Affective Implications of Human–Animal Relationship on Pig Welfare: Integrating Non-Linear Heart Rate Variability Measures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
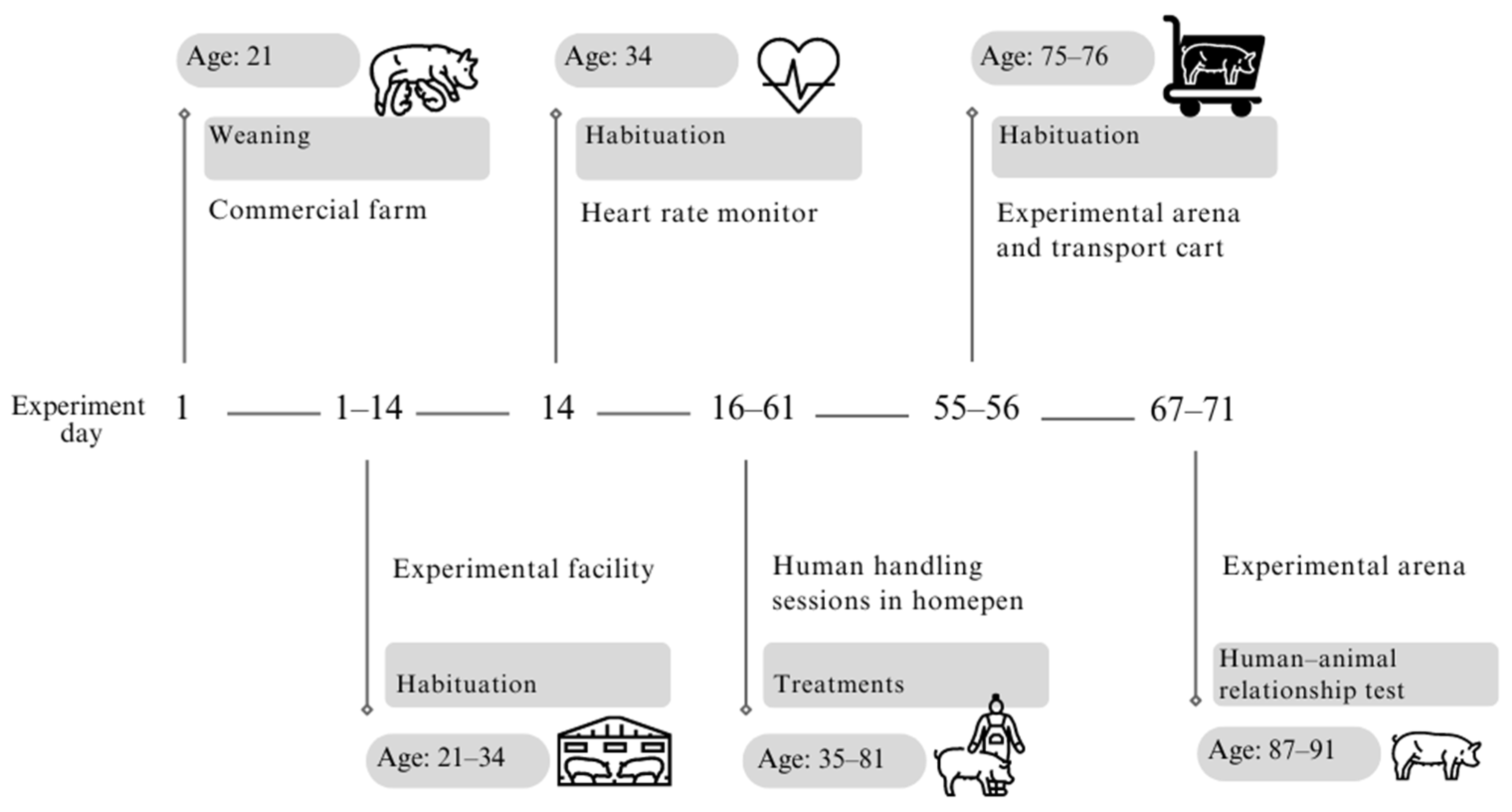
2.1. Animals and Housing
2.2. Treatments
2.2.1. Positive Human Handling (PHH)
2.2.2. Minimal Human Handling (MHH)
2.2.3. Negative Human Handling (NHH)
2.3. Human–Animal Relationship Test
2.4. Behavioral Measurements
2.5. Measurement of Cardiac Activity
| Parameter | Description | |
|---|---|---|
| Linear domains | Time domain | |
| Mean RR interval (ms) | Mean interval between adjacent heart beats (RR) over a period [25]. | |
| SDNN (ms) | Standard deviation of inter-beats intervals, which is driven by both sympathetic and parasympathetic activity [26,27]. | |
| RMSSD (ms) | The root mean square of successive inter-beat intervals over a period, which reflects parasympathetic activity. Greater values indicate an increased parasympathetic input [15,26]. | |
| RMSSD/SDNN | Ratio that reflects the overall balance of the autonomic nervous system. Greater values indicate an increase in parasympathetic activity or a decrease in the activation of the sympathetic system [4,28,29]. | |
| Frequency domain | ||
| LF | Low frequency band, which reflects both parasympathetic and sympathetic regulation [11]. | |
| HF | High frequency band, which reflects parasympathetic regulation, where greater values indicate an increased parasympathetic input [30]. | |
| LF/HF | Ratio that reflects the overall balance of the autonomic nervous system, with an increase in the LF/HF ratio being interpreted as a regulatory shift towards sympathetic dominance [11,25]. | |
| Non-linear domains | SampEn | Sample entropy, which measures the unpredictability of fluctuations and indicates the regularity of data patterns. Lower values indicate increased regularity in the HRV data [25,31]. |
| DFAα1 | Short-term detrended fluctuation analysis used for short-term measures of RR fluctuations at various time lengths to evaluate HR signal self-similarity. Values closer to zero indicate lower stress [16]. | |
| % REC | Percent recurrence, which is determined through recurrence quantification analysis, the percentage of recurrent points (within some radius) in the recurrence plot. Greater values indicate increased HR regularity and greater physiological stress [27,32]. | |
| %DET | Percent determinism, which is determined through recurrence quantification analysis, the percentage of recurrent points that form a diagonal line in the recurrence plot. Greater values indicate greater incidence of periodicity in the HRV data and greater physiological stress [27,32]. | |
