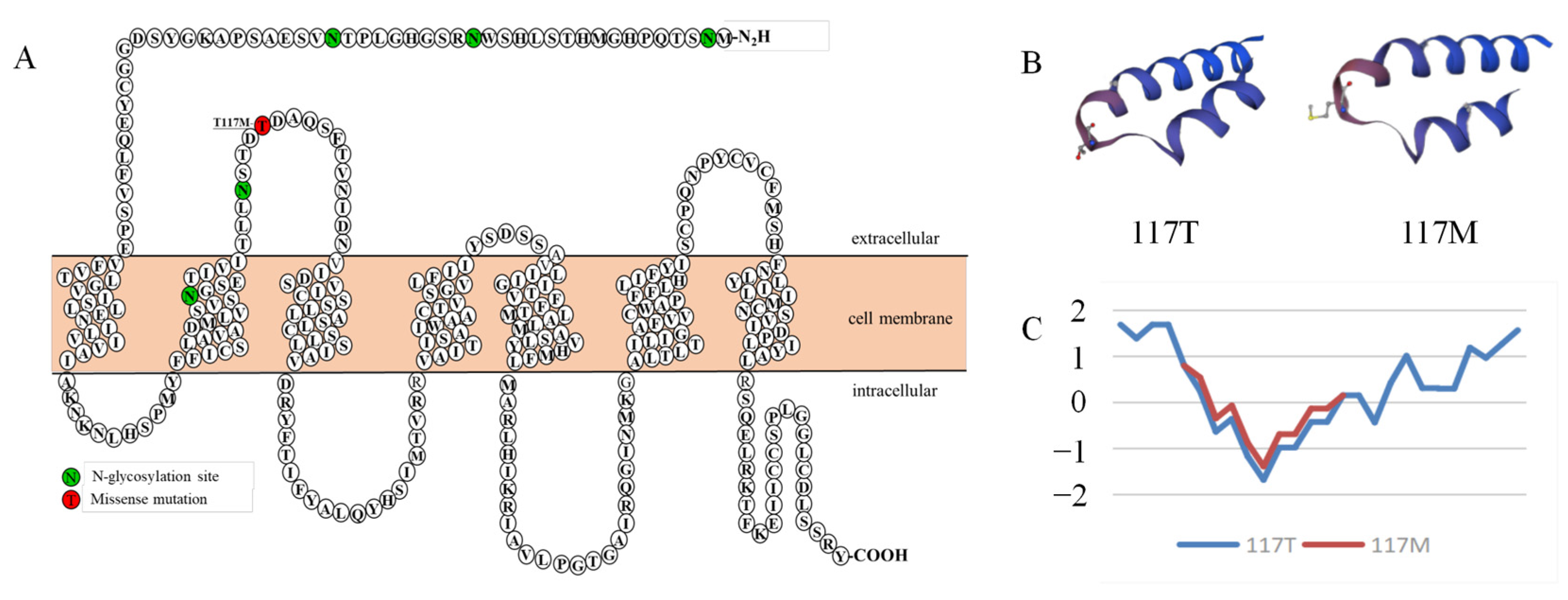
2.3. Protein 3D Structure Prediction
2.4. Sample Collection and DNA Extraction
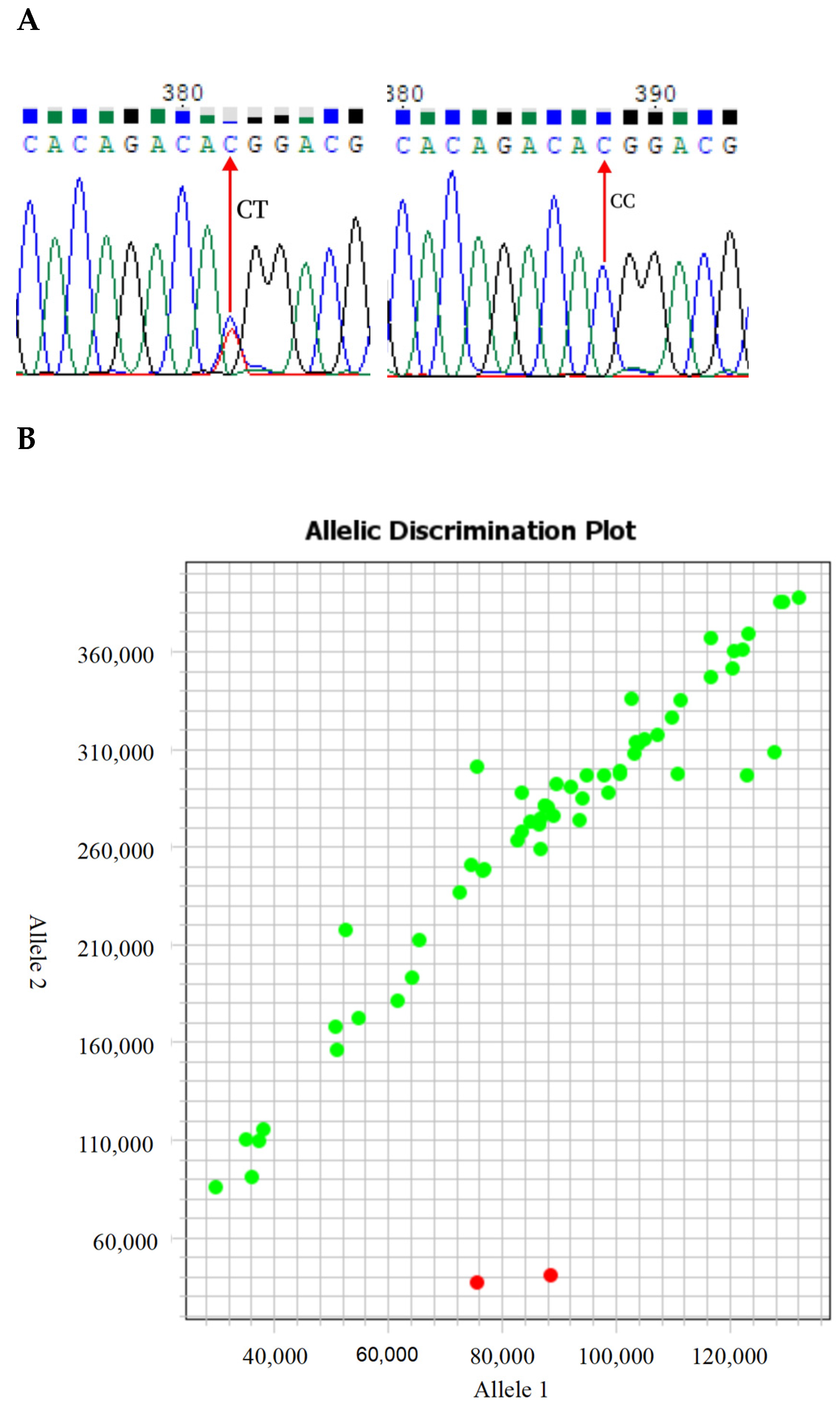
2.5. Primer Design and PCR Amplification
2.6. KASP Fast Typing
2.7. Vector Construction
2.8. Double Luciferase Reporter Gene Experiment
2.9. Statistical Analysis
3. Results
3.1. The MC4R p.T117M Mutation Is a Low-Frequency Mutation in Several Sheep Breeds
3.2. The Schematic Structure and Hydrophilicity of Variants
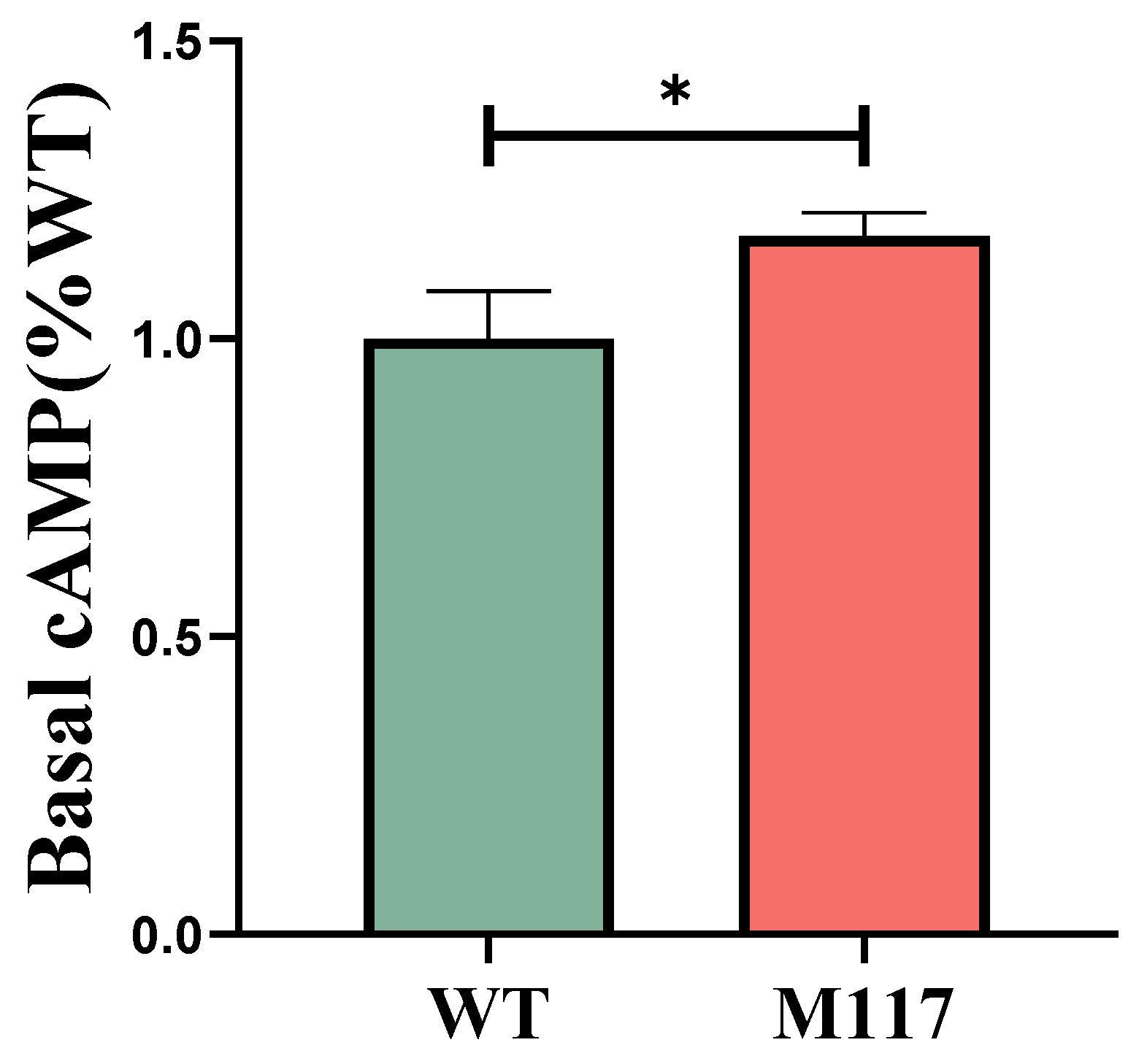
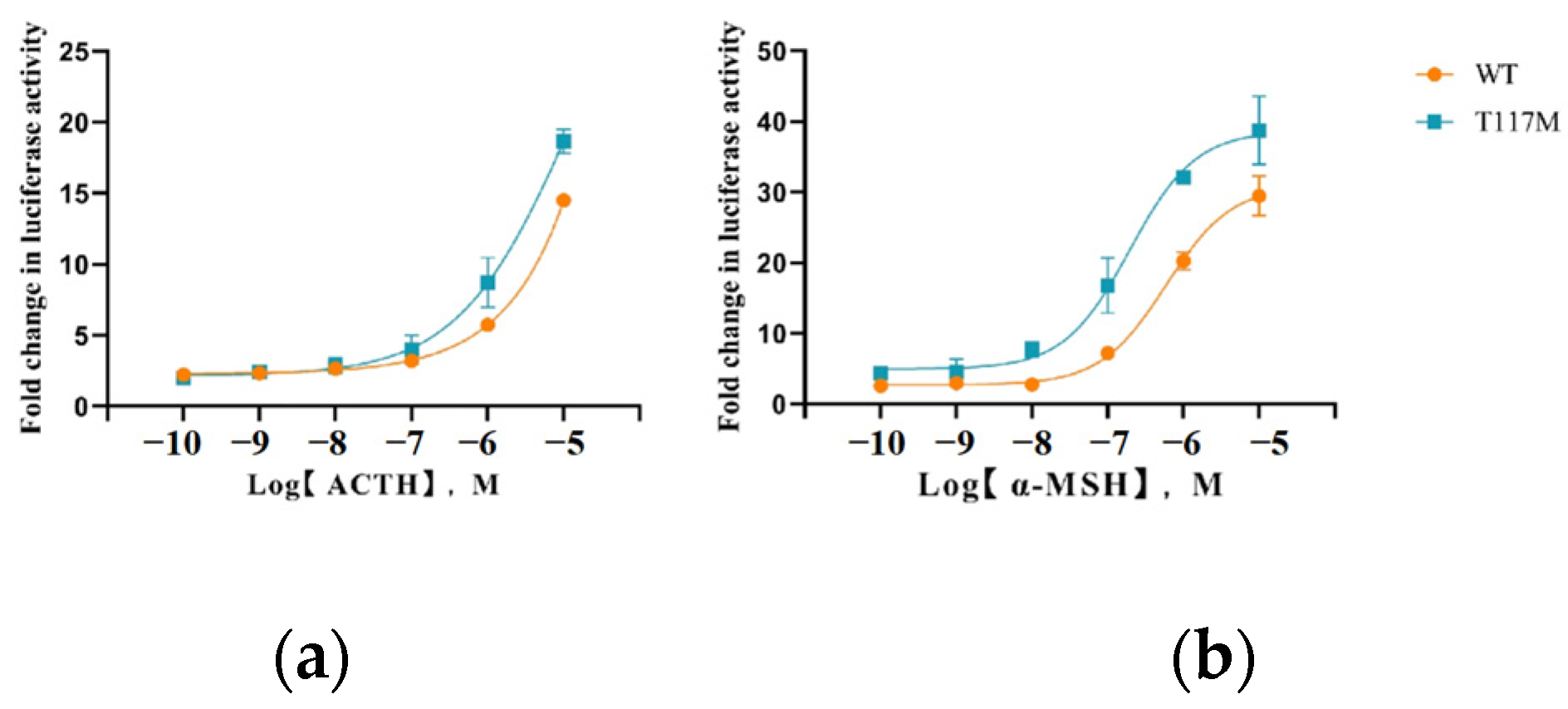
3.3. The T117M Mutation in MC4R Mediates Enhanced cAMP Signaling Activation
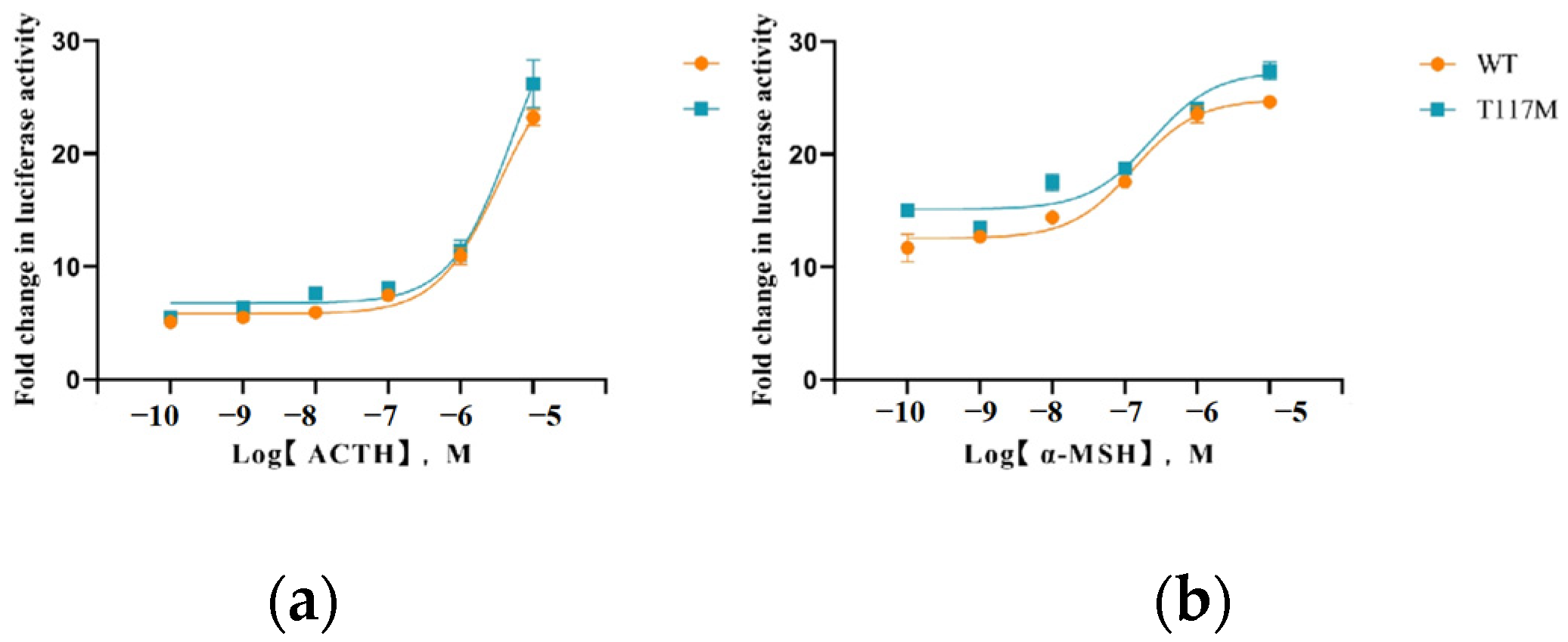
3.4. Activation of the MAPK/ERK Pathway Mediated by MC4R WT and T117M
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, A.A. The melanocortin system and energy balance. Peptides 2006, 27, 281–290. [Google Scholar] [CrossRef]
- Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Cone, R.D. The cloning of a family of genes that encode the melanocortin receptor. Science 1992, 257, 1248–1251. [Google Scholar] [CrossRef]
- Tao, Y. The melanocortin-4 receptor: Physiology, pharmacology, and pathophysiology. Endocr. Rev. 2010, 31, 506–543. [Google Scholar] [CrossRef]
- Gantz, I.; Fong, T.M. The melanocortin system. Am. J. Physiol.-Endocrinol. Metab. 2003, 284, E468–E474. [Google Scholar] [CrossRef]
- Emeson, R.B.; Eipper, B.A. Characterization of pro-ACTH/endorphin-derived peptides in rat hypothalamus. J. Neurosci. Off. J. Soc. Neurosci. 1986, 6, 837–849. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar]
