Heat Shock Related Protein Expression in Abdominal Testes of Asian Elephant (Elephas maximus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Elephant Fibroblast Cells
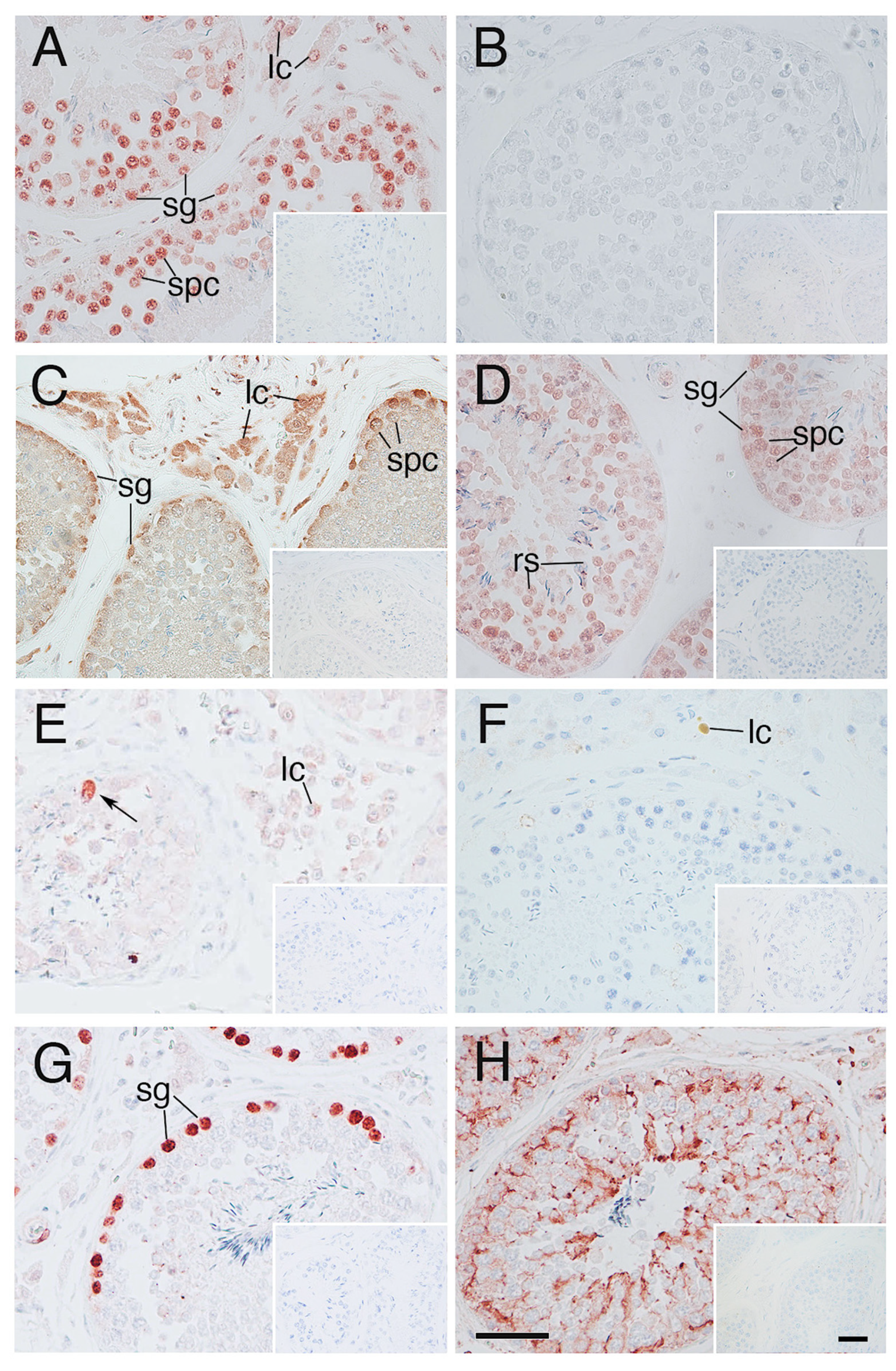
2.2. Tissues of Elephant Testis
2.3. Heat Shock Experiment
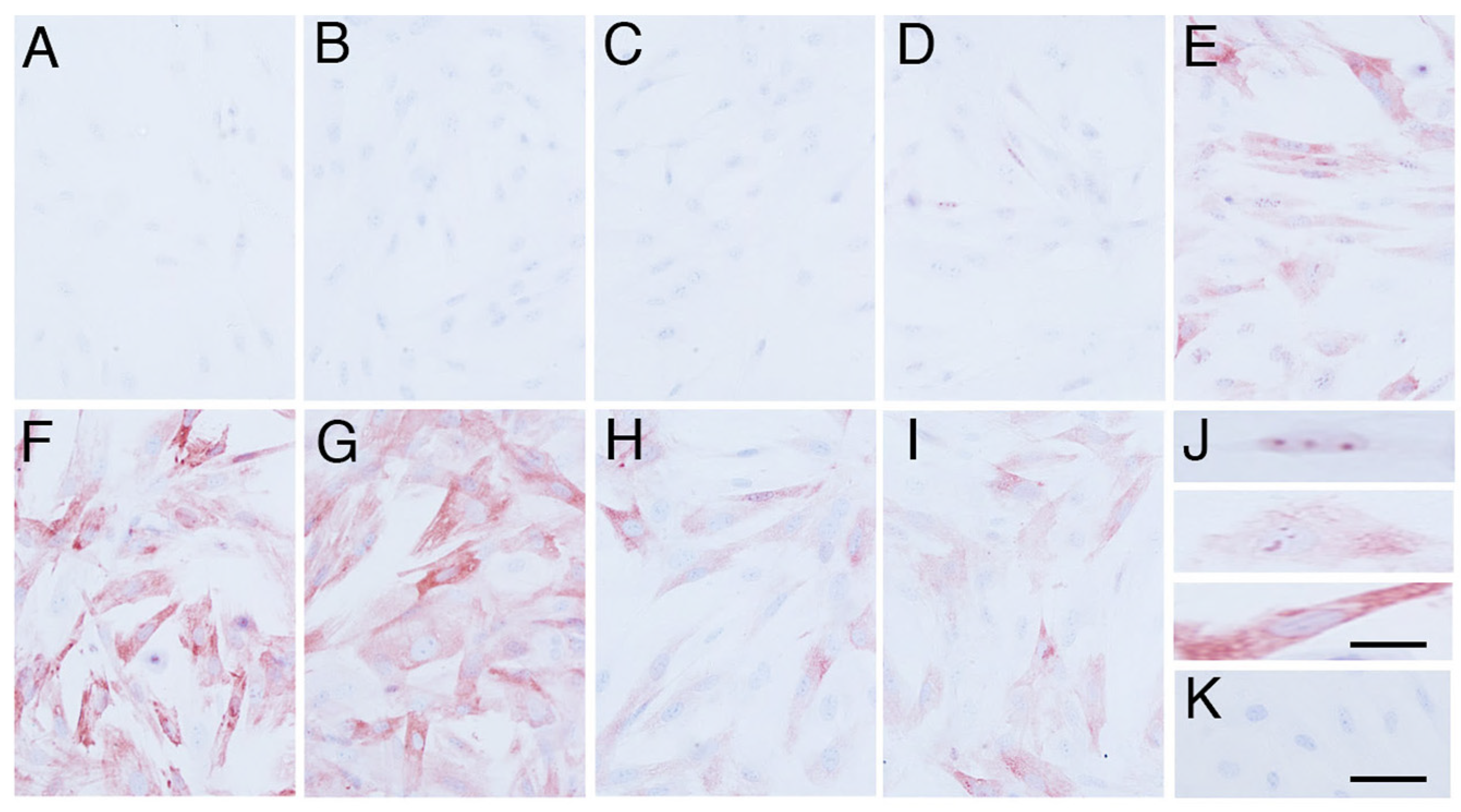
2.4. Immunocytochemistry and Immunohistochemistry
2.5. TUNEL Assay
2.6. Semi-Quantitative Image Analysis of Immunolabeling Cells
2.7. Statistical Analysis
3. Results
3.1. Effects of Heat Shock Stimulus on Elephant Fibroblasts
3.2. Heat Shock Related Molecules Immunoexpression in the Tissues of Elephant Testes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinberger, A. Effects of Temperature on the Biochemistry of the Testis; Zorgniotti, A.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 286, pp. 33–47. [Google Scholar] [CrossRef]
- Kandeel, F.R.; Swerdloff, R.S. Role of temperature in regulation of spermatogenesis and the use of heating as a method of contraception. Fertil. Steril. 1988, 49, 1–23. [Google Scholar] [CrossRef]
- Chowdhury, A.K.; Steinberger, E. A quantitative study of the effect of heat on germinal epithelium of rat testes. Am. J. Anat. 1964, 115, 509–524. [Google Scholar] [CrossRef]
- Kocak, I.; Dundar, M.; Hekimgil, M.; Okyay, P. Assessment of germ cell apoptosis in cryptorchid rats. Asi. J. Androl. 2002, 4, 183–201. [Google Scholar]