| Lmean (beats) | Mean line length of diagonal line, which is determined through recurrence quantification analysis. Greater values indicate periodicities with longer durations in the HRV data and greater physiological stress [33,34]. | |
2.6. Statistical Analysis
3. Results
3.1. Behavioral Reactions during Human–Animal Relationship Test
3.1.1. Phase 1: Habituation
3.1.2. Phase 2: Stationary Standing Human
3.1.3. Phase 3: Stationary Sitting Human
3.1.4. Phase 4: Forced Human Interaction
3.2. Cardiac Activity of Pigs during the Test
3.2.1. Linear Parameters of Heart Rate Variability
3.2.2. Non-Linear Parameters of Heart Rate Variability
4. Discussion
4.1. Pigs’ Behavioral Reactions during the Human–Animal Relationship Test
4.2. Cardiac Response of Pigs during the Human–Animal Relationship Test
4.3. Integrative Interpretation of Affective States and Welfare Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; Waiblinger, S.; Boivin, X.; Hemsworth, P. The Power of a Positive Human–Animal Relationship for Animal Welfare. Front. Vet. Sci. 2020, 7, 590867. [Google Scholar] [CrossRef] [PubMed]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the Human–Animal Relationship in Farmed Species: A Critical Review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Luna, D.; González, C.; Byrd, C.J.; Palomo, R.; Huenul, E.; Figueroa, J. Do Domestic Pigs Acquire a Positive Perception of Humans through Observational Social Learning? Animals 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Barnett, J.L.; Hansen, C. The Influence of Handling by Humans on the Behavior, Growth, and Corticosteroids in the Juvenile Female Pig. Horm. Behav. 1981, 15, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Barnett, J.L.; Coleman, G.J.; Hansen, C. A Study of the Relationships between the Attitudinal and Behavioural Profiles of Stockpersons and the Level of Fear of Humans and Reproductive Performance of Commercial Pigs. Appl. Anim. Behav. Sci. 1989, 23, 301–314. [Google Scholar] [CrossRef]
- Brajon, S.; Laforest, J.P.; Schmitt, O.; Devillers, N. The Way Humans Behave Modulates the Emotional State of Piglets. PLoS ONE 2015, 10, e0133408. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.P.; Paul, E.S. An Integrative and Functional Framework for the Study of Animal Emotion and Mood. Proc. R. Soc. B Biol. Sci. 2010, 277, 2895–2904. [Google Scholar] [CrossRef]
- Reefmann, N.; Wechsler, B.; Gygax, L. Behavioural and Physiological Assessment of Positive and Negative Emotion in Sheep. Anim. Behav. 2009, 78, 651–659. [Google Scholar] [CrossRef]