- Garfield, A.S.; Lam, D.D.; Marston, O.J.; Przydzial, M.J.; Heisler, L.K. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol. Metab. 2009, 20, 203–215. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, Y.H.; Chen, H.; Lan, X.-Y.; Lei, C.-Z.; Fang, X.-T. Association between variants in the 5′-untranslated region of the bovine MC4R gene and two growth traits in Nanyang cattle. Mol. Biol. Rep. 2009, 36, 1839–1843. [Google Scholar] [CrossRef]
- Liu, H.Y.; Tian, W.Q.; Zan, L.S.; Wang, H.; Cui, H. Association of MC4R gene variants with carcass and meat quality traits in Qinchuan cattle. Afr. J. Biotechnol. 2009, 8, 3666–3671. [Google Scholar]
- Szyndler-Nedza, M.; Tyra, M.; Blicharski, T.; Piórkowska, K. Effect of mutation in MC4R gene on carcass quality in Pulawska pig included in conservation breeding programme. Anim. Sci. Pap. Rep. 2010, 28, 37–45. [Google Scholar]
- Dvorakova, V.; Stupka, R.; Sprysl, M.; Čítek, J.; Okrouhlá, M.; Kluzáková, E.; Kratochvílová, H. Effect of the missense mutation Asp298Asn in MC4R on growth and fatness traits in commercial pig crosses in the Czech Republic. Czech J. Anim. Sci. 2011, 56, 176–180. [Google Scholar] [CrossRef]
- Kwon, K.; Cahyadi, M.; Park, H.B.; Seo, D.W.; Jin, S.; Kim, S.; Choi, Y.; Kim, K.S.; Gotoh, T.; Lee, J.H. Association of variation in the MC4R gene with meat quality traits in a commercial pig population. J. Fac. Agric. Kyushu Univ. 2015, 60, 113–118. [Google Scholar] [CrossRef]
- Zuo, B.Y.; Liu, G.Q.; Peng, Y.Q.; Qian, H.; Liu, J.; Jiang, X.; Mara, A. Melanocortin-4 receptor (MC4R) polymorphisms are associated with growth and meat quality traits in sheep. Mol. Biol. Rep. 2014, 41, 6967–6974. [Google Scholar] [CrossRef]
- Liu, H.; Tian, W.; Zan, L.; Wang, H.; Cui, H. Mutations of MC4R gene and its association with economic traits in Qinchuan cattle. Mol. Biol. Rep. 2010, 37, 535–540. [Google Scholar] [CrossRef]