- Rommel, S.A.; Pabst, D.A.; McLellan, W.A.; Mead, J.G.; Potter, C.W. Anatomical evidence for a countercurrent heat exchanger associated with dolphin testes. Anat. Rec. 1992, 232, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Johnson, O.W.; Buss, I.O. The testes of the African elephant (Loxodonta africana). I. Histological features. J. Reprod. Fertil. 1967, 13, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Short, R.V.; Mann, T.; Hay, M.F. Male reproductive organs of the African elephant, Loxodonta africana. J. Reprod. Fertil. 1967, 13, 517–536. [Google Scholar] [CrossRef]
- Creasy, D.M. Evaluation of testicular toxicity in safety evaluation studies: The appropriate use of spermatogenic staging. Toxicol. Pathol. 1997, 25, 119–131. [Google Scholar] [CrossRef]
- Hayashida, N.; Inouye, S.; Fujimoto, M.; Tanaka, Y.; Izu, H.; Takaki, E.; Ichikawa, H.; Rho, J.; Nakai, A. A novel HSF1-mediated death pathway that is suppressed by heat shock proteins. EMBO J. 2006, 25, 4773–4783. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Z.L.; Qian, X.J.; Qiu, W.Y.; Huang, H. Expression of Hsf1, Hsf2, and Phlda 1 in Cells undergoing cryptorchid-induced apoptosis in Rat Testes. Mol. Reprod. Dev. 2011, 78, 283–291. [Google Scholar] [CrossRef]
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef]