- Kuhne, F.; Hößler, J.C.; Struwe, R. Emotions in Dogs Being Petted by a Familiar or Unfamiliar Person: Validating Behavioural Indicators of Emotional States Using Heart Rate Variability. Appl. Anim. Behav. Sci. 2014, 161, 113–120. [Google Scholar] [CrossRef]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart Rate Variability as a Measure of Autonomic Regulation of Cardiac Activity for Assessing Stress and Welfare in Farm Animals—A Review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Tallet, C.; Sy, K.; Prunier, A.; Nowak, R.; Boissy, A.; Boivin, X. Behavioural and Physiological Reactions of Piglets to Gentle Tactile Interactions Vary According to Their Previous Experience with Humans. Livest. Sci. 2014, 167, 331–341. [Google Scholar] [CrossRef]
- Leliveld, L.M.C.; Düpjan, S.; Tuchscherer, A.; Puppe, B. Behavioural and Physiological Measures Indicate Subtle Variations in the Emotional Valence of Young Pigs. Physiol. Behav. 2016, 157, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Social Support in Pigs with Different Coping Styles. Physiol. Behav. 2014, 129, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.J.; Johnson, J.S.; Radcliffe, J.S.; Craig, B.A.; Eicher, S.D.; Lay, D.C. Nonlinear Analysis of Heart Rate Variability for Evaluating the Growing Pig Stress Response to an Acute Heat Episode. Animal 2020, 14, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.-K.; Stanley, H.E. Fractal Dynamics in Physiology: Alterations with Disease and Aging. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. S1), 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.E.; Hemsworth, L.M.; Morrison, R.S.; Tilbrook, A.J.; Hemsworth, P.H. Positive Human Contact and Housing Systems Impact the Responses of Piglets to Various Stressors. Animals 2021, 11, 1619. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine, 11th ed.; Revised; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Koba, Y.; Tanida, H. How Do Miniature Pigs Discriminate between People? The Effect of Exchanging Cues between a Non-Handler and Their Familiar Handler on Discrimination. Appl. Anim. Behav. Sci. 1999, 61, 239–252. [Google Scholar] [CrossRef]
- Brajon, S.; Laforest, J.P.; Bergeron, R.; Tallet, C.; Hötzel, M.J.; Devillers, N. Persistency of the Piglet’s Reactivity to the Handler Following a Previous Positive or Negative Experience. Appl. Anim. Behav. Sci. 2015, 162, 9–19. [Google Scholar] [CrossRef]
- Poletto, R.; Janczak, A.M.; Marchant-Forde, R.M.; Marchant, J.N.; Matthews, D.L.; Dowell, C.A.; Hogan, D.F.; Freeman, L.J.; Lay, D.C. Identification of Low and High Frequency Ranges for Heart Rate Variability and Blood Pressure Variability Analyses Using Pharmacological Autonomic Blockade with Atropine and Propranolol in Swine. Physiol. Behav. 2011, 103, 188–196. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 28 July 2024).