- Seong, J.; Suh, D.S.; Park, K.D.; Lee, H.K.; Kong, H.S. Identification and analysis of MC4R polymorphisms and their association with economic traits of Korean cattle (Hanwoo). Mol. Biol. Rep. 2012, 39, 3597–3601. [Google Scholar] [CrossRef]
- Saini, B.L.; Gaur, G.K.; Sahoo, N.R.; Mendiratta, S.K.; Kumar, A.; Naha, B.C.; Baranwal, A.; Yadav, V.; Jaiswal, R.K. Polymorphism distribution of RYR1, PRKAG3, HFABP, MYF-5 and MC4R genes in crossbred pigs. Mol. Biol. Rep. 2018, 45, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jin, S.K.; Jeong, Y.H.; Jung, Y.C.; Jung, J.H.; Shim, K.S.; Choi, Y.I. Relationships between single nucleotide polymorphism markers and meat quality traits of Duroc breeding stocks in Korea. Asian-Australas. J. Anim. Sci. 2016, 29, 1229–1238. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, Z.; Bruce, H.L.; Janz, J.; Goddard, E.; Moore, S.; Plastow, G.S. Associations between single nucleotide polymorphisms in 33 candidate genes and meat quality traits in commercial pigs. Anim. Genet. 2014, 45, 508–516. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Aggag, S. Association of single nucleotide polymorphism in melanocortin receptor gene with egg production traits in Lohmann Brown Chickens. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 145–148. [Google Scholar]
- Switonski, M.; Mankowska, M.; Salamon, S. Family of melanocortin receptor (MCR) genes in mammals-mutations, polymorphisms and phenotypic effects. J. Appl. Genet. 