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004, 5, 781–791. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.R.; Xiao, X.; Shao, L.; Graves, K.; Benjamin, I.J. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 1998, 273, 7523–7528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Zhang, J.; Moskophidis, D.; Mivechi, N.F. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J. Cell. Biochem. 2002, 86, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Katsuki, K.; Izu, H.; Fujimoto, M.; Sugahara, K.; Yamada, S.-I.; Shinkai, Y.; Oka, Y.; Katoh, Y.; Nakai, A. Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperature. Mol. Cell. Biol. 2003, 23, 5882–5895. [Google Scholar] [CrossRef] [PubMed]
- Kurt, F.; Garai, M.E. The Asian Elephant in Captivity: A Field Study; Cambridge University Press India Pvt. Ltd.: New Delhi, India, 2007; 52p. [Google Scholar]
- Dumonceaux, G.A. Digestive System; Fowler, M., Mikota, S., Eds.; Biology, Medicine, and Surgery of Elephants; Blackwell Publishing: Ames, IA, USA, 2006; pp. 299–307. [Google Scholar]
- Miller, M.; Terrell, S. Elephant TAG/SSP research and necropsy protocol (Elephas maximus and Loxodonta africana). The American Zoo and Aquarium Association Elephant Species Survival Plan. 2016. Available online: https://www.aphis.usda.gov/animal_welfare/downloads/elephant/ELEPHANT-NECROPSY-PROTOCOL-March%202016.pdf (accessed on 2 July 2024).