- Wallot, S. Recurrence Quantification Analysis of Processes and Products of Discourse: A Tutorial in R. Discourse Process 2017, 54, 382–405. [Google Scholar] [CrossRef]
- Byrd, C.J.; Radcliffe, J.S.; Craig, B.A.; Eicher, S.D.; Lay, D.C. Measuring Piglet Castration Pain Using Linear and Non-Linear Measures of Heart Rate Variability. Anim. Welf. 2020, 29, 257–269. [Google Scholar] [CrossRef]
- de Jong, I.C.; Sgoifo, A.; Lambooij, E.; Korte, S.M.; Blokhuis, H.J.; Koolhaas, J.M. Effects of Social Stress on Heart Rate and Heart Rate Variability in Growing Pigs. Can. J. Anim. Sci. 2000, 80, 273–280. [Google Scholar] [CrossRef]
- Mohr, E.; Langbein, J.; Nürnberg, G. Heart Rate Variability. A Noninvasive Approach to Measure Stress in Calves and Cows. Physiol. Behav. 2002, 75, 251–259. [Google Scholar] [CrossRef]
- Zebunke, M.; Langbein, J.; Manteuffel, G.; Puppe, B. Autonomic Reactions Indicating Positive Affect during Acoustic Reward Learning in Domestic Pigs. Anim. Behav. 2011, 81, 481–489. [Google Scholar] [CrossRef]
- Tamioso, P.R.; Maiolino Molento, C.F.; Boivin, X.; Chandèze, H.; Andanson, S.; Delval, É.; Hazard, D.; da Silva, G.P.; Taconeli, C.A.; Boissy, A. Inducing Positive Emotions: Behavioural and Cardiac Responses to Human and Brushing in Ewes Selected for High vs Low Social Reactivity. Appl. Anim. Behav. Sci. 2018, 208, 56–65. [Google Scholar] [CrossRef]
- Byrd, C.J.; Mcconn, B.R.; Gaskill, B.N.; Schinckel, A.P.; Green-Miller, A.R.; Lay, D.C.; Johnson, J.S. Characterizing the Effect of Incrementally Increasing Dry Bulb Temperature on Linear and Nonlinear Measures of Heart Rate Variability in Nonpregnant, Mid-Gestation, and Late-Gestation Sows. J. Anim. Sci. 2022, 100, skac004. [Google Scholar] [CrossRef]
- Parois, S.P.; Cabezón, F.A.; Schinckel, A.P.; Johnson, J.S.; Stwalley, R.M.; Marchant-Forde, J.N. Effect of Floor Cooling on Behavior and Heart Rate of Late Lactation Sows under Acute Heat Stress. Front. Vet. Sci. 2018, 5, 223. [Google Scholar] [CrossRef]
- Schlenker, J.; Socha, V.; Riedlbauchová, L.; Nedělka, T.; Schlenker, A.; Potočková, V.; Malá, Š.; Kutílek, P. Recurrence Plot of Heart Rate Variability Signal in Patients with Vasovagal Syncopes. Biomed. Signal Process Control 2016, 25, 1–11. [Google Scholar] [CrossRef]
- Mohebbi, M.; Ghassemian, H. Prediction of Paroxysmal Atrial Fibrillation Using Recurrence Plot-Based Features of the RR-Interval Signal. Physiol. Meas. 2011, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Dua, S.; Du, X.; Vinitha Sree, S.; Thajudin Ahamed, V.I. Novel Classification of Coronary Artery Disease Using Heart Rate Variability Analysis. J. Mech. Med. Biol. 2012, 12, 1240017. [Google Scholar] [CrossRef]
- Krause, A.; Puppe, B.; Langbein, J. Coping Style Modifies General and Affective Autonomic Reactions of Domestic Pigs in Different Behavioral Contexts. Front. Behav. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, N.; Griffin, G.; Gauthier, C. The Welfare of Animals Used in Science: How the “Three Rs” Ethic Guides Improvements. Can. Vet. J. 2009, 50, 523–530. [Google Scholar] [PubMed]
- Friard, O.; Gamba, M. BORIS: A Free, Versatile Open-Source Event-Logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R. J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Villain, A.; Guérin, C.; Tallet, C. The Use of Pigs Vocalisation to Assess the Quality Of-Pig Relationship. Peer Community J. 2023, 3, e36. [Google Scholar] [CrossRef]