2013, 54, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Analysis of MC4R, POMC, CSN3 and BLG Gene Polymorphisms and Thier Association with Growth Traits in Hu Sheep and East Friesian Crossbred Sheep; Nanjing Agricultral University: Nanjing, China, 2014. [Google Scholar]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Yan, H.L.; Wang, K.; Cui, Y.; Chen, R.; Liu, J.; Zhu, H.; Qu, L.; Pan, C. Goat SPEF2: Expression profile, indel variants identification and association analysis with litter size. Theriogenology 2019, 139, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, H.; Mo, H.; Lan, X.; Pan, C.; Wang, L.; Zhao, H.; Zhou, J.; Li, Y. Functional Characterization of Melanocortin-3 Receptor in a Hibernating Cavefish Onychostoma macrolepis. Animals 2022, 12, 38. [Google Scholar] [CrossRef]
- Matsumura, S.; Miyakita, M.; Miyamori, H.; Kyo, S.; Ishikawa, F.; Sasaki, T.; Jinno, T.; Tanaka, J.; Fujita, K.; Yokokawa, T.; et al. CRTC1 deficiency, specifically in melanocortin-4 receptor-expressing cells, induces hyperphagia, obesity, and insulin resistance. FASEB J. 2022, 36, e22645. [Google Scholar] [CrossRef] [PubMed]
- Szyndler-Nedza, M.; Ropka-Molik, K.; Piorkowska, K. Changes in body weight and fatness of sows during reproductive activity depending on LEPR and MC4R genes polymorphism. Livest. Sci. 2016, 192, 25–32. [Google Scholar] [CrossRef]
- Kubota, S.; Vandee, A.; Keawnakient, P.; Molee, W.; Yongsawatdikul, J.; Molee, A. Effects of the MC4R, CAPN1, and ADSL genes on body weight and purine content in slow-growing chickens. Poult. Sci. 2019, 98, 4327–4337. [Google Scholar] [CrossRef] [PubMed]
- Shishay, G.; Liu, G.; Jiang, X.; Yu, Y.; Teketay, W.; Du, D.; Jing, H.; Liu, C. Variation in the promoter region of the MC4R gene elucidates the association of body measurement traits in Hu Sheep. Int. J. Mol. Sci. 2019, 20, 240. [Google Scholar] [CrossRef]