- Pushpakumara, A.; Thitaram, C.; Brown, J.L. Elephants. In Veterinary. Reproduction & Obstetrics, 10th ed.; Noakes, D., Parkinson, T., England, G., Eds.; Saunders Ltd.: Philadelphia, PA, USA, 2018; pp. 724–744. [Google Scholar]
- Sananmuang, T.; Phutikanit, N.; Nguyen, C.; Manee-In, S.; Techakumphu, M.; Tharasanit, T. In vitro culture of feline embryos increases stress-induced heat shock protein 70 and apoptotic related genes. J. Reprod. Dev. 2013, 59, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshida, K.; Nozawa, S.; Yoshiike, M.; Arai, M.; Otoi, T.; Iwamoto, T. Establishment of adult mouse Sertoli cell lines by using starvation method. Reproduction 2013, 145, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nozawa, S.; Yoshiike, M.; Otoi, T.; Iwamoto, T. Glycoconjugates recognized by peanut agglutinin lectin in the inner acellular layer of the lamina propria of seminiferous tubules in human testes showing impaired spermatogenesis. Hum. Reprod. 2012, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Koji, T.; Hishikawa, Y. Germ cell apoptosis and its molecular trigger in mouse testes. Arch. Histol. Cytol. 2003, 66, 1–16. [Google Scholar] [CrossRef]
- Sarge, K.D. Male germ cell-specific alteration in temperature set point of the cellular stress response. J. Biol. Chem. 1995, 270, 18745–18748. [Google Scholar] [CrossRef]
- Izu, H.; Inouye, S.; Fujimoto, M.; Shiraishi, K.; Naito, K.; Nakai, A. Heat shock transcription factor 1 is involved in quality-control mechanisms in male germ cells. Biol. Reprod. 2004, 70, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahmoubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zuo, X.; Davis, A.A.; McMillan, D.R.; Curry, B.B.; Richardson, J.A.; Benjamin, I.J. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999, 18, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liw, X.; Lei, K.; Liw, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, A.A.; Inge-moller, R.; Bateman, P.W.; Kotze, A.; Scantlebury, M. Body temperature daily rhythm adaptations in African savanna elephants (Loxodonta africana). Physiol. Behav. 2007, 92, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.F.; Bakken, G.S.; Ratliff, J.J.; Langman, V.A. Heat storage in Asian elephants during submaximal exercise: Behavioral regulation of thermoregulatory constraints on activity in endothermic gigantothermy. J. Exp. Biol. 2013, 216, 1774–1785. [Google Scholar] [CrossRef]
- Valeri, A.; Mianne, D.; Merouze, F.; Bujan, L.; Altobelli, A.; Masson, J. Scrotal temperature in 258 healthy men, randomly selected from a population of men aged 18 to 23 years old. Statistical analysis, epidemiologic observations, and measurement of the testicular diameters. Progrè. Urol. 1993, 3, 444–452. [Google Scholar]
- Garolla, A.; Torino, M.; Miola, P.; Caretta, N.; Pizzol, D.; Menegazzo, M.; Bertoldo, A.; Foresta, C. Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Hum. Reprod. 2015, 30, 1006–1013. [Google Scholar] [CrossRef]