- Tallet, C.; Brajon, S.; Devillers, N.; Lensink, J. Pig-Human Interactions: Creating a Positive Perception of Humans to Ensure Pig Welfare. In Advances in Pig Welfare; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 381–398. ISBN 9780081011195. [Google Scholar]
- Acharya, R.Y.; Hemsworth, P.H.; Coleman, G.J.; Kinder, J.E. The Animal-Human Interface in Farm Animal Production: Animal Fear, Stress, Reproduction and Welfare. Animals 2022, 12, 487. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Bi, Y.; Zhao, P.; Sun, H.; Li, J.; Liu, H.; Zhang, R.; Li, X.; Bao, J. Effects of Long-Term Gentle Handling on Behavioral Responses, Production Performance, and Meat Quality of Pigs. Animals 2020, 10, 2–12. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L. The Effects of Aversively Handling Pigs, Either Individually or in Groups, on Their Behaviour, Growth and Corticosteroids. Appl. Anim. Behav. Sci. 1991, 30, 61–72. [Google Scholar] [CrossRef]
- de Oliveira, D.; Paranhos da Costa, M.J.R.; Zupan, M.; Rehn, T.; Keeling, L.J. Early Human Handling in Non-Weaned Piglets: Effects on Behaviour and Body Weight. Appl. Anim. Behav. Sci. 2015, 164, 56–63. [Google Scholar] [CrossRef]
- Briefer, E.F.; Sypherd, C.C.R.; Linhart, P.; Leliveld, L.M.C.; Padilla de la Torre, M.; Read, E.R.; Guérin, C.; Deiss, V.; Monestier, C.; Rasmussen, J.H.; et al. Classification of Pig Calls Produced from Birth to Slaughter According to Their Emotional Valence and Context of Production. Sci. Rep. 2022, 12, 3409. [Google Scholar] [CrossRef] [PubMed]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of Positive and Negative Emotions and Emotional Contagion in Pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Battini, M.; Barbieri, S.; Waiblinger, S.; Mattiello, S. Validity and Feasibility of Human-Animal Relationship Tests for on-Farm Welfare Assessment in Dairy Goats. Appl. Anim. Behav. Sci. 2016, 178, 32–39. [Google Scholar] [CrossRef]
- Henry, S.; Hemery, D.; Richard, M.A.; Hausberger, M. Human–Mare Relationships and Behaviour of Foals toward Humans. Appl. Anim. Behav. Sci. 2005, 93, 341–362. [Google Scholar] [CrossRef]
- Miura, A.; Tanida, H.; Tanaka, T.; Yoshimoto, T. The Influence of Human Posture and Movement on the Approach and Escape Behaviour of Weanling Pigs. Appl. Anim. Behav. Sci. 1996, 49, 247–256. [Google Scholar] [CrossRef]
- Coulon, M.; Nowak, R.; Peyrat, J.; Chandèze, H.; Boissy, A.; Boivin, X. Do Lambs Perceive Regular Human Stroking as Pleasant? Behavior and Heart Rate Variability Analyses. PLoS ONE 2015, 10, e0118617. [Google Scholar] [CrossRef]
- Kremer, L.; Klein Holkenborg, S.E.J.; Reimert, I.; Bolhuis, J.E.; Webb, L.E. The Nuts and Bolts of Animal Emotion. Neurosci. Biobehav. Rev. 2020, 113, 273–286. [Google Scholar] [CrossRef]
- Ernst, G. Heart-Rate Variability—More than Heart Beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef]
- Shi, H.; Yang, L.; Zhao, L.; Su, Z.; Mao, X.; Zhang, L.; Liu, C. Differences of Heart Rate Variability Between Happiness and Sadness Emotion States: A Pilot Study. J. Med. Biol. Eng. 2017, 37, 527–539. [Google Scholar] [CrossRef]
- Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology Guidelines. Heart Rate Variability Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Kitajima, K.; Oishi, K.; Miwa, M.; Anzai, H.; Setoguchi, A.; Yasunaka, Y.; Himeno, Y.; Kumagai, H.; Hirooka, H. Effects of Heat Stress on Heart Rate Variability in Free-Moving Sheep and Goats Assessed With Correction for Physical Activity. Front. Vet. Sci. 2021, 8, 658763. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 528. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Simitzis, P.E. Positive Welfare Indicators in Dairy Animals. Dairy. 2022, 3, 814–841. [Google Scholar] [CrossRef]