- Naser, A.A.; Miyazaki, T.; Wang, J.; Takabayashi, S.; Pachoensuk, T.; Tokumoto, T. MC4R mutant mice develop ovarian teratomas. Sci. Rep. 2021, 11, 3483. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, W.; Yang, G.; Lv, F.; Liu, M.; Li, W.; Liu, Y.; Li, J.; Wang, F.; Shen, Z.; et al. Y chromosome haplotype diversity of domestic sheep (Ovis aries) in northern Eurasia. Anim. Genet. 2014, 45, 903–907. [Google Scholar] [CrossRef]
- Gantz, I.; Konda, Y.; Tashiro, T.; Shimoto, Y.; Miwa, H.; Munzert, G.; Watson, S.; DelValle, J.; Yamada, T. Molecular-cloning of a novel melanocortin receptor. J. Biol. Chem. 1993, 268, 8246–8250. [Google Scholar] [CrossRef]
- Chai, B.; Li, J.; Zhang, W.; Newman, E.; Ammori, J.; Mulholland, M.W. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides 2006, 27, 2846–2857. [Google Scholar] [CrossRef]
- Vongs, A.; Lynn, N.M.; Rosenblum, C.I. Activation of MAP kinase by MC4-R through PI3 kinase. Regul. Pept. 2004, 120, 113–118. [Google Scholar] [CrossRef]
- Metzger, P.; Carlson, B.; Sun, H.; Cui, Z.; Gavrilova, O.; Chen, M.; Weinstein, L. OR12-3 Mice with MC4R site mutation (F51L) develop severe obesity independent of Gs-alpha/cAMP signaling. J. Endocr. Soc. 2019, 3 (Suppl. S1), R12–R13. [Google Scholar] [CrossRef]
- Inoue, A.; Raimondi, F.; Kadji, F.M.N.; Singh, G.; Kishi, T.; Uwamizu, A.; Ono, Y.; Shinjo, Y.; Ishida, S.; Arang, N.; et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 2019, 177, 1933–1947. [Google Scholar] [CrossRef]
- Li, Y.; Shrestha, Y.; Pandey, M.; Chen, M.; Kablan, A.; Gavrilova, O.; Offermanns, S.; Weinstein, L.S. Gq/11α and Gsα mediate distinct physiological responses to central melanocortins. J. Clin. Investig. 2016, 126, 40–49. [Google Scholar] [CrossRef]