- Hjollund, N.H.I.; Storgaard, L.; Ernst, E.; Bonde, J.P.; Olsen, J. Impact of diurnal scrotal temperature on semen quality. Reprod. Toxicol. 2002, 16, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Degabriele, R. The physiology of the Koala. Sci. Am. 1980, 243, 110–117. [Google Scholar] [CrossRef]
- Thongtip, N.; Saikhun, J.; Mahasawangkul, S.; Kornkaewrat, K.; Pongsopavijitr, P.; Songsasen, N.; Pinyopummin, A. Potential factors affecting semen quality in the Asian elephant (Elephas maximus). Reprod. Biol. Endocrinol. 2008, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thongphakdee, A.; Kiatsomboon, S.; Noimoon, S.; Kongporm, U.; Boonorana, I.; Karoon, S.; Thawnern, J.; Sakulthai, A.; Sombuoputorn, P.; Sukmak, M.; et al. Semen characteristics and second successful artificial insemination of Asian elephant (Elephas maximus) in Thailand). Vet. World 2022, 15, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Bronson, F.H.; Heideman, P.D. Failure of cryptorchidism to suppress fertility in a tropical rodent. Biol. Reprod. 1993, 48, 1354–1359. [Google Scholar] [CrossRef] [PubMed]




| control | NH0h | NH1h | NH2h | NH4h | NH6h | NH12h | NH18h | NH24h | |
|---|---|---|---|---|---|---|---|---|---|
| HSF1 | 9.38 ± 1.23 | 11.28 ± 1.43 | 11.06 ± 2.25 | 11.61 ± 1.02 | 11.15 ± 1.13 | 10.74 ± 0.87 | 9.27 ± 0.74 | ||
| HSP70 | 9.34 ± 0.44 | 11.61 ± 0.77 | 11.2 ± 0.31 | 10.76 ± 0.39 | 9.48 ± 0.43 | 10.08 ± 0.18 | 10.84 ± 0.40 | 9.49 ± 0.31 | 9.46 ± 0.28 |
| TDAG51 | 1.39 ± 0.23 | 1.35 ± 0.11 | 1.15 ± 0.09 | 1.59 ± 0.16 | 1.12 ± 0.12 | 1.16 ± 0.11 | 1.53 ± 0.23 | 1.07 ± 0.09 | 1.61 ± 0.16 |
| TUNEL | 1.13 ± 0.14 | 1.10 ± 0.36 | 1.42 ± 0.11 | 0.60 ± 0.12 | 0.94 ± 0.11 | 0.81 ± 0.09 | 0.39 ± 0.10 | 0.56 ± 0.11 | 1.04 ± 0.14 |
| b-act | 13.76 ± 0.47 | 13.07 ± 1.51 | 13.42 ± 0.93 | 13.09 ± 1.70 | 9.51 ± 0.77 | 12.12 ± 0.95 | 11.91 ± 1.43 | 9.38 ± 0.90 | 10.90 ± 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Tharasanit, T.; Thitaram, C.; Somgird, C.; Mahasawangkul, S.; Thongtip, N.; Chatdarong, K.; Tiptanavattana, N.; Taniguchi, M.; Otoi, T.; et al. Heat Shock Related Protein Expression in Abdominal Testes of Asian Elephant (Elephas maximus). Animals 2024, 14, 2211. https://doi.org/10.3390/ani14152211
Sato Y, Tharasanit T, Thitaram C, Somgird C, Mahasawangkul S, Thongtip N, Chatdarong K, Tiptanavattana N, Taniguchi M, Otoi T, et al. Heat Shock Related Protein Expression in Abdominal Testes of Asian Elephant (Elephas maximus). Animals. 2024; 14(15):2211. https://doi.org/10.3390/ani14152211
Chicago/Turabian StyleSato, Yoko, Theerawat Tharasanit, Chatchote Thitaram, Chaleamchat Somgird, Sittidet Mahasawangkul, Nikorn Thongtip, Kaywalee Chatdarong, Narong Tiptanavattana, Masayasu Taniguchi, Takeshige Otoi, and et al. 2024. "Heat Shock Related Protein Expression in Abdominal Testes of Asian Elephant (Elephas maximus)" Animals 14, no. 15: 2211. https://doi.org/10.3390/ani14152211