- Khairudin, R.; Nasir, R.; Halim, F.W.; Zainah, A.Z.; Wan Shahrazad, W.S.; Ismail, K.; Valipour, G.M. Emotion and Explicit Verbal Memory: Evidence Using Malay Lexicon. Asian Soc. Sci. 2012, 8, 38. [Google Scholar] [CrossRef]
- Anderson, D.J.; Adolphs, R. A Framework for Studying Emotions across Species. Cell 2014, 157, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of Human-Animal Relationship on Animal Productivity and Welfare. J. Anim. Behav. Biometeorol. 2020, 8, 196–205. [Google Scholar] [CrossRef]
- Scopa, C.; Greco, A.; Contalbrigo, L.; Fratini, E.; Lanatà, A.; Scilingo, E.P.; Baragli, P. Inside the Interaction: Contact with Familiar Humans Modulates Heart Rate Variability in Horses. Front. Vet. Sci. 2020, 7, 582759. [Google Scholar] [CrossRef]
- Lange, A.; Bauer, L.; Futschik, A.; Waiblinger, S. Talking to Cows: Reactions to Different Auditory Stimuli During Gentle Human-Animal Interactions. Front. Psychol. 2020, 11, 579346. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neroscience: The Foundations of Human and Animal Emotion; OUP: New York, NY, USA, 1998; ISBN 9780195096736. [Google Scholar]
- Paul, E.S.; Mendl, M.T. Animal Emotion: Descriptive and Prescriptive Definitions and Their Implications for a Comparative Perspective. Appl. Anim. Behav. Sci. 2018, 205, 202–209. [Google Scholar] [CrossRef] [PubMed]
- De Waal, F.; Andrews, K. The Question of Animal Emotions. Science 2022, 375, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Gonyou, H.W.; Hemsworth, P.H.; Barnett, J.L. Effects of Frequent Interactions with Humans on Growing Pigs. Appl. Anim. Behav. Sci. 1986, 16, 269–278. [Google Scholar] [CrossRef]
- Alonso, M.E.; González-Montaña, J.R.; Lomillos, J.M. Consumers’ Concerns and Perceptions of Farm Animal Welfare. Animals 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Reimert, I.; Rodenburg, B.; Ursinus, W.W.; Duijvesteijn, N.; Camerlink, I.; Kemp, B.; Bolhuis, J.E. Backtest and Novelty Behavior of Female and Castrated Male Piglets, with Diverging Social Breeding Values for Growth. J. Anim. Sci. 2013, 91, 4589–4597. [Google Scholar] [CrossRef]
- Kanitz, E.; Tuchscherer, M.; Otten, W.; Tuchscherer, A.; Zebunke, M.; Puppe, B. Coping Style of Pigs Is Associated With Different Behavioral, Neurobiological and Immune Responses to Stressful Challenges. Front. Behav. Neurosci. 2019, 13, 173. [Google Scholar] [CrossRef]


| Behavior | Description | Phase 1 |
|---|---|---|
| Locomotor activity | Number of quadrants that the pig crossed with both of its front legs. | 1, 4 |
| Latency to first physical contact | Time (seconds) required for the pig to make physical contact with any part of the handler’s body. | 2, 3 |
| Time in physical contact with the handler | Time (%) the pig remained in physical contact with any part of the handler’s body, either touching or smelling. | 2, 3 |
| Accepted strokes | Strokes (%) accepted by each pig based on the total number of attempts made by the handler. | 3, 4 |
| Attempts needed to accept the first stroke | Number of attempts made by the handler until the pig accepted the first stroke. | 4 |
| High-pitched vocalizations | Number of screams, squeals or grunt–squeals. | 1, 2, 3, 4 |
| Low-pitched vocalizations | Number of short or long grunts. | 1, 2, 3, 4 |
| Treatments | ||||
|---|---|---|---|---|
| Behavior | PHH (n = 12) | MHH (n = 12) | NHH (n = 12) | p-Value |
| Phase 1 | ||||
| Locomotor activity | 19.16 ± 2.53 | 21.50 ± 2.13 | 20.50 ± 2.28 | 0.81 |