- Nijenhuis, W.; Oosterom, J.; Adan, R. AgRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol. Endocrinol. 2001, 15, 164–171. [Google Scholar]
- Biebermann, H.; Krude, H.; Elsner, A.; Chubanov, V.; Gudermann, T.; Grüters, A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes 2003, 52, 2984–2988. [Google Scholar] [CrossRef]
- Haskell-Luevano, C.; Monck, E.K. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul. Pept. 2001, 99, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Thompson, D.A.; Dickinson, C.J.; Wilken, J.; Barsh, G.S.; Kent, S.B.; Gantz, I. Characterization of agouti-related protein binding to melanocortin receptors. Mol. Endocrinol. 1999, 13, 148–155. [Google Scholar] [CrossRef]
- Schiöth, H.B.; Muceniece, R.; Wikberg, J.E.S.; Chhajlani, V. Characterisation of melanocortin receptor subtypes by radioligand binding analysis. Eur. J. Pharmacol. Mol. Pharmacol. 1995, 288, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.C.; Jia, W.P.; Cai, S.B.; Shao, X.-Y.; Zhang, R.; Wang, C.-R.; Bao, Y.-Q.; Xiang, K.-S. Intracellular retention of human melanocortin-4 receptor: A molecular mechanism underlying early-onset obesity in F261S pedigree of Chinese. Biomed. Environ. Sci. 2008, 21, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Tao, Y. Activation of MAPK by inverse agonists in six naturally occurring constitutively active mutant human melanocortin-4 receptors. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Tao, Y. Defect in MAPK signaling as a cause for monogenic obesity caused by inactivating mutations in the Melanocortin-4 Receptor gene. Int. J. Biol. Sci. 2014, 10, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ogawa, Y.; Shintani, M.; Ebihara, K.; Shimodahira, M.; Iwakura, T.; Hino, M.; Ishihara, T.; Ikekubo, K.; Kurahachi, H.; et al. A novel homozygous missense mutation of Melanocortin-4 Receptor (MC4R) in a Japanese woman with severe obesity. Diabetes 2002, 51, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tao, Y. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J. Cell. Mol. Med. 2009, 13, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Mokrosinski, J.; de Oliveira, E.M.; Li, C.; Sharp, S.J.; Luan, J.; Brouwers, B.; Ayinampudi, V.; Bowker, N.; Kerrison, N.; et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 2019, 177, 597. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Nunes, C.N.; Ronan, J.J.; Harper, C.; Beall, M.; Hanaway, M.; Fairhurst, A.; Van der Ploeg, L.; Maclntyre, D.; Mellin, T. Melanocortin mediated inhibition of feeding behavior in rats. Neuropeptides 1998, 32, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Strader, A.D.; Schiöth, H.B.; Buntin, J.D. The role of the melanocortin system and the melanocortin-4 receptor in ring dove (Streptopelia risoria) feeding behavior. Brain Res. 2003, 960, 112–121. [Google Scholar]
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef]

| Primers | Sequence (5′–3′) |
|---|---|
| sheep-MC4R-F | GGGAGGTTCAGTCAGTCCAGA |
| sheep-MC4R-R | TCTCTTAAGCTTGTGTTTGGCAC |
| KASP-Forward primer FAM (5′–3′) | AAGGTGACCAAGTTCATGCTTCACCCTGCTGAACAGCACAGACAC |
| KASP-Forward primer VIC (5′–3′) | AAGGTCGGAGTCAACGGATTTCACCCTGCTGAACAGCACAGACAT |
| KASP-Reverse co-primer (5′–3′) | GTCAATATTCACCGTGAAGCTCTGCGCGT |
| Breeds | Genotypes (Size) | Frequency | Ho | He | Ne | PIC | χ2 (p-Value) | |
|---|---|---|---|---|---|---|---|---|
| Genotypes | Alleles | |||||||
| Hu sheep | CC (182) | 0.989 | 0.995 (C) | 0.989 | 0.011 | 1.011 | 0.011 | 0.005 (p = 0.997) |
| CT (2) | 0.011 | 0.005 (T) | ||||||