| High-pitched vocalizations | 0.16 ± 0.11 | 0 | 0 | 0.12 |
| Low-pitched vocalizations | 18.25 ± 4.12 | 14.58 ± 3.52 | 13.75 ± 3.12 | 0.64 |
| Phase 2 | ||||
| Latency to first contact (s) | 12.04 ± 5.54 b | 49.60 ± 5.45 a | 40.22 ± 5.36 a | <0.001 † |
| Time in physical contact (%) | 33.20 ± 10.09 a | 3.16 ± 2.17 b | 9.86 ± 3.37 b | 0.006 |
| Locomotor activity | 9.58 ± 2.53 | 13.16 ± 2.13 | 13.75 ± 2.28 | 0.39 |
| High-pitched vocalizations | 0.08 ± 0.08 | 1.25 ± 1.25 | 2.75 ± 2.31 | 0.47 |
| Low-pitched vocalizations | 20.75 ± 4.34 | 24.5 ± 3.99 | 22.25 ± 4.17 | 0.81 |
| Phase 3 | ||||
| Latency to first contact (s) | 2.81 ± 1.72 b | 6.91 ± 3.87 b | 39.58 ± 13.83 a | 0.003 ‡ |
| Time in physical contact (%) | 41.00 ± 6.19 a | 49.46 ± 8.20 a | 16.55 ± 7.37 b | 0.008 |
| Accepted strokes (%) | 94.76 ± 2.63 a | 70.48 ± 7.42 b | 5.12 ± 2.64 c | <0.001 |
| Locomotor activity | 23.75 ± 2.75 | 20.91 ± 1.71 | 25.25 ± 3.35 | 0.52 |
| High-pitched vocalizations | 0 (0–0) | 0 (0–0) | 0 (0–9.75) | 0.06 # |
| Low-pitched vocalizations | 46.75 ± 12.04 | 57.08 ± 12.31 | 50.50 ± 12.14 | 0.83 |
| Phase 4 | ||||
| Accepted strokes (%) | 96.11 ± 1.80 a | 76.84 ± 3.25 b | 20.91 ± 3.89 c | <0.001 |
| Attempts until accepting first stroke | 1.25 ± 0.25 a | 1 ± 0.35 a | 8.50 ± 1.50 b | <0.001 ‡ |
| High-pitched vocalizations | 0 (0–0) b | 0.5 (0–1,75)b | 5 (0–11) a | 0.002 # |
| Low-pitched vocalizations | 34.41 ± 8.66 b | 40.08 ± 8.10 b | 76.00 ± 13.57 a | 0.02 |
| Locomotor activity | 41.25 ± 5.47 b | 42.41 ± 5.47 b | 63.50 ± 5.47 a | 0.01 |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | PHH (n = 12) | MHH (n = 12) | NHH (n = 12) | p-Value |
| RR Interval (ms) | 380.75 ± 6.38 a | 349.08 ± 6.23 b | 370.33 ± 6.32 a | 0.004 |
| SDNN (ms) | 26.90 ± 1.50 | 21.80 ± 1.59 | 29.13 ± 2.96 | 0.058 |
| RMSSD (ms) | 14.06 ± 1.03 a | 9.45 ± 0.62 b | 10.78 ± 0.79 b | 0.001 |
| RMSSD/SDNN (ms) | 0.51 ± 0.01 a | 0.43 ± 0.01 b | 0.39 ± 0.02 b | <0.001 |
| LF | 502.17 ± 79.70 ab | 311.67 ± 36.77 b | 763.25 ± 163.92 a | 0.02 |
| HF | 227.91 ± 30.81 | 151.91 ± 25.89 | 169.75 ± 34.43 | 0.17 |
| LF/HF | 2.17 ± 0.14 b | 2.55 ± 0.49 b | 4.68 ± 0.76 a | 0.002 |
| SampEn | 1.50 ± 0.03 a | 1.37 ± 0.05 ab | 1.24 ± 0.09 b | 0.02 |
| DFAα1 | 1.36 ± 0.03 | 1.42 ± 0.02 | 1.42 ± 0.02 | 0.23 |
| %REC (%) | 0.05 ± 0.001 | 0.05 ± 0.0009 | 0.05 ± 0.0006 | 0.90 |
| %DET (%) | 0.45 ± 0.01 c | 0.50 ± 0.01 b | 0.63 ± 0.02 a | <0.001 |
| Lmean (beats) | 2.50 ± 0.02 c | 2.73 ± 0.05 b | 3.04 ± 0.10 a | <0.001 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Amor, J.; Zuleta, B.; Ceballos, M.C.; Cartes, D.; Byrd, C.J.; Lecorps, B.; Palomo, R.; Guzmán-Pino, S.A.; Siel, D.; Luna, D. Affective Implications of Human–Animal Relationship on Pig Welfare: Integrating Non-Linear Heart Rate Variability Measures. Animals 2024, 14, 2217. https://doi.org/10.3390/ani14152217
Calderón-Amor J, Zuleta B, Ceballos MC, Cartes D, Byrd CJ, Lecorps B, Palomo R, Guzmán-Pino SA, Siel D, Luna D. Affective Implications of Human–Animal Relationship on Pig Welfare: Integrating Non-Linear Heart Rate Variability Measures. Animals. 2024; 14(15):2217. https://doi.org/10.3390/ani14152217
Chicago/Turabian StyleCalderón-Amor, Javiera, Belén Zuleta, Maria Camila Ceballos, Daniel Cartes, Christopher J. Byrd, Benjamin Lecorps, Rocío Palomo, Sergio A. Guzmán-Pino, Daniela Siel, and Daniela Luna. 2024. "Affective Implications of Human–Animal Relationship on Pig Welfare: Integrating Non-Linear Heart Rate Variability Measures" Animals 14, no. 15: 2217. https://doi.org/10.3390/ani14152217