| TT (0) | 0 | |||||||
| LXBH | CC (91) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| LFT | CC (77) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| GSFW | CC (47) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| YS | CC (40) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| Tan sheep | CC (40) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| Mongolian sheep | CC (47) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| WN | CC (47) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| AUW | CC (82) | 1.000 | 1.000 (C) | 1.000 | 0 | 1.000 | 0 | |
| CT (0) | 0 | 0 (T) | ||||||
| TT (0) | 0 | |||||||
| Ligand | cAMP Response | ERK1/2 Response | |
|---|---|---|---|
| EC50 (µM) | EC50 (µM) | ||
| ACTH (1–24) | WT | 6.142 ± 0.835 a | 3.623 ± 0.303 |
| T117M | 3.933 ± 0.255 b | 2.169 ± 0.919 | |
| α-MSH | WT | 0.848 ± 0.443 | 0.716 ± 0.126 a |
| T117M | 0.058 ± 0.009 | 0.193 ± 0.014 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Yang, Y.; Liu, P.; Yan, T.; Li, R.; Pan, C.; Li, Y.; Lan, X. A Critical Functional Missense Mutation (T117M) in Sheep MC4R Gene Significantly Leads to Gain-of-Function. Animals 2024, 14, 2207. https://doi.org/10.3390/ani14152207
Zhao Z, Yang Y, Liu P, Yan T, Li R, Pan C, Li Y, Lan X. A Critical Functional Missense Mutation (T117M) in Sheep MC4R Gene Significantly Leads to Gain-of-Function. Animals. 2024; 14(15):2207. https://doi.org/10.3390/ani14152207
Chicago/Turabian StyleZhao, Ziyi, Yuta Yang, Peiyao Liu, Taotao Yan, Ran Li, Chuanying Pan, Yang Li, and Xianyong Lan. 2024. "A Critical Functional Missense Mutation (T117M) in Sheep MC4R Gene Significantly Leads to Gain-of-Function" Animals 14, no. 15: 2207. https://doi.org/10.3390/ani14152207